Abstract
Laminoplasty has been gradually accepted as a treatment for choice for cervical compression myelopathy. The historical perspective of laminoplasty is described. The aims of laminoplasty are to expand the spinal canal, to secure spinal stability, to preserve the protective function of the spine, and to preserve spinal mobility. Laminoplasty is indicated in myelopathic patients with a developmentally narrow spinal canal or multiple-level involvement combined with a relatively narrow canal. Several laminoplasty techniques and supplementary techniques are described, together with expected outcomes and complications of surgery.
Keywords: Laminectomy, Laminoplasty, Cervical myelopathy, History, Surgical technique
Laminectomy was the sole procedure by which to access the spinal canal until Robinson and Smith [24], and Cloward [2] devised the anterior procedures, and was a choice of treatment for cervical spondylotic myelopathy (CSM) or ossification of the posterior longitudinal ligament (OPLL). However, laminectomy for these conditions was not always rewarded. The following reasons for undesirable surgical results can be enumerated:
1. Spinal cord injury during and immediately after surgery. The insertion of surgical instruments such as a Kerrison rongeur or a curette into the spinal canal without awareness of canal narrowness, or uneven decompression of the spinal cord during resection of the laminae, can impinge or distort the spinal cord and result in worsening of neurological function. Several authors have pointed out the hazard of postoperative loss of neural function due to surgical intervention [1, 4, 18]. Hematoma in association with swelled nuchal muscles may compress spinal cord that has lost the protective shield of laminae.
2. Instability and malalignment of the cervical spine. Instability and malalignment are notorious as a reason for deterioration of neurological symptoms after laminectomy. The thick scar formation—so-called laminectomy membrane—occasionally seen subsequent to postlaminectomy hematoma may increase cord compression due to kyphotic deformity.
3. Inadequate decompression caused by limited laminectomy. Adequate posterior shift of the spinal cord cannot be expected if the laminectomy is limited [25].
Because of these shortcomings of laminectomy, many surgeons switched from posterior to anterior access to the spinal canal, and this led to the development of anterior techniques such as subtotal corpectomy and recent expansion of cervical plate systems. At the same time, several surgeons continued to try to improve the shortcomings of laminectomy. Kirita developed extensive simultaneous decompression laminectomy to avoid distorting the spinal cord by the edges of the resected laminae [20]. Hattori devised an expansive Z-shaped laminoplasty in which the posterior wall of the spinal canal was preserved by Z-plasty of the thinned laminae [23]. He attempted in this way to prevent the invasion of scar tissue, which was believed to be a cause of late neurological deterioration. He also expected that the laminae reconstructed by Z-plasty would preserve function of the cervical spine as a supportive structure. The introduction of a high-speed air-driven bar allowed the successful development of these procedures.
In 1977, Hirabayashi developed an epoch-making laminoplasty, the expansive open-door laminoplasty [7]. He described the advantages of this procedure; multiple levels of the spinal cord can be decompressed simultaneously; better postoperative support of the neck allows earlier mobilization of the patients; postoperative kyphotic deformity of the cervical spine can be prevented; and mobility of the cervical spine is reduced postoperatively, which helps to prevent late neurological deterioration as well as the progression of OPLL.
Subsequent to Hirabayashi's laminoplasty, various modifications and supplementary procedures have been devised for further improvement of the safety and efficacy of decompression, as well as the stability of the spine, especially by Japanese orthopedic surgeons. The high incidence of OPLL and CSM in Japan may have promoted the evolution of cervical spine surgery in this country. The mean developmental anteroposterior canal diameter of the cervical spine has been reported to be smaller in the Japanese than that in white Western populations.
Aims, advantages, and disadvantages of laminoplasty
Aims
The aims of laminoplasty are to expand the spinal canal, to secure spinal stability, and to preserve the protective function of the spine. Preservation of spinal mobility is also the goal of this procedure.
Nuchal muscles and spinal ligaments which were totally or partially detached to expose laminae can be reattached to preserved posterior spinal structures, and this may prevent development of the cervical instability which often happens after laminectomy, particularly in those subjects below 50 years of age. The spared laminae preserve the protective function of the spine, shielding the spinal cord from pressure from hematoma during the early postoperative period and preventing the invasion of scar tissue subsequent to hematoma in the late convalescent period. Development of kyphosis in combination with a thick peridural scar following laminectomy is a notorious cause of late neurological deterioration in laminectomy.
Advantages
Basically, no instrument needs to be inserted into the canal for laminotomy. Furthermore, the site of the laminotomy or hinge for laminoplasty is uniformly at the junction of the lamina and facet, whereas in laminectomy the site of the laminotomy is variable. Both these factors make laminoplasty more predictable and safer.
Expansion of the spinal canal is obtained without much loss of spinal stability, as mentioned above.
Decompression of the spinal cord is accomplished without removal of spondylotic protrusion impinging on the neural tissue. Removal of the osteocartilaginous protrusion or ossified ligament encroaching on the already compromised neural tissue is known to be the most hazardous part of the procedure when surgeons use the anterior approach to treat CSM and OPLL respectively.
Supplementary procedures for nerve root decompression or reinforcement of spinal stability can easily be performed. Facetectomy for nerve root decompression is optional except for the facets on the hinge side of the laminae. Bone grafting for stabilization either in single or multiple segments is easily applicable.
Disadvantages
Upper extremity palsy. Details are described in the complications section.
Neck discomfort. The incidence of neck pain after laminoplasty is reported to be high, and this is one of the most discouraging complications [11]. The pathomechanism of postoperative neck discomfort has not yet been clarified, although several hypotheses have been advocated such as prolonged neck immobilization, facet joint damage, and nuchal muscle damage.
Reduction of mobility of the cervical spine. Although preservation of spinal mobility is one of the aims of laminoplasty, the range of motion (ROM) usually decreases by 30–70% of the preoperative range. This becomes more marked when laminotomy or hinges are located at the facet in either expansive open-door laminoplasty or spinous process splitting laminoplasty.
Indications
Laminoplasty is indicated for myelopathy secondary to:
Developmental spinal canal stenosis (an anteroposterior canal diameter less than 13 mm)
Continuous or mixed type of OPLL
Multisegmental spondylosis associated with a relatively narrow spinal canal(13–14 mm) [32, 35]
Distal type of cervical spondylotic amyotrophy [3] with canal stenosis
For younger patients laminoplasty should be borne in mind. Laminoplasty is preferable to laminectomy because it can be lessen postoperative kyphosis and instability. Laminoplasty with stabilization (fusion) has been widely indicated for myelopathy secondary to multilevel subaxial subluxation in patients with rheumatoid arthritis.
At present, no type of laminoplasty can correct a fixed kyphotic deformity into a lordotic curve. Accordingly, a kyphotic cervical spine is an absolute contraindication for posterior decompression. Suda and his coauthors reported that patients having local kyphosis exceeding 13° showed poor surgical results and recommended anterior surgery or posterior decompression with correction of kyphosis [26]. For (radiculo)myelopathy secondary to multisegmental spondylosis associated with athetoid cerebral palsy, laminoplasty combined with a proper fusion procedure can be indicated, provided that the athetoid movements of the neck can be properly controlled with a halo vest in the postoperative period.
Laminoplasty can be indicated for myelopathy secondary to soft disc herniation when the condition is associated with a developmentally narrow canal. Spontaneous withdrawal of disc fragments after laminoplasty has been reported [14].
Techniques of laminoplasty and supplementary procedures
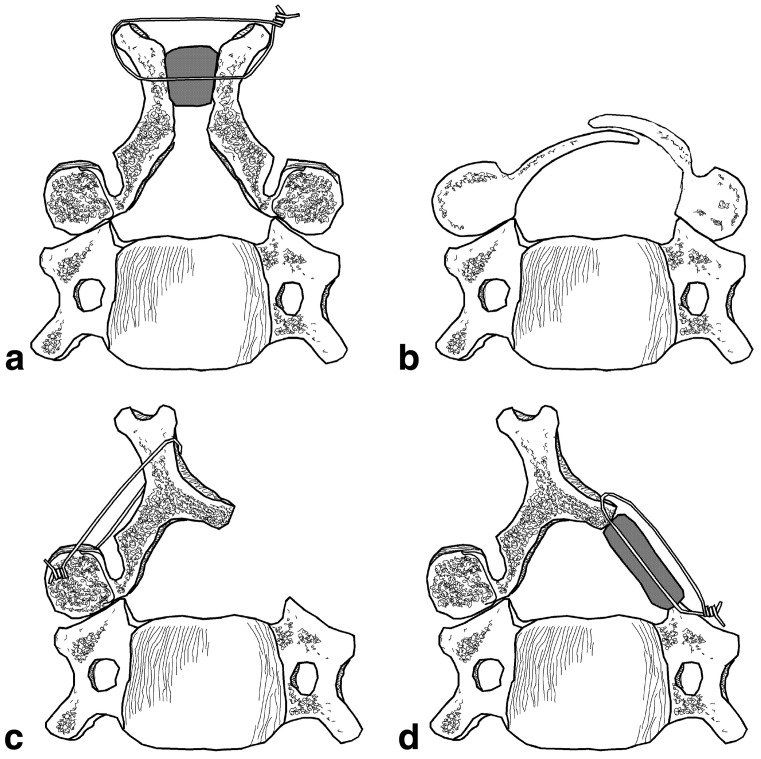
Although several types of laminoplasty have been reported, most of them can be classified into two types: the open-door type of Hirabayashi [7] and the French-door type of Kurokawa [10]. The basic concept of most of procedures is similar to one of these two procedures (Fig. 1). Hence these two procedures are described in more detail. For further details of other procedures, the reader is referred to the original articles [5, 10, 13, 16, 17].
Fig. 1a–d.
Various types of laminoplasty. a Kurokawa; b Hattori; c Hirabayashi; d Itoh and Tsuji
The patient is placed in the prone position on a laminectomy frame to decrease the abdominal pressure. A three-point pin fixation device such as Mayfield's tongs is recommended to secure the head and maintain cervical alignment in the neutral position or slight extension. When spinal fusion is required, the cervical spine is adjusted to its proper alignment after laminoplasty.
Through a posterior midline incision, the nuchal ligament is divided at the midline. Typically, in CSM, the extent of decompression is from C3 to C7 for complete posterior migration of the spinal cord. Decompression should also be wide enough for the spinal cord to migrate posteriorly. When an ossified lesion extends up to C2, undercutting of the C2 lamina (dome-shaped laminoplasty) or a spinous process splitting type of laminoplasty is added.
Unilateral hinge laminoplasty (Hirabayashi)
For the expansive open-door laminoplasty of Hirabayashi [7, 9], the spinous processes are exposed from C2 to T1, with care being taken not to damage the supraspinous and interspinous ligaments. After exposure of the laminae from C2 to T1, a gutter is made at the junction of the articular processes and the laminae. We employ a steel burr to cut the outer cortex and cancellous bone of the laminae, and then make the inner cortex progressively thinner using a diamond burr. With adequate irrigation and suction, the color of the cortex can be seen to change from ivory to dark red as the epidural venous plexus becomes evident. The cranial part of each lamina is thicker than the caudal part and is covered by the caudal portion of the lamina above, so it needs more grinding than the caudal part to equalize the thickness of the inner cortex. Then a scalp clip holder is inserted into the gutter and opened to separate both edges of the gutter by fracturing the thinned inner cortex. With this technique, no instruments need to be inserted into the canal. After laminotomy, another gutter is made on the hinge side with a steel burr, being set more laterally than the gutter on the opening side. Opening of the laminae is secured by sutures placed on the facet joint capsules on the hinge side and the corresponding laminae.
If the lifted laminae are not fixed firmly, loss of enlargement of the spinal canal can occur. In order to avoid this loss, a prop bone graft from C7 and T1 spinous process supporting the lifted lamina was devised by Itoh and Tsuji [13], known as en-bloc laminoplasty.
Foraminotomy on the opening side can be combined with this technique. If spinal fusion is required, a block bone graft from the ilium is placed to bridge the segments to be fused and is secured with wire.
Bilateral hinge laminoplasty
In the spinous process splitting laminoplasty of Kurokawa [10], the dorsal part of each spinous process is removed and the fragments are used as bone grafts in the space made by spinous process splitting. Gutters for the hinge are produced as in the expansive open-door laminoplasty. The laminae are then cut at the midline with a diamond burr and bone grafts from the spinous processes or ilium are shaped to fit the spaces. When spinal fusion is required, a long bone block is positioned to connect the desired spinous processes. A ceramic spacer can also be substituted for an autogenous bone graft [5, 10, 22].
Procedures supplementary to laminoplasty
The nuchal muscles and ligaments are believed to be an indispensable structure helping to stabilize the cervical spine in lordosis. In fact, the nuchal muscles are displaced laterally and ventrally in patients who develop kyphotic deformity after laminectomy, indicating that these muscles and ligaments play an important role in stabilization of the cervical spine. The following procedure attempting soft tissue reconstruction for restoration of stability in lordosis has been practiced, although its clinical significance has not yet been fully clarified.
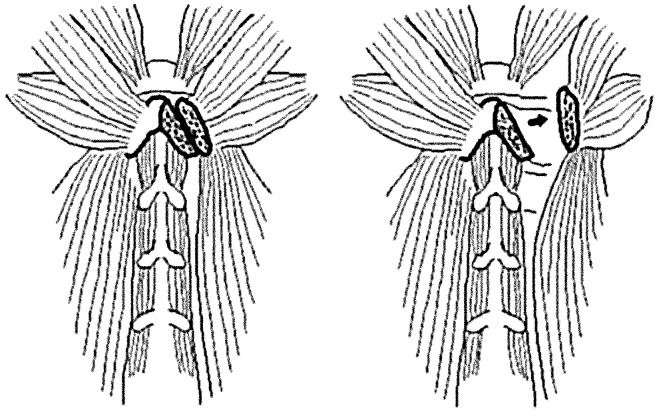
For reattachment of the nuchal muscles to the spinous process of the axis (Fig. 2), the rectus major, inferior oblique, and semispinalis cervicis muscles attached to the spinous process of the axis are detached from their origins along with small fragments of the spinous process tip, and the lamina and articular processes of C3 are exposed by retracting the semispinalis cervicis muscles laterally. After laminoplasty, the muscles are reattached to their origins by suturing the bony fragments to the tip of the spinous process. The other nuchal muscles are also repositioned and sutured to each other in order to form a suspensory nuchal ligament.
Fig. 2.
Reattachment of the nuchal muscles to the spinous process of the axis
Postoperative management
A couple of days after surgery, patients are allowed to leave bed without wearing a collar. When a patient complains of neck pain, a collar is recommended until the patient can stand the pain. If a patient does not feel pain, a gentle active ROM exercise of the neck is recommended. Three weeks after surgery, isometric neck muscle exercises are started. When spinal fusion is required, immobilization of the neck with the collar should last until consolidation of the graft is confirmed roentgenographically.
Surgical results and complications
Neurological results
Since the Japanese Orthopaedic Association proposed a scoring system for cervical myelopathy in 1975, Japanese orthopaedic surgeons have used this system (the JOA system). Recently, a new scoring system has been proposed to improve assessment of shoulder and elbow function and to subdivide the assessment scale for sensory function. Inter- and intrarater reliability of the system has been verified [36]. In most of the Japanese studies, neurological results were assessed by the JOA system and evaluated from the postoperative score and the recovery rate determined by the method of Hirabayashi [7].
There are many reports on surgical results after various types of laminoplasty, and the mean recovery rate ranges from 53% to 86%, with a median of approximately 65%. The duration of symptoms is reported to be one of the most significant prognostic factors. The transverse area of the spinal cord at the maximally compressed level is another significant prognostic factor; an area less than 35 mm2 indicates poor surgical results. The significance of a high-signal-intensity area in the cord on T2-weighted magnetic resonance imaging is still a controversial subject.
Several comparative studies of anterior surgery and posterior surgery have been conducted [12, 14, 29, 35]. However, no conclusion that has statistical significance has been drawn.
Regarding laminoplasty techniques, no procedure has been proven to be statistically superior to any others with regard to the neurological and roentgenographical results. Few studies have been done with respect to this question. Tsuzuki and coauthors employed six different procedures for CSM and reported that there was no difference in the degrees of improvement of long-tract signs of CSM [28]. We compared the results of two procedures, en-bloc laminoplasty with reconstruction of the nuchal muscle attachments and spinous process splitting laminoplasty in patients with multisegmental spondylotic myelopathy. There was no significant difference between the two groups regarding to postoperative score and recovery rate.
Concerning the difference of surgical results by disease, Miyazaki and his colleagues found that 86.8% of patients with OPLL treated by extensive simultaneous laminectomy showed useful improvement, compared with 75.5% of CSM patients after the same procedure [21]. They also reported that increased instability of the spine after laminectomy influenced the surgical results, and they therefore added posterolateral fusion to laminectomy. When posterolateral fusion was added, the results were improved, and were better than those for OPLL. Kawai and his associates analyzed the results of expansive Z-laminoplasty and reported that outcomes for spondylotic myelopathy were better than those for OPLL [16]. Therefore, better surgical results could be expected in spondylotic myelopathy if the laminoplasty is properly carried out. Of course, the severity of the myelopathy will certainly influence the surgical results.
Decompression of the nerve roots is usually impossible when the bilateral hinge type of laminoplasty is selected. With the unilateral hinge type of laminoplasty, however, foraminotomy or facetectomy can be done on the open side. Herkowitz compared the results of anterior cervical fusion, laminectomy, and laminoplasty for multiple level spondylotic radiculopathy, and concluded that although anterior cervical fusion provides the best results, laminoplasty provides an effective alternative to anterior fusion [6].
Roentgenographic outcome
Kyphotic deformity after laminectomy is a notorious problem, especially when the procedure is carried out in young patients [31, 33]. The incidence of kyphotic deformity after laminectomy for CSM, developmental cervical canal stenosis, and OPLL is probably lower than has been believed. Mikawa and his coworkers reported that no deformity developed after multilevel laminectomy for spondylosis, and that deformity developed more often in OPLL [19]. In our series, 21% of laminectomy patients showed deterioration of neurological symptoms due to cervical spine instability. Approximately half of them had a straight spine before surgery and their symptoms worsened in association with the development of kyphosis triggered by minor trauma.
All of the patients with kyphotic alignment before surgery showed worsening of their alignment after laminoplasty, while no patient with lordotic alignment before surgery showed deterioration after en-bloc laminoplasty. The degree of lordosis in the patients with lordotic alignment before surgery decreased slightly, but no patient developed kyphotic deformity of the cervical spine. After spinous process splitting laminoplasty in our series, 26.9% of the patients showed deterioration of spinal alignment. Hirabayashi and his associates did not note any postoperative malalignment of the cervical spinal lateral curvature after expansive open-door laminoplasty [8]. The difference between the procedures is not clear.
A cervical spine with OPLL tends to be kyphotic, although the reason for this is not clear. Fortunately, few patients deteriorated due to kyphotic deformity after laminoplasty. Formed laminae prevent infiltration of scar tissue into the spinal canal and maintain room for the spinal cord.
Hirabayashi and coworkers reported the progression of OPLL after laminectomy and suggested the usefulness of laminoplasty in this respect [7]. Although progression of OPLL (defined as 2 mm or more growth in thickness or length) was also observed in about 60% of laminoplasty patients, none of them complained of worsening of their neurological symptoms secondary to this progression.
Regarding listhesis, little is known because originally laminoplasty was not indicated for patients with marked spinal instability. In our series, all of the patients having instability as defined by White and Panjabi [30] revealed improvement of stability, and their results were not different from those of the patients without instability.
No procedure has been proven to completely prevent further progression of kyphotic deformity of the cervical spine and to create cervical lordosis in patients with a pre-existing kyphotic deformity. Except when supplemented with spinal fusion, no type of laminoplasty is able to guarantee lifelong spinal stability. To obtain a more consistent outcome after laminoplasty, procedures to reconstruct the supporting soft tissues and rehabilitation programs should be improved.
Postoperatively, the ROM of the neck usually decreases, with the extent of the decrease ranging from 30% to 70%. The type of laminoplasty, the extent of exposure, the position of the laminotomy, the use of bone graft, and the postoperative rehabilitation program including the period of neck immobilization may all influence the degree of loss of ROM. Many surgeons believe that the loss of ROM has a favorable effect on the neurological outcome through a partial stabilization of the spine. Few patients complain of the inconvenience of decreased ROM of the neck, which generally occurs after multisegmental anterior spinal fusion. Hence, patient complaints of ROM reduction after laminoplasty may derive from a combination of stiffness and neck discomfort.
Long-term results
Laminoplasty was developed in the late 1970s, and various modifications were reported in the early 1980s. As yet, only a few follow-up studies over 10 years have been published [15, 29]. Miyazaki and coworkers carried out a study with a mean follow-up of 12 years and 11 months, and reported that improvement after surgery was maintained [21]. Kawai and his associates followed up patients who had undergone a Z-laminoplasty for 10 years on average, and reported that spondylotic myelopathy was stable, in contrast to the results for OPLL [16]. The reasons for this difference were not described in detail. However, OPLL patients frequently have diabetes mellitus and ossification of spinal ligaments in the thoracic and lumbar spine, which also causes myelopathy. These factors might influence the long-term results of surgical treatment to varying degrees.
Complications
Generally, the complications of laminoplasty are similar to those of laminectomy. However, nonneurological complications are relatively rare compared with other procedures including laminectomy. Delayed healing or dehiscence of the surgical wound may occur slightly more frequently after laminoplasty than with laminectomy, and this may be related to the bulk of the elevated laminae. The incidence of neurological complications attributed to this operation is less in laminoplasty because of simultaneous decompression and the use of air-driven instruments. There are, however, complications characteristic to this procedure, which are nerve root palsy and axial (neck and shoulder) pain.
Neurological deterioration due to hematoma has decreased since the reconstructed or preserved laminae still have a protective function to diminish blood pooling and soft tissue swelling after surgery. We have experienced this complication in only 0.3% of laminoplasty patients in contrast to 2.4% of laminectomy patients [34].
Fracture of a hinge or loss of spinal canal enlargement due to insufficient fixation of the lifted lamina is reported to cause nerve root or spinal cord palsy when a lamina migrates into the spinal canal. Computerized tomography (CT) is useful for delineating the pathology in this case, and total or partial removal of the lifted lamina is necessary. The prognosis is usually good if salvage is carried out promptly. For prevention, the inner cortex of the lamina destined to be the hinge should be thinned step by step, while assessing its mobility, until the surgeon is very familiar with the procedure.
Nerve root palsy due to thermal damage or mechanical injury to the nerve root is known to develop occasionally following posterior decompression, and a different type of nerve root palsy is reported to occur after laminoplasty [27, 28, 34]. The initial symptom is severe pain in the shoulder and upper arm, which is followed by paresis or paralysis of the deltoid and biceps brachii muscles. There is a motor-dominant type of nerve root paralysis. The former symptom is the more frequent form of this complication. It occurs on the 1st, 2nd, or 3rd postoperative day, and not immediately after surgery. The fifth cervical nerve root is most frequently involved, followed by the sixth and seventh, in that order. The eighth nerve root is rarely affected. Out of 239 laminoplasty patients in our series, 12 patients developed fifth or sixth nerve root palsy, 3 patients had seventh nerve root involvement, and 1 patient had an eighth root complication. The long-tract signs and symptoms are usually improved, and no regression of the long-tract signs and symptoms can be detected.
The incidence of this complication varies between surgeons and procedures. Tsuzuki and his coworkers studied its incidence in relation to the surgical procedure in their own series [28]. A higher incidence of this complication was encountered in both closed types of laminoplasty with foraminectomy (C4/5, 5/6), while the closed laminoplasties without foraminectomy or facetectomy showed a lower incidence.
This complication has been rarely reported to occur after laminectomy, and the mechanism of this complication has not yet been fully clarified. Nerve root tethering due to posterior migration of the spinal cord has been suggested to be the major cause [26, 27, 33].
This entity may be differentiated from nerve root or spinal cord palsy due to mechanical compression by CT scanning with or without contrast medium. Pain can be controlled with nonsteroid anti-inflammatory drugs and/or analgesics. Neck traction in the neutral position may also reduce pain. The motor paralysis usually recovers to normal or good grade within 12 months. Severe spondylotic changes, especially at the root tunnel, and spinal cord atrophy are thought to be predisposing factors for this complication. Although the alignment of the cervical spine, the relative position of the facets to the vertebral body, and the distance from the cord to the dura–nerve root junction were all analyzed, no factor was proven to be a sole predictor of this complication.
Foraminotomy or facetectomy has not been proven to be a preventive measure. However, controlled opening of the lamina can prevent this problem—although a definitive method for control of opening has not been found.
Postoperatively, patients with laminoplasty complain of various axial symptoms such as nuchal pain and stiffness of the neck and shoulder muscles. Neck stiffness usually appeared on the hinge side in our en-bloc laminoplasty series. In our series, 59.7% of laminoplasty patients complained of some axial symptoms within 1 year after surgery, in contrast to 27.2% of laminectomy patients and 19.2% of subtotal corpectomy and fusion patients. After spinous process splitting laminoplasty, a few of the patients complained of neck and/or shoulder pain. The symptoms were usually distributed on both sides. The causes of these symptoms are not clear. However, changes in and around the facet joints caused by surgical intervention may be the cause. The symptoms resolved by about 1 year after surgery in most patients. However, axial symptoms are the chief complaint in some patients, and their cause should also be clarified. Thermal therapy and active mobilization of the neck and shoulder is recommended for treatment. Nonsteroidal anti-inflammatory agents and muscle-relaxant drugs have little effect. Recently, several surgeons have started to assess the usefulness of various postoperative muscle exercises and neck motion programs to prevent these complaints as well as to maintain or create a cervical lordosis after laminoplasty, but none of these programs has been proven to be useful so far.
References
- 1.Bohlman Spine. 1977;2:151. [Google Scholar]
- 2.Cloward J Neurosurg. 1958;15:602. doi: 10.3171/jns.1958.15.6.0602. [DOI] [PubMed] [Google Scholar]
- 3.Ebara Spine. 1988;13:785. doi: 10.1097/00007632-198807000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Graham JJ (1989) Complications of cervical spine surgery. In: Shark HH, et al (eds) The cervical spine, 2nd edn. Lippincott, Philadelphia, pp 831–837
- 5.Hase Spine. 1991;16:1269. doi: 10.1097/00007632-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Herkowitz Spine. 1988;3:774. doi: 10.1097/00007632-198807000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi Spine. 1981;6:354. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hirabayashi Spine. 1983;8:693. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hirabayashi K (1994) Expansive open door laminoplasty. In: Shark HH, et al (eds) The cervical spine. An atlas of surgical procedures. Lippincott, Philadelphia, pp 233–250
- 10.Hoshi Spinal Cord. 1996;34:725. doi: 10.1038/sc.1996.132. [DOI] [PubMed] [Google Scholar]
- 11.Hosono Spine. 1996;21:1969. doi: 10.1097/00007632-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y (1985) Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg 67-B:609–615 [DOI] [PubMed]
- 13.Itoh Spine. 1985;10:729. doi: 10.1097/00007632-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki Spine. 1996;21:32. doi: 10.1097/00007632-199601010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K (2002) Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow-up. J Neurosurg (Spine) 96:180–189 [PubMed]
- 16.Kawai Spine. 1988;13:1245. [PubMed] [Google Scholar]
- 17.Matsuzaki Spine. 1989;14:1198. doi: 10.1097/00007632-198911000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Mayfield Clin Neurosurg. 1976;23:435. doi: 10.1093/neurosurgery/23.cn_suppl_1.435. [DOI] [PubMed] [Google Scholar]
- 19.Mikawa Spine. 1987;12:6. [Google Scholar]
- 20.Miyazaki Spine. 1986;11:531. [Google Scholar]
- 21.Miyazaki J Jpn Spine Res Soc. 1994;5:167. [Google Scholar]
- 22.Nakano Spine. 1992;17:S41. doi: 10.1097/00007632-199203001-00009. [DOI] [PubMed] [Google Scholar]
- 23.Oyama Chubuseisaisi. 1973;16:792. [Google Scholar]
- 24.Robinson Johns Hopkins Med J. 1955;96:223. [Google Scholar]
- 25.Rogers J Bone Joint Surg. 1961;43B:3. [Google Scholar]
- 26.Suda Spine. 2003;28:1258. doi: 10.1097/00007632-200306150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Tsuzuki Eur Spine J. 1993;2:191. [Google Scholar]
- 28.Tsuzuki Eur Spine J. 1993;2:197. doi: 10.1007/BF00299446. [DOI] [PubMed] [Google Scholar]
- 29.Wada Spine. 2001;26:1443. doi: 10.1097/00007632-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 30.White AA, Panjabi MM (1990) The problem of clinical instability in the human spine. Clinical biomechanics of the spine, 2nd edn. Lippincott, Philadelphia, pp 302–326
- 31.Yasuoka J Neurosurg. 1982;57:441. doi: 10.3171/jns.1982.57.4.0441. [DOI] [PubMed] [Google Scholar]
- 32.Yonenobu Spine. 1985;10:710. doi: 10.1097/00007632-198510000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Yonenobu Spine. 1986;11:818. doi: 10.1097/00007632-198610000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Yonenobu Spine. 1991;16:1277. doi: 10.1097/00007632-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Yonenobu Spine. 1992;17:1281. [PubMed] [Google Scholar]
- 36.Yonenobu Spine. 2001;26:1890. doi: 10.1097/00007632-200109010-00014. [DOI] [PubMed] [Google Scholar]