Abstract
There is a relatively high prevalence of osteoporotic vertebral compression fractures (VCFs) in the elderly population, especially in women aged 50 or older. The result of these VCFs is increased morbidity and mortality in the short and long term. Medical treatment of these fractures includes bed rest, orthotics, analgesic medication and time. Percutaneous vertebroplasty (PVP) consists of percutaneous injection of biomaterial, such as methylmethacrylate, into the VCF to produce stability and pain relief. Biomechanical testing has shown that PVP can restore strength and stiffness of the vertebral body to the pre-fracture levels. Clinical results show immediate and maintained pain relief in 70–95% of the patients. Possible major complications include cement leakage into the spinal canal or into the venous system. Additionally, percutaneous vertebroplasty may alter the normal loading behavior of the adjacent vertebral body, and there is an increased risk of adjacent segment VCF. Kyphoplasty is a new technique, which introduces a balloon into the vertebral body transpedicularly to reduce the VCF while creating a cavity for the cement injection. This technique has the benefit of kyphosis reduction as well as less cement leakage. Research continues into the development of injectable biomaterials that are resorbable and allow for new bone formation. Vertebroplasty and kyphoplasty are safe and effective in the treatment of osteoporotic VCFs. They may allow for a faster return to function, and thus avoid the morbidity associated with medical treatment.
Keywords: Vertebral compression fracture, Osteoporotic compression fracture, Percutaneous vertebroplasty, Vertebroplasty, Kyphoplasty
Introduction
Osteoporosis is a disorder of decreased bone mass, microstructural collapse, and fragility fractures. It can affect people of all ethnic backgrounds and can result in challenging complications, ranging from compression fractures of vertebral bodies to femoral neck fractures [59]. The geriatric population is especially at risk for such osteoporotic fractures, as bone mass decreases with age [53]. A loss of one standard deviation of bone mass doubles the risk of spine fractures [34, 56, 59]. It is estimated that 90% of hip and spine fractures occurring in the elderly are attributable to osteoporosis [45]. The consequences of such osteoporotic vertebral fractures are diverse and include back pain, functional limitations and impairment of mood [11, 37, 58].
A recent study in Canada examined the health-related quality of life (HRQL) in women aged 50 years and older with osteoporosis [1]. Subjects who had experienced a vertebral fracture had lower HRQL scores than participants without fracture in total score, symptoms, physical function, activities of daily living, and leisure. Acute complications of osteoporotic vertebral fractures include transient ileus, urinary retention, nausea, abdominal pain and chest pain [41, 49]. Long-term effects of osteoporotic fractures include increased kyphosis, deconditioning, insomnia and depression [14, 32, 41, 49]. Physiologic changes include significant diminution of pulmonary function in patients with spinal osteoporotic fractures and increased kyphosis. The degree of pulmonary function reduction correlates with the severity of the kyphosis [55]. In addition to the increased morbidity, mortality may also increase after osteoporotic vertebral fractures. A study from the Mayo Clinic found the estimated survival at 5 years after spine fractures in the elderly to be 61% compared with the expected value of 78% [12]. Treatment of osteoporosis to prevent such fractures is thus justified.
While physicians are aware of the risks of osteoporosis and fractures, the disease remains under-diagnosed and under-treated. A survey of physicians who treated elderly patients residing in long-term care facilities found that while the physicians are well aware of the prevalence of osteoporosis in their patients, 45% of the physicians did not routinely assess their patients for the disease and 26% did not routinely treat it [44]. One can only assume the use of preventive measures is even lower, which leads to a higher prevalence of osteoporotic vertebral compression fractures (VCFs). The prevalence of these fractures in women aged 50 or older has been estimated at 26% [58].
Historically, the painful VCF has been treated medically. Surgery in these patients has been limited because of its inherent risks, invasiveness and the poor quality of osteoporotic bone. However, surgery is indicated in patients with instability or neurological deficit [16]. The medical treatment of stable osteoporotic fractures without neurological involvement includes bed rest, orthotic management, narcotic analgesia, and time. Each of these modalities has side effects: bed rest over time results in loss of muscle mass, bone density and resultant deconditioning [10], braces are poorly tolerated [30], and narcotic medication can lead to mood or mental alteration. As a result, there has been a search for alternative ways to treat VCFs. Percutaneous vertebroplasty has become a very popular, safe, and effective treatment for this condition.
Percutaneous vertebroplasty (PVP) is a minimally invasive technique consisting of percutaneous injection of biomaterial, usually methylmethacrylate, into the pathologic fractured area, stabilizing the fracture and more importantly decreasing pain and improving function. It was first developed by Deramond in France in the late 1980s [19]. Initially it was used for treatment of aggressive hemangiomas and osteolytic neoplasms. However, as it proved successful with these lesions, the indications also expanded to include osteoporotic compression fractures refractory to medical treatment. The initial experience with vertebroplasty for the treatment of osteoporotic fractures has shown 70–95% pain relief [3, 13, 15, 18, 22, 23, 26, 28, 29, 31, 33, 46, 50, 51, 61]. The mechanism by which PVP achieves its palliative effect is not known. It may be due to the initial stability that it provides or due to neuronal damage caused by heat liberated during polymerization [17].
Vertebroplasty: technique with polymethylmethacrylate
PVP is performed under fluoroscopic guidance. The patient is under conscious sedation and is positioned prone on a radiolucent table. Adequate and clear pictures must be obtained prior to the start of the procedure, as it is crucial to be able to visualize the cement being injected into the vertebral body. The back is then prepped and local anesthetic is injected over the area of needle placement. Under fluoroscopic guidance, an 11-G bone marrow biopsy needle is introduced into the fractured vertebra via a transpedicular approach (Fig. 1a,b). In the thoracic spine, one can opt to enter the vertebral body extrapedicularly, between the rib head and the lateral aspect of the pedicle. The needle is then advanced to the anterior half of the vertebral body. At this point, an optional intraosseous venogram can be performed to aid in placement of the needle out of the venous flow path to avoid embolization to the lungs. Additionally, the intraosseous venogram can aid in determination of the flow pattern in the vertebral body, which may allow for cement leaks. Once the needle is in the correct position, the cement is injected. The cement should be radio opaque, with addition of barium powder or tungsten powder. Each kit of polymethylmethacrylate (PMMA) cement can be mixed with 5.0 g barium sulfate and 2.0 g tungsten powder [3]. The cement is allowed to achieve a paste-like consistency prior to injection. Using a 1-cc or 3-cc syringe, the cement is injected into the vertebral body under fluoroscopic guidance. Filling of the posterior one-third of the vertebral body should signal the end of the injection to avoid overfilling (Fig. 2). Typical volumes for cement injection are 2–3 cc for thoracic and 3–5 cc for lumbar vertebrae [3]. Usually there is symmetrical filling of the vertebral body, but if it is asymmetrical, then the contralateral pedicle can be used for further delivery of the cement. After the procedure, the patients are allowed to ambulate as tolerated.
Fig. 1. a,b.
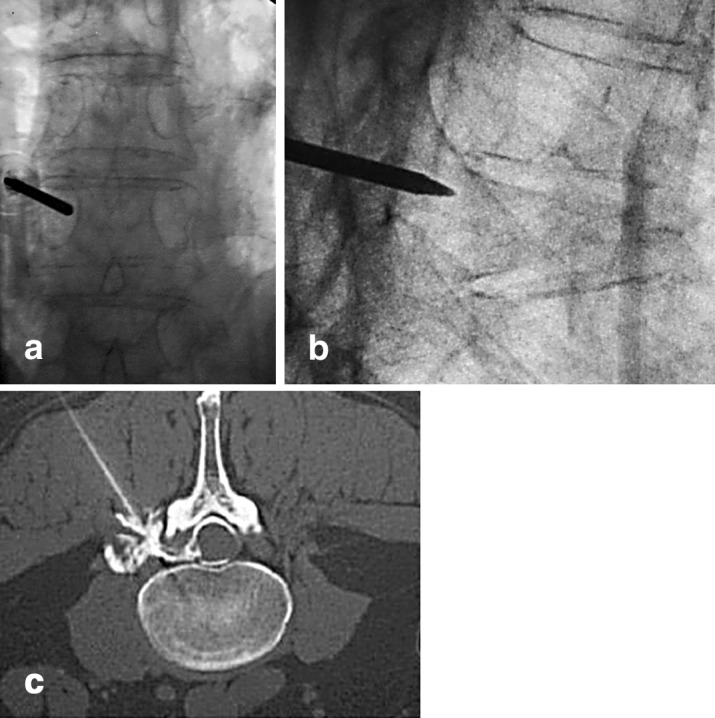
Radiograph of an osteoporotic fracture with a needle in the fractured vertebra. c Computed tomography scan showing needle positioning
Fig. 2.
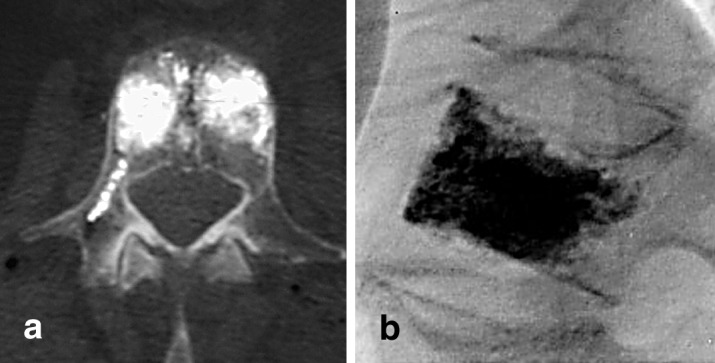
a Computed tomography scan showing cement filling after bilateral needle injection. b Lateral view radiographic control
Biomechanical considerations
There is a continual effort being made to optimize the technique of PVP. Biomechanical and clinical studies have been performed to determine the characteristics of different cements, the role of cement volume, and differences in the approach used (unipedicular vs bipedicular). Presently acrylic cement such as methylmethacrylate is used most frequently for PVP. Use of cement in a fractured vertebra has been shown to increase vertebral body strength and stiffness [4, 8, 25, 40]. Other materials, like glass-ceramic matrix [4], calcium phosphate [40], and hydroxyapatite [8, 25] have also been compared to methylmethacrylate and have shown similar biomechanical properties. The theoretical clinical benefit of using calcium phosphate or hydroxyapatite is that they are osteoconductive and can undergo remodeling, although the ability of pathologic osteoporotic bone to regenerate or, for that matter, to remodel is questionable.
The effect of different cement volumes on the biomechanical properties of the vertebrae depends on the type of cement used. Belkoff et al. [6] showed that when using Orthocomp, thoracic and thoracolumbar vertebrae needed 4 cc and lumbar vertebrae needed 6 cc to restore stiffness to the pre-fracture levels. For simplex P, the volumes needed were 6 cc and 8 cc, respectively. Using anatomically accurate finite-element models, it has been shown that approximately 15% volume fraction or approximately 3.5 cc is needed to restore stiffness of the vertebra to pre-fracture levels and that overfilling can increase the stiffness beyond that of the intact state. Overfilling has several other disadvantages: it can cause asymmetrical distribution and lead to single-sided load transfer and toggle, it can lead to leakage of cement into the epidural space [54], and in the long term it can cause increased stress on adjacent vertebrae, leading to increased risk of adjacent level fractures [9].
Whether to perform a bipedicular or unipedicular approach depends on the individual case. In biomechanical controlled studies, no significant difference has been found between the two techniques in terms of strength and stiffness [6, 39]. Further analysis, however, shows that while providing the same strength and stiffness, the use of a unipedicular approach leads to a medial-lateral bending motion or toggle toward the untreated side with uniform loading [39]. The clinical significance of this toggle is not known. Clinically, the two techniques have been shown to give similar results. The unipedicular approach can result in filling across the midline in 96% of cases [33]. The mean opacification of the vertebral body did not differ between the groups. More importantly, there was no difference in the amount of pain relief achieved with the two techniques.
Clinical results: literature review
The clinical results of PVP from the United States, Europe, and Asia show a 70–95% success rate in relieving pain. Most reports in the literature are retrospective, although a few prospective studies have been published. The main indication for the procedure is pain persisting despite nonoperative treatment of osteoporotic compression fractures. One series bravely included four burst fractures treated with PVP [46]. The majority of the cases are around the thoracolumbar area. The largest retrospective study [18] was a collaboration between seven centers in the US, where 488 consecutive patients underwent PVP for vertebral compression fractures. A telephone questionnaire was conducted with 245 patients at median of 7 months' follow-up. Questions were designed to measure pain, ambulation, and ability to perform activities of daily living. The pain decreased from a mean of 8.9 pre PVP to 3.4 post PVP. Ability to ambulate was impaired in 72% pre PVP and in 28% post PVP. Ability to perform activities of daily living improved significantly post PVP. There was a 4.9% rate of minor complications.
In another study, Barr et al. [3] studied 38 patients with 70 symptomatic fractures who had failed to respond to medical treatment. After undergoing PVP, 63% reported marked to complete relief and 32% had moderate relief of pain. Peh et al. [50] retrospectively studied 37 patients with 48 compression fractures treated with PVP. At a mean follow-up of 11 months, pain relief was complete in 47% and partial in 50%.
More recently, prospective studies have shown similar success with PVP. The largest prospective study [43] reported on 100 patients who underwent PVP for vertebral compression fractures. At final follow-up averaging 21 months, 97% of the patients reported significant pain reduction, with the VAS improving from 8.9 to 2.0. Cortet et al. [13] added to the literature by reporting on 16 patients with 20 VCFs of more than 3 months' duration not responding to medical treatment. They all underwent PVP and showed a statistically significant improvement in VAS pain score immediately after the procedure, which remained at 30, 90, and 180 days after the procedure. Additionally, there was a significant improvement in the general health status as assessed by Nottingham Health Profile, which includes pain, mobility, emotional reaction, social isolation, and energy.
The longest follow-up has been reported by Perez-Higueras et al. [51], who followed 13 patients with VCFs for at least 5 years following PVP. The VAS improved significantly from a score of 9 pre PVP to 2 immediately post PVP, to 1 at 3 months. At 5 years, the VAS was 2.2. Significant improvement after treatment with PVP was also noted on the McGill Questionnaire.
The safety and efficacy of the procedure in the upper thoracic spine was reported by Kallmes et al. [29], who studied 41 patients with 63 vertebral compression fractures from T4 to T8. There was a significant pain reduction, as the mean VAS decreased from 9.7 pre PVP to 1.7 post PVP. There was one case of a pedicle fracture and no cases of pneumothorax.
The issue of timing of vertebroplasty was reviewed by Kaufman et al. [31]. Seventy-five patients with 122 VCFs underwent PVP. The age of the fracture at time of PVP was not independently associated with post PVP pain or activity. The procedure was efficacious in reducing pain and improving mobility in patients, regardless of the age of the fracture. However, the authors found that increasing age of the fracture was independently associated with increased needs of analgesia post PVP. Whether the delay in carrying out PVP leads to tolerance of and dependence on pain medication, leading to higher requirements post PVP, is not known.
Complications
While these clinical studies have shown good success rates in improving pain and function, the procedure is not without risks and complications. Most series report a complication rate of between 4 and 6% [3, 15, 18, 28]. Reported complications associated with the insertion of the needle include rib fractures [28], neuritis [3], pedicle fracture [29], and infection [29]. The most feared complication is the potential for leakage of cement into the spinal canal (Fig. 3) or into the venous system. Cement leakage into the spinal canal has been reported in a small number of patients without causing any clinical symptoms [46], while there have been reports of transient neuropathy [28] and one case of paraplegia associated with PVP of T11 [36]. We have consulted on a patient in whom PVP was performed for burst fracture of L2 with cement leakage into the spinal canal causing symptoms of spinal stenosis. The patient underwent a decompression and removal of cement from the spinal canal.
Fig. 3.
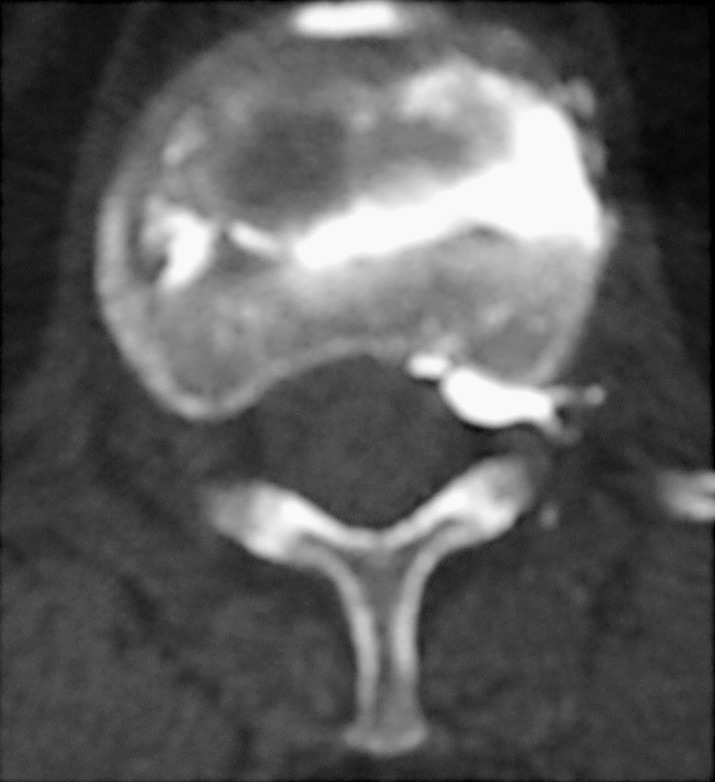
Cement leakage in the foramen
Leakage of cement into the venous system can have a spectrum of clinical consequences, from being asymptomatic [51], causing pulmonary embolism [27, 47], or causing a paradoxical cerebral artery embolization in a patient with patent foramen ovale [57]. In a recent study [46], 17 patients had CT scans performed immediately after undergoing PVP. Cement in the epidural veins adjacent to the vertebra was found in 48% of the cases, with only one patient developing a transient neuritis. The risk of cement leakage into the spinal canal or venous system is increased with higher volumes of injected cement [54]. This problem is so feared that some have advocated the use of pre PVP venography to assess the risk of cement leakage.
Venography can document sites of potential leakage during cement injection [21, 42, 63]. In one study [42], venography was performed prior to vertebroplasty, and the results retrospectively reviewed. Venography could predict the flow characteristics of cement within the vertebral body and within the venous structures. While venography could predict cement leakage into endplates or central defects in 100% of cases, it could only predict leakage into the venous structures in 29% of the cases. Another study [63] specifically looked at 205 PVP procedures in 137 patients without antecedent venography, and found only one cement leakage causing symptoms of radiculopathy. The value of antecedent venography will need to be determined with prospective studies.
A topic of interest is the occurrence of new vertebral body fractures after PVP in patients with osteoporosis [2, 9, 62]. This was noted in a follow-up of 25 patients who underwent PVP. The average follow-up was 48 months. The authors found a significantly increased risk of vertebral fractures adjacent to a cemented vertebra, with the odds ratio of 2.27, whereas the odds ratio for sustaining a vertebral fracture next to an uncemented fracture was 1.44 [23]. In another report [62], 177 patients treated with PVP for osteoporotic fractures were followed for a minimum of 2 years. Twenty-two patients (12.4%) developed a total of 36 new vertebral body fractures. Two-thirds (67%) of the new fractures involved a vertebra adjacent to a previously treated vertebra.
New developments for treatment of osteoporotic spine
Kyphoplasty
Vertebroplasty carries its share of risks and complications, but it does lead to significant pain reduction and improved function. It does not, however, improve the sagittal balance or the kyphosis caused by the fracture. Kyphoplasty is a new technique, which tries to address this issue. Kyphoplasty is similar to vertebroplasty except that it calls for introduction of an inflatable bone tamp into the vertebral body which, when inflated, tries to restore the vertebral body height back to its original height while creating a cavity that can be filled with cement (Fig. 4). This technique is performed via a bipedicular approach for a uniform restoration of the compression. Why might reduction of the kyphosis be important in these patients? It has been shown that patients with spinal osteoporotic fractures have significantly diminished pulmonary function compared to those without fractures. More importantly, the reduction in the pulmonary functions has been shown to correlate significantly with severity of the spinal deformity [55]. Furthermore, it has been shown that, if left untreated, the thoracic compression fracture can lead to worsening of the kyphosis over 3 months and further deterioration at 3 years [14]. If the kyphosis can be corrected, pulmonary functions may improve and further collapse may be avoided.
Fig. 4.
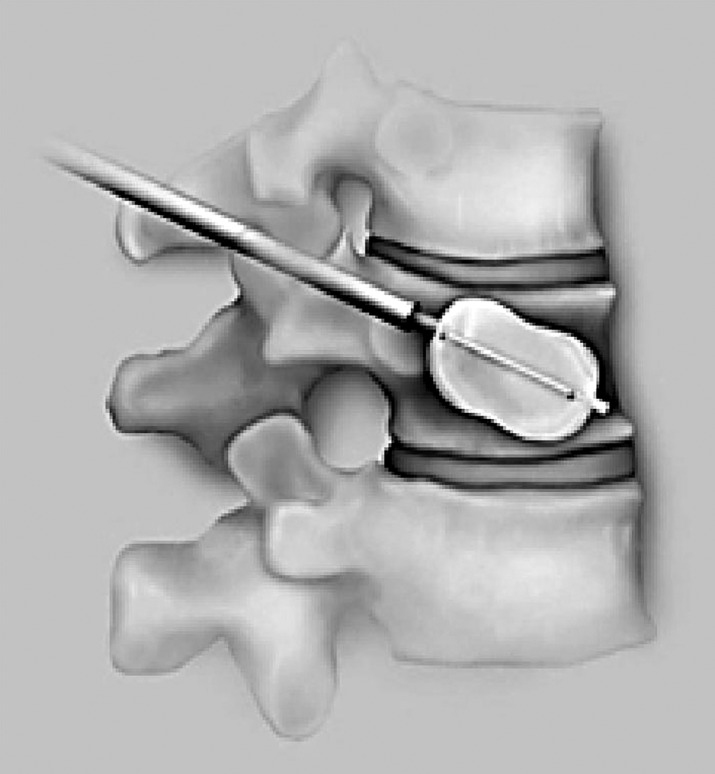
The inflated balloon restores vertebral body height, while creating a cavity that can be filled with cement (kyphoplasty)
An ex vivo biomechanical evaluation comparing vertebroplasty to kyphoplasty showed that both techniques result in significantly stronger vertebral bodies relative to the initial fractured state. Kyphoplasty was able to restore vertebral height to 97% of the original height. Vertebroplasty resulted in a significantly lower restoration of vertebral height, to 30% of the original height [5]. The ability to restore vertebral body height has been shown in other laboratory studies as well [7, 64]. Clinical studies have shown increased vertebral height, but not the level of increase obtained in the laboratory. Lieberman et al. [38] reported on 70 consecutive kyphoplasties performed on 30 patients for painful VCFs with a mean duration of symptoms of 5.9 months. The patients were followed prospectively for 3 months. In 70% of the patients, height was restored to 46.8% of predicted values. In 30% of the patients there was no restoration of height. Pain and physical functional scores significantly improved after kyphoplasty. Although no conclusions could be made with regards to the age of the fracture and the ability to regain height, the authors got the "impression" that they were able to restore height more predictably in fractures less than 3 months old. A balloon failure rate of 20% and cement leakage rate of 8.6% was also reported.
Since the approval of kyphoplasty by the FDA in 1998, a multi-center study in the US has been initiated, with results reported for 2,194 kyphoplasty procedures in 1,439 patients [20]. In fractures less than 3 months old, the average fractured vertebral body height improved from the 71% pre treatment to 92% after treatment. In fractures more than 3 months old, the height improved from 74% pre treatment to 84% after treatment. Ninety percent of the patients had relief of their pain as they returned to the pre-fracture level of pain medication use. There were three thoracic level parapareses related to instrument insertion through the medial wall of the seventh pedicle with cord injuries, and there was one case of epidural hematoma in a patient on anticoagulation medication. These complications occurred in the first 100 fractures treated. Since technique adjustment, no neurological complications have been reported.
Kyphoplasty has the added benefit of less cement leakage. When the balloon is inserted and inflated, it forms a contained cavity that can then be injected. As the cement travels along the path of less resistance, it will then fill this empty cavity rather than flowing into the surrounding osseous or venous structures. In an in vivo comparison of the potential for extravertebral cement leakage after vertebroplasty and kyphoplasty, there were significantly lower rates of leakage of contrast material with kyphoplasty [52]. In the recent US experience, there was only one cement embolus, without breathing consequences [20].
Vertebroplasty using Cortoss
Cortoss is a new synthetic bone void filler that contains bis glycidyl methyl-methacrylate, bisphenol, a polyethylene glycol diether dimethylacrylate, triethyleneglycol dimethylacrylate monomer and bioactive glass ceramic [60]. It is provided in a double lumen cartridge with specially designed tips for mixing. After the composite is expressed through these tips, polymerization begins and the material is ready for use. The monomer is not volatile and Cortoss polymerizes in a three-dimensional network, which minimizes the chances of leaking. After mixing, the material has the consistency of toothpaste, and stays that way until it polymerizes quickly, in a matter of seconds. This characteristic provides a consistent tactile feedback and allows for an even injection. The polymerization has a much lower exotherm than PMMA (63°C vs 84°C), which reduces the risk of thermal necrosis. The modulus of elasticity of Cortoss is close to that of bone [60]. This composite is bioactive, and in animal studies the cement-bone interface continues to be strengthened over time with bone apposition occurring at the interface without any fibrous interposition. Cortoss cement appears well suited for use in the treatment of VCFs. The aliquot delivery system allows for accurate amounts of cement to be injected directly into the region of interest.
A prospective clinical study has been conducted at our institution with Cortoss [48]. To participate, patients had to have fracture-related pain measuring at least 50/100 on the VAS, which also caused a change in lifestyle or disability. Patients were scheduled for follow-up at 4 days, 1 week, and 1, 3, and 6 months after the procedure. Two metal trocars of 10 G diameter were introduced through the pedicles at each level treated. Twenty-four patients with osteoporotic fractures were enrolled. The average pain scores were 69 preoperatively and 38 at 4 days postoperatively. The scores continued to decrease, to 33 at 1 week and 29 at 1 month, and then returned to 33 at 6 months. This represents a reduction of pain of 46% at 6 months. The quality of life has been evaluated with the short form 1 (SF-12) questionnaire. Ability to ambulate was impaired in 75% preoperatively and in 28% at 6 months postoperatively. Ability to perform activities of daily living improved significantly post PVP. There was a 3% rate of minor complications, and no leakage into the spinal canal. Results indicate that Cortoss addresses the shortcomings of PMMA for vertebroplasty augmentation. This cement is a fixed composition material with less variability than current variations of PMMA, and in conjunction with the Aliquot delivery system can be accurately delivered in incremental doses without excessive material waste.
Bone substitutes in vertebroplasty
As requested by Heini [24], bone substitutes for vertebroplasty need the following properties: injectability, radiopacity, adapted viscosity, long setting time, good mechanical properties for the load (compressive strength/stiffness), biocompatibility, bioactivity, and slow degradation. Calcium phosphate cement meets these criteria well. In their ceramic form they cannot be used as injectable device. Tetracalcium phosphate with dicalcium hydroxy apatite and amorphous calcium phosphate also meet the criteria and are readily available. They can be injected through a 10- or 11-G needle. The results of animal tests are very promising, and in vitro experimental studies have shown interesting resistance in compression, of around 45 MPa. As reported by Le Huec [35], these resorbable calcium phosphates provide the calcium for local bone formation and are of great interest for the treatment of osteoporotic fractures. Clinical applications on humans are in progress, but the results of these studies have not yet been published. Also yet to be reported on is the effect of combining the use of resorbable calcium phosphates with bone morphogenic protein as a carrier, which is a promising technique to promote bone healing in fracture cases.
Conclusion
Kyphoplasty and vertebroplasty are safe and effective in the treatment of osteoporotic VCFs that do not respond to conservative medical treatment. Kyphoplasty has the potential benefit of restoring the height of the vertebral body and reducing kyphosis, but the clinical benefit of this needs to be studied by prospective randomized trials comparing the two techniques. The other question remaining is whether we should perform vertebroplasty or kyphoplasty in patients with osteoporotic fractures in an acute setting, or wait until failure of medical treatment before carrying out the procedure. This question is also best addressed by conducting a prospective randomized trial comparing conservative treatment to vertebroplasty and kyphoplasty. Bone substitutes are promising devices to treat osteoporotic fractures, but more experimental and clinical data are required to assess their efficacy in this application.
References
- 1.Adachi BMC Musculoskeletal Disord. 2002;3:11. doi: 10.1186/1471-2474-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebli Spine. 2002;27:460. doi: 10.1097/00007632-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Barr Spine. 2000;25:923. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Belkoff Spine. 2000;25:1061. doi: 10.1097/00007632-200005010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Belkoff Spine. 2001;26:151. doi: 10.1097/00007632-200101150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Belkoff Spine. 2001;26:1537. [Google Scholar]
- 7.Belkoff Spine. 2002;27:1640. doi: 10.1097/00007632-200208010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Belkoff AJNR. 2002;23:1647. [PMC free article] [PubMed] [Google Scholar]
- 9.Berlemann J Bone Joint Surg. 2002;84:748. doi: 10.1302/0301-620x.84b5.11841. [DOI] [PubMed] [Google Scholar]
- 10.Convertino Med Sci Sports Exerc. 1997;29:187. doi: 10.1097/00005768-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cook Arthritis Rheum. 1993;36:750. doi: 10.1002/art.1780360603. [DOI] [PubMed] [Google Scholar]
- 12.Cooper Am J Epidemiol. 1993;137:1001. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 13.Cortet J Rheumatol. 1999;26:2222. [PubMed] [Google Scholar]
- 14.Cortet Joint Bone Spine. 2002;69:201. doi: 10.1016/S1297-319X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 15.Cyteval AJR. 1999;173:1685. doi: 10.2214/ajr.173.6.10584820. [DOI] [PubMed] [Google Scholar]
- 16.Denis Spine. 1983;8:817. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Deramond Bone. 1999;25:17S. doi: 10.1016/S8756-3282(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 18.Evans Radiology. 2003;226:366. doi: 10.1148/radiol.2262010906. [DOI] [PubMed] [Google Scholar]
- 19.Galibert Neurochirurgie. 1987;33:166. [Google Scholar]
- 20.Garfin S (2001) A retrospective review of early outcomes of balloon kyphoplasty. In: Proceedings of the North American Spine Society, Seattle, Washington
- 21.Gaughen AJNR. 2002;23:594. [Google Scholar]
- 22.Gaughen AJNR. 2002;23:1657. [PMC free article] [PubMed] [Google Scholar]
- 23.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P (2000) Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 39:1410–1414 [DOI] [PubMed]
- 24.Heini Eur Spine J. 2001;10:S205. doi: 10.1007/s005860100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchon J Neurosurg. 2001;95:215. [Google Scholar]
- 26.Hiwatashi AJNR. 2003;24:185. [Google Scholar]
- 27.Jang Spine. 2002;27:E416. doi: 10.1097/00007632-200210010-00021. [DOI] [PubMed] [Google Scholar]
- 28.Jensen AJNR. 1997;18:1897. [PMC free article] [PubMed] [Google Scholar]
- 29.Kallmes AJNR. 2002;23:1117. [PMC free article] [PubMed] [Google Scholar]
- 30.Karjalainen Ann Chir Gynaecol. 1991;80:45. [PubMed] [Google Scholar]
- 31.Kaufmann AJNR. 2001;22:1860. [Google Scholar]
- 32.Keller Spine. 2003;28:455. doi: 10.1097/00007632-200303010-00009. [DOI] [Google Scholar]
- 33.Kim Radiology. 2002;222:737. doi: 10.1148/radiol.2223010718. [DOI] [PubMed] [Google Scholar]
- 34.Lane Clin Orthop. 2000;372:139. doi: 10.1097/00003086-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Le Huec J (1998) Evolution of the local calcium content around irradiated beta-tricalcium phosphate ceramic implants. Biomaterials 733–738 [DOI] [PubMed]
- 36.Lee Spine. 2002;27:E419. doi: 10.1097/00007632-200210010-00022. [DOI] [PubMed] [Google Scholar]
- 37.Leidig-Bruckner J Bone Miner Res. 1997;12:663. doi: 10.1359/jbmr.1997.12.4.663. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman Spine. 2001;26:1631. [Google Scholar]
- 39.Liebschner Spine. 2001;26:1547. doi: 10.1097/00007632-200107150-00009. [DOI] [PubMed] [Google Scholar]
- 40.Lim Spine. 2002;27:1297. doi: 10.1097/00007632-200206150-00010. [DOI] [PubMed] [Google Scholar]
- 41.Lukert Geriatrics. 1994;49:22. [PubMed] [Google Scholar]
- 42.McGraw J Vasc Interv Radiol. 2002;13:149. [Google Scholar]
- 43.McGraw J Vasc Interv Radiol. 2002;13:883. doi: 10.1016/s1051-0443(07)61770-9. [DOI] [PubMed] [Google Scholar]
- 44.McKercher Can Fam Physician. 2000;46:2228. [PMC free article] [PubMed] [Google Scholar]
- 45.Melton J Bone Miner Res. 1997;12:16. [Google Scholar]
- 46.Nakano J Neurosurg. 2002;97:287. doi: 10.3171/spi.2002.97.3.0287. [DOI] [PubMed] [Google Scholar]
- 47.Padovani AJNR. 1999;20:375. [PMC free article] [PubMed] [Google Scholar]
- 48.Palussiere (2003) The clinical use of Cortoss synthetic bone void filler in the repair of fractures of the vertebral body. In: Szpalski M, Gunzburg R (eds) Vertebral osteoporotic compression fractures. Lippincott Williams and Wilkins, pp 151–158
- 49.Patel Br J Rheumatol. 1991;30:418. doi: 10.1093/rheumatology/30.6.418. [DOI] [PubMed] [Google Scholar]
- 50.Peh Radiology. 2002;223:121. doi: 10.1148/radiol.2231010234. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Higueras Neuroradiology. 2002;44:950. doi: 10.1007/s00234-002-0856-1. [DOI] [PubMed] [Google Scholar]
- 52.Phillips Spine. 2002;27:2173. [Google Scholar]
- 53.Ryan Ann Rheum Dis. 1992;51:1063. doi: 10.1136/ard.51.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryu J Neurosurg. 2002;96:56. doi: 10.3171/spi.2002.96.1.0056. [DOI] [PubMed] [Google Scholar]
- 55.Schlaich Osteoporos Int. 1998;8:261. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 56.Schousboe J Clin Densitom. 2002;5:239. doi: 10.1385/jcd:5:3:239. [DOI] [PubMed] [Google Scholar]
- 57.Scroop AJNR. 2002;23:868. [Google Scholar]
- 58.Silverman Bone. 1992;13:S27. doi: 10.1016/8756-3282(92)90193-z. [DOI] [PubMed] [Google Scholar]
- 59.Sinaki M (1998) Musculoskeletal challenges of osteoporosis. Aging (Milano) 10:249–262 [DOI] [PubMed]
- 60.Szpalski M, Gunzburg R, Deramond H (2003) Percutaneous injection of Cortoss synthetic bone void filler in the repair of fractures in the vertebral body. In: Szpalski M, Gunzburg R (eds) Vertebral osteoporotic compression fractures. Lippincott Williams and Wilkins, pp 171–177
- 61.Tsou Ann Acad Med Singapore. 2002;31:15. [PubMed] [Google Scholar]
- 62.Uppin Radiology. 2003;226:119. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 63.Vasconcelos AJNR. 2002;23:913. [PMC free article] [PubMed] [Google Scholar]
- 64.Verlaan Spine. 2002;27:543. doi: 10.1097/00007632-200203010-00021. [DOI] [PubMed] [Google Scholar]


