Abstract
Cervical spondylotic myelopathy is a clinical entity that manifests itself due to compression and ischemia of the spinal cord. The goal of treatment is to decompress the spinal cord and stabilize the spine in neutral, anatomical position. Since the obstruction and compression of the cord are localized in front of the cord, it is obvious that an anterior surgical approach is the preferred one. The different surgical procedures, complications, and outcome are discussed here.
Keywords: Cervical spondylotic myelopathy, Anterior surgery, Fusion, Decompression
Introduction
In cervical spondylotic myelopathy (CSM) there is dysfunction of the spinal cord because of degenerative changes in the spine. The pathophysiology of neural loss is still a subject of some debate. Essentially there are two major mechanisms which cause myelopathy: direct compression of the cord and ischemic changes because of alterations in the local blood flow [10, 14, 41, 42, 55]. Since studies have demonstrated that the pathology of CSM is located predominantly anteriorly [47], it seems logical to approach the spine where the lesion is and choose an anterior approach. Removal of extruding intervertebral disc, spurs, osteophytes and calcified posterior longitudinal ligament relieves the compression of the anterior cord and improves to some extent the blood supply to the cord. The surgical approach as described by Smith and Robinson [86] covers the area between the vertebral bodies of C2 and T1. In patients with long slender necks the vertebral body of T3 may be within reach by this approach. The Smith and Robinson approach allows atraumatic dissection of the anterior aspect of the cervical spine. There is a low potential risk for injuries of the esophagus, trachea, the recurrent laryngeal nerve, and the carotid artery. The direct visualization of the offending pathology allows atraumatic and extensive decompression.
Surgical strategy
The goal of surgical treatment is to achieve a maximum of decompression without compromising the spinal stability and respecting the sagittal profile of the spine. Depending on the affected area the decompression may be executed through a simple discectomy, with or without fusion, or through extensive vertebrectomy with grafting and internal fixation. There are reports in the literature, advocating a discectomy without fusion [60, 90], but the majority of patients included in those studies had disc herniation and not CSM. The nonfusion discectomy eliminates the radicular symptoms in most of the cases but results for a long time in axial neck pain and compromises the lordotic curvature of the spine. This is the reason why discectomy is predominantly combined with interbody fusion today.
In a systematic review covering the literature until 1996 we were not able to identify the anterior interbody fusion as a gold standard for the treatment of degenerative disc disease [56] Nevertheless, the anterior discectomy and interbody fusion is the time-honored procedure in treatment of degenerative conditions of the cervical spine. This procedure is predictable with respect to decompression and symptom relief. It is suitable for addressing stenotic changes at single or multiple levels. Restoration of the intervertebral height and the lordotic curvature is possible when approaching each level separately (Fig. 1). On the other hand, this may result in increased risk for symptomatic pseudarthrosis because of the large number areas to fuse [39, 54, 83]. Since the degenerative changes in CSM cover a large area of the subaxial spine, corpectomy and grafting may be advocated [9, 10, 58]. Various terms have been adopted to describe the partial vertebral body resection, including complete or partial vertebrectomy, anterior corpectomy, and partial corpectomy. Basically all the terms refer to a partial resection of the vertebral body without removal of the transverse processes, pedicles, lateral masses, or other posterior elements. Resection of the lateral part of the uncovertebral joints must also be avoided to prevent injury of the vertebral artery. After decompression the spine must be reconstructed using strut grafts or artificial devices with or without internal fixation [21, 31, 36, 38, 44, 51, 63, 66, 94, 95].
Fig. 1.
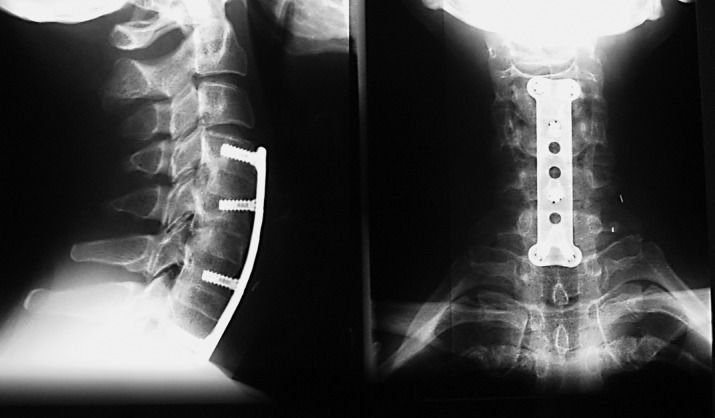
Three level decompression and fusion with iliac crest grafts and internal fixation. Note the restoration of lordosis
Surgical technique
In monosegmental decompression and stabilization it is essential to have sufficient view of the posterior part of the intervertebral space. After excision of the intervertebral disc and resection of the posterior longitudinal ligament the osteophytes must be recognized and entirely removed. Use of the diamond bur is recommended, together with Kerrison rongeurs and curettes. To ensure sufficient distraction of the intervertebral space a strong interlaminar spreader may be used. Use of the Caspar distractor is also recommended. It must be recognized that this distractor has limited ability to mobilize collapsed segments. When performing partial vertebrectomy it is essential to have a wide trough, positioned symmetrically in the midline. The width of the trough is up to 18 mm and may include the medial part of the uncovertebral joints [65]. Some authors do not advocate entire removal of the midsection of the posterior wall of the vertebral body [33].
Grafts, bone substitutes, devices, internal fixation
Structural autografts harvested from the anterior iliac crest or from the fibula are used in anterior fusion of the cervical spine. The grafts must enhance stability and substitute for the regenerative capacity of bone. Fresh autologous grafts posess some osteogenic potential and have osteoinductive and osteoconductive properties [62]. Structural corticocancellous grafts from the anterior iliac crest are commonly used, and their mechanical strength is greater than that of the posterior crest [89]. Iliac crest grafts are used in mono- and bisegmental interbody fusion and also after corpectomy involving no more then two levels. They are considered the biological and biomechanical standard for mono- and bisegmental reconstruction of the anterior cervical spine [3, 11, 17, 73, 75, 86, 98, 102, 103, 107]. In longer fusions after corpectomies a structural fibula graft is appropriate. There are different techniques for stabilizing the strut graft within the decompressed site [7, 47, 78, 105, 106]. Vascularized fibula grafts may accelerate the process of fusion in the case of multiple vertebrectomies [80, 100]. Additional internal fixation may provide immediate intrinsic stability in long strut graft constructs [15, 16, 46, 67, 92]. There are disadvantages when using autologous grafts such as potential donor site morbidity, increased operative time, and hospital stay.
To avoid these disadvantages allografts may be considered. There are also disadvantages concerning the use of allografts, such as risk of transmitting infections from the donor, prolonged healing, and compatibility problems [26, 30, 34, 49, 74, 82, 88, 99, 107]. The use of allografts in multilevel reconstructions is associated with a nonunion rate up to 41%. This nonunion rate is significantly higher than that with autologous grafts, which is estimated at 27% [24]. Allografts may be preserved as fresh-frozen or freeze-dried [27, 52, 87]. Both processes are effective in suppressing antigenicity and retain some osteoinductive ability and osteoconductive properties [62]. Other methods, including sterilization with ethylene oxide gas and high-dose γ-irradiation are effective but decrease significantly the osteoinductive properties and mechanical integrity of the graft [69, 81].
Demineralized bone matrix is composed material, consisting from some collagen proteins and bone growth factors [45]. There are some osteoinductive and osteoconductive properties established [81]. Since demineralized bone matrix lacks mechanical properties that resist forces, it is not suitable for reconstruction of large defects in the cervical spine.
Bioceramics are calcium phosphate materials processed by sintering. Hydroxyapatite and β-tricalcium phosphate are examples of the ceramics which may be used in reconstructive surgery. Hydroxyapatite is almost unresorbable while β-tricalcium phosphate degrades and resorbs 6–12 weeks after surgery [40, 70]. The bioceramics are mechanically stable, but the material is brittle and not suitable for use as a stand-alone device. Combined with a rigid anterior fixation bioceramics may be very successful in anterior interbody fusion [91].
Interposition devices (cages)
The introduction of interbody spacers, so-called cages, is the answer to donor site morbidity and optimalization of the fusion construct. There are two major types of cages: threaded hollow cylinders and rectangular cages. There is a fundamental difference in mode of action. The threaded cages are introduced and screwed through the endplates of the vertrebral bodies, whereas the rectangular cages mimic the intervertebral space dimensions and are in accordance with the anatomy of the endplates. In long fusions cylindrical mesh cages are employed, filled with autologous bone. Most cages are made of titanium, carbon fiber of poly-ether-ether-keton. The cages may be used empty or filled with autologous bone or bone substitutes. Good results have been reported by different authors [35, 50]. Our experience with rectangular cages made of poly-ether-ether-keton and filled with β-tricalcium phosphate (Cervios and Chronos, Mathys Medical, Bettlach, Switzerland) is extremely good. In a study to be published, we report that the TCP inserts are resorbed and restored by trabecular bone within 9 months after surgery (Fig. 2).
Fig. 2.
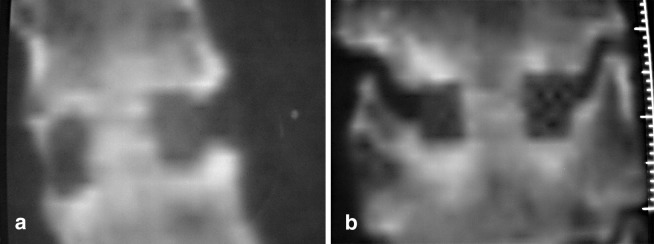
Computed tomography 6 months after C5–C6 segmental fusion with cage (Cervios, Mathys, Bettlach, Switzerland) prefilled with β-tricalcium phosphate. Note the restitution of β-tricalcium phosphate by bone. a Sagittal plane. b Coronal plane
Internal fixation
Internal fixation after decompression and fusion of the cervical spine provides high intrinsic stability of the construct, maintains alignment, and allows early functional recovery [2]. However, there is no substantial evidence to demonstrate higher fusion rates in plated fusion [1, 18, 96, 109, 110]. On the other hand, there are reports of improved maintenance of the sagittal profile of the spine after instrumented fusion [48, 93, 97]. Internal fixation is used by many surgeons today for mono- and bisegmental anterior interbody fusion [29, 76, 85]. In multilevel fusion after corpectomy (three or more levels), however, high rates of complications and pseudarthrosis have been reported [12, 20]. Di Angelo et al. [19] described the adverse effect of rigid anterior fixation on the stability of the construct. They concluded that the anterior plating reverses strut graft loading mechanics and excessively loads the graft in retroflexion. The stress shielding phenomenon has been observed by using rigid plates and screws with fixed angular orientation [108]. To improve some shortcomings of rigid fixation systems the concept of dynamic fixation has been introduced [1]. The "old" Caspar plates (Aesculaap, Braun, Tuttlingen, Germany) and Orozco (Synthes, Switzerland) are the first examples of noncontroled dynamic fixation on the cervical spine. Numerous different systems have been introduced to permit controlled dynamization of anterior fixation. Early reports are promising but not sufficiently convincing.
Complications
Mono- or bisegmental interbody fusion is usually not complication prone. The major complaints with autologous iliac crest grafts are from the donor site. Morbidity of up to 25% has been reported [79], and residual pain may persist for as long as 24 months after surgery [6]. The major advantage with cages filled with bone substitutes is the avoidance of any donor site morbidity. Multisegmental corpectomy and strut graft reconstructions contribute to the majority of complications regarding anterior surgery of the cervical spine. Some authors have reported perioperative complication rates up to 60% [8, 15, 53, 58, 68, 71, 78, 94, 106]. Most of these are due to inadequate soft tissue exposure and careless handling of vessels, nerves, and esophagus. Neural injuries are usually transient and involve the relatively short C5 nerve roots [77]. Complications related to bone grafting after multiple corpectomies are very common [7, 13, 24, 39]. Graft extrusion has been reported in 5–20%, even when internal fixation is used [25]. There are even reports of increased complication rate when using internal fixation [58, 68]. In instrumented multilevel corpectomies the construct failure that is observed is due to pistoning of the graft. This occurs because rigid anterior plating reverses graft-loading mechanics and excessively loads the graft in retroflexion. This load is higher then the resistive strength of the endplates, and therefore the strut graft subsides [4, 5, 28, 61, 101]. Using titanium mesh cage, Hee et al. [38] reported a high fusion rate of 95% for multilevel corpectomies but still an overall complication rate of 33%.
Outcome
Since there are no reliable data on the natural history of CSM, its treatment remains controversial. However, the anterior decompression and stabilization of the stenotic cervical spine reliably arrests myelopathy progression, and there is measurable objective improvement [7, 13, 15, 23, 25, 37, 57, 59]. Other authors report even a cure rate in excess of 50% and a regression rate of 5% [77]. A mean morbidity rate of 31% has been reported, which emphasizes the challenging nature of this kind of surgery [64, 84, 92, 106]. In an independent matched-cohort analysis comparing corpectomy vs. laminoplasty for multiple cervical myelopathy Edwards et al. [22] reported similar clinical outcome in the two cohorts, with fewer complications in the laminoplasty group. In the long term surgical benefits are maintained but functional capacity deteriorates. This is age related and may be an expression of a slow progression of cord dysfunction [104]. The surgical outcome from anterior decompression of the myelopathic spine is predictable. In monosegmental procedures the fusion rate is high, and the pseudarthrosis rate ranges from 4 to 6%. In the multilevel segmental fusion the pseudarthrosis rate increases due to the increased number of surfaces to fuse [39, 55, 83]. Preliminary experience in our clinic with anatomically shaped cages suggests a significant decrease in pseudarthrosis rate in multisegmental decompression and fusion. After solving the early complications with strut grafts in multilevel corpectomies the surgical outcome seems to be successful. In different series fusion rates above 90% have been reported without respect to plating as well [25, 23, 43, 72, 106].
In conclusion, the anterior approach to the myelopathic cervical spine is a logical answer to a specific pathological substrate. It is a challenging and rewarding surgery, which must be tailored to the individual patient.
References
- 1.Abraham Orthop Clin North Am. 1998;29:731. doi: 10.1016/s0030-5898(05)70044-4. [DOI] [PubMed] [Google Scholar]
- 2.An HS, Simpson JM (1994) Surgery of the cervical spine. In: An H, Simpson J (eds) Spinal instrumentation. Dunitz, London
- 3.An Spine. 1995;20:2211. [PubMed] [Google Scholar]
- 4.Anderson PA, Budorick TE, Easton KB, Henley MB, Salciccioli GG (1991) Failure of halo vest to prevent in vivo motion in patients with injured cervical spines. Spine 16 [Suppl 10]:S501–S505 [DOI] [PubMed]
- 5.Askins Spine. 1997;22:1193. doi: 10.1097/00007632-199706010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Banwart Spine. 1995;20:1055. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Bernard Clin Orthop. 1987;221:149. [Google Scholar]
- 8.Bernhardt J Bone Joint Surg Am. 1993;5:119. doi: 10.2106/00004623-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt J Bone Joint Surg Am. 1993;75:119. doi: 10.2106/00004623-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Bohlman Instr Course Lect. 1995;44:81. [PubMed] [Google Scholar]
- 11.Bohlman J Bone Joint Surg Am. 1993;75:1298. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bolesta Spine. 2000;25:2040. doi: 10.1097/00007632-200008150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Boni Spine. 1984;9:358. [PubMed] [Google Scholar]
- 14.Brain Brain. 1952;75:187. [Google Scholar]
- 15.Brown Spine. 1988;13:236. doi: 10.1097/00007632-198803000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Caspar W (1987) Anterior stabilization with trapezoid osteosynthetic technique in cervical spine injuries. In: Kehr P, Weidnner A (eds) Cervical spine. Springer, Berlin Heidelberg New York, pp 198–204
- 17.Clements Spine. 1990;15:1023. doi: 10.1097/00007632-199015100-00008. [DOI] [PubMed] [Google Scholar]
- 18.Connolly J Spinal Disord. 1996;9:202. [PubMed] [Google Scholar]
- 19.DiAngelo Spine. 2000;25:783. doi: 10.1097/00007632-200004010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Doh ES, Heller JG (1998) Multi-level anterior cervical reconstruction: comparison of surgical techniques and results. Program and abstracts of Cervical Spine Research Society 26th Annual Meeting, 3–5 December 1998, Atlanta
- 21.Ebraheim Orthopedics. 1995;18:141. doi: 10.3928/0147-7447-19950201-12. [DOI] [PubMed] [Google Scholar]
- 22.Edwards Spine. 2002;27:1168. doi: 10.1097/00007632-200206010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Emery J Bone Joint Surg Am. 1998;80:941. doi: 10.1302/0301-620X.80B6.9517. [DOI] [PubMed] [Google Scholar]
- 24.Fernyhough Spine. 1991;16:561. doi: 10.1097/00007632-199110001-00022. [DOI] [PubMed] [Google Scholar]
- 25.Fessler Neurosurgery. 1998;43:257. [Google Scholar]
- 26.Friedlaender GE, Huo M (1991) Bone grafts and bone graft substitutes. In: Frymoyer JW (ed) The adult spine: principles and practice. Raven, New York, pp 565–574
- 27.Friedlander Instr Course Lect. 1979;30:36. [PubMed] [Google Scholar]
- 28.Garfin J Bone Joint Surg Am. 1986;8:320. [PubMed] [Google Scholar]
- 29.Geer Clin Neurosurg. 1999;45:25. [PubMed] [Google Scholar]
- 30.Goldberg Clin Orthop. 1987;225:7. [Google Scholar]
- 31.Gore Spine. 1984;9:667. doi: 10.1097/00007632-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Grob Spine. 1998;23:2674. doi: 10.1097/00007632-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Groff Spine. 2003;28:14. doi: 10.1097/00007632-200301010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Guha Br J Plast Surg. 1983;36:305. doi: 10.1016/s0007-1226(83)90048-6. [DOI] [PubMed] [Google Scholar]
- 35.Hacker Spine. 2000;25:2646. doi: 10.1097/00007632-200010150-00017. [DOI] [PubMed] [Google Scholar]
- 36.Hall DJ, Webb JK (1991) Anterior plate fixation in spine tumor surgery: indications, technique and results. Spine 16 [Suppl]:80–83 [DOI] [PubMed]
- 37.Hanai K, Fujiyoshi F, Kamei K (1986) Subtotal vertebrectomy and spinal fusion for cervical spondylotic myelopathy. Spine 11310–315 [DOI] [PubMed]
- 38.Hee HT, Majd ME, Holt RT, Whitecloud TS III, Pienkowski D (2003) Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spin Disorders Techn 16:1:1–9 [DOI] [PubMed]
- 39.Herkowitz Clin Orthop. 1989;239:94. [Google Scholar]
- 40.Hoogendoorn Clin Orthop. 1984;187:281. [PubMed] [Google Scholar]
- 41.Hukuda Spine. 1988;13:15. doi: 10.1097/00007632-198801000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Ito Spine. 1996;21:827. doi: 10.1097/00007632-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 43.Jamjoom Br J Neurosurg. 1991;5:249. doi: 10.3109/02688699109005184. [DOI] [PubMed] [Google Scholar]
- 44.Johnston J Neurosurg. 1995;82:234. doi: 10.3171/jns.1995.82.2.0234. [DOI] [PubMed] [Google Scholar]
- 45.Kale Am J Orthop. 1995;24:752. [Google Scholar]
- 46.Kalfas IH (1996) The anterior cervical spine locking plate: a technique for surgical decompression and stabilization. In: Fessler RG, Haid RW (eds) Techniques in spinal stabilization. McGraw-Hill, New York, pp 25–33
- 47.Kang Curr Opin Orthop. 1996;7I:13. [Google Scholar]
- 48.Katsuura J Spinal Disord. 1996;9:470. [PubMed] [Google Scholar]
- 49.Kaufmann Neurosurgery. 1989;24:264. doi: 10.1227/00006123-198902000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Kettler A, Wilke HJ, Claes L (2001) Effects of neck movements on stability and subsidence in cervical interbody fusion: an in vitro study. J Neurosurg 94 [Suppl 1]:97–107 [DOI] [PubMed]
- 51.Kostuik Spine. 1993;18:1273. doi: 10.1097/00007632-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Kreuz J Bone Joint Surg Am. 1951;33:863. [PubMed] [Google Scholar]
- 53.Law J Bone Joint Surg Am. 1994;6:1420. [Google Scholar]
- 54.Law Instr Course Lect. 1995;44:99. [PubMed] [Google Scholar]
- 55.Levine J Neurol Neurosurg Psychiatry. 1997;62:334. doi: 10.1136/jnnp.62.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limbeek J van, Jacobs WCH, Anderson PG, Pavlov PW (2000) A systematic literature review to identify the best method for a single level anterior cervical interbody fusion. Eur Spine J 9:129–136 [DOI] [PMC free article] [PubMed]
- 57.Lunsford J Neurosurg. 1980;53:1. doi: 10.3171/jns.1980.53.1.0001. [DOI] [PubMed] [Google Scholar]
- 58.MacDonald J Neurosurg. 1997;86:990. doi: 10.3171/jns.1997.86.6.0990. [DOI] [PubMed] [Google Scholar]
- 59.Majd Spine. 1999;24:1604. doi: 10.1097/00007632-199908010-00016. [DOI] [PubMed] [Google Scholar]
- 60.Maurice-Williams Br J Neurosurg. 1996;10:261. doi: 10.1080/02688699650040115. [DOI] [PubMed] [Google Scholar]
- 61.Müller Manual of internal. 1991;fixation:techniques. [Google Scholar]
- 62.Muschler G, Lane JM (1992) Spinal fusion: principles of bone fusion. In: Rothmann RH, Simeone FA (eds) The spine, vol 2, 3rd edn. Saunders, Philadelphia
- 63.O'Brien Orthop Trans. 1997;24:474. [Google Scholar]
- 64.Okada J Bone Joint Surg Am. 1991;73:352. [PubMed] [Google Scholar]
- 65.Orr Clin Orthop. 1999;359:58. [PubMed] [Google Scholar]
- 66.Panjabi Spine. 1999;24:2383. doi: 10.1097/00007632-199911150-00016. [DOI] [PubMed] [Google Scholar]
- 67.Papadopoulos SM, Kalfas IH, Sonntag VKH (1993) Anterior Instrumentation of the cervical spine. In Whitecloud TS III, Dunsker SB (eds) Anterior cervical spine surgery. Raven, New York, pp 89–103
- 68.Paramore J Neurosurg. 1996;84:957. doi: 10.3171/jns.1996.84.6.0957. [DOI] [PubMed] [Google Scholar]
- 69.Pelker Orthop Clin North Am. 1984;18:235. [PubMed] [Google Scholar]
- 70.Rejeda J Bioeng. 1977;1:93. [Google Scholar]
- 71.Riew KD, Palumbo M, Hilibrand A, Carlson G, Bohlman H (1998) Complications of anterior cervical corpectomy in postlaminectomy patients. Federation of Spine Associations, Thirteenth Annual Meeting, New Orleans, Louisiana, March 1998
- 72.Riew Spine. 1999;24:2404. doi: 10.1097/00007632-199911150-00019. [DOI] [PubMed] [Google Scholar]
- 73.Riley J Neurosurg. 1969;30:127. [Google Scholar]
- 74.Rish Surg Neurol. 1976;5:119. [PubMed] [Google Scholar]
- 75.Robinson J Bone Joint Surg Am. 1962;4:1569. [Google Scholar]
- 76.Rushton Spinal Fusion. 1998;29:755. [Google Scholar]
- 77.Russegger Eur Spine J. 1997;6:70. doi: 10.1007/BF01676577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunders J Neurosurg. 1991;74:163. [Google Scholar]
- 79.Sawin J Neurosurg. 1998;88:255. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 80.Schaffer Clin Orthop. 1985;197:32. [Google Scholar]
- 81.Schimandle JH, Boden SD (1992) Bone grafts and bone graft substitutes for spinal fusion. In Rothman RH, Simeone FA (eds) The spine, vol 2, 3rd edn. Saunders, Philadelphia, pp 1610–1629
- 82.Schneider JR, Bright RW (1976) Anterior cervical fusion using preserved bone allografts. Transplant Proc 8 [Suppl]:73–6 [PubMed]
- 83.Seifert Acta. 1995;Neurochir:105. doi: 10.1007/BF02187753. [DOI] [PubMed] [Google Scholar]
- 84.Seifert Neurosurgery. 1991;29:498. [PubMed] [Google Scholar]
- 85.Shapiro J Neurosurg. 1996;84:161. doi: 10.3171/jns.1996.84.2.0161. [DOI] [PubMed] [Google Scholar]
- 86.Smith J Bone Joint Surg Am. 1958;40:607. [PubMed] [Google Scholar]
- 87.Stahlman GC, Hanley EN, Phillips E (1992) Allograft bone in spinal surgery. In Rothman RH, Simeone FA (eds) The spine, vol 2, 3rd edn. Saunders, Philadelphia, pp 1601–1606
- 88.Summers J Bone Joint Surg Br. 1989;71:677. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 89.Takeda Clin Orthop. 1985;193:120. [PubMed] [Google Scholar]
- 90.Tegos Eur Spine J. 1994;3:62. doi: 10.1007/BF02221441. [DOI] [PubMed] [Google Scholar]
- 91.Thalgott Spine. 1999;24:1295. doi: 10.1097/00007632-199907010-00005. [DOI] [PubMed] [Google Scholar]
- 92.Tippets Neurosurgery. 1988;22:1008. doi: 10.1227/00006123-198806010-00006. [DOI] [PubMed] [Google Scholar]
- 93.Vaccaro Clin Orthop. 1997;335:112. [PubMed] [Google Scholar]
- 94.Vaccaro J Spinal Disord. 1998;11:410. [PubMed] [Google Scholar]
- 95.Vanichkachorn Spine. 1998;23:2462. doi: 10.1097/00007632-199811150-00023. [DOI] [PubMed] [Google Scholar]
- 96.Wang J Spinal Disord. 1999;12:476. [Google Scholar]
- 97.Wang Spine. 2000;25:41. doi: 10.1097/00007632-200001010-00009. [DOI] [PubMed] [Google Scholar]
- 98.Watters Spine. 1994;19:2343. doi: 10.1097/00007632-199410150-00016. [DOI] [PubMed] [Google Scholar]
- 99.Weikel Plast Reconstr Surg. 1977;60:572. [PubMed] [Google Scholar]
- 100.Weiland Plast Reconstr Surg. 1984;74:368. [PubMed] [Google Scholar]
- 101.White AA, Panjabi MM (eds) (1990) Clinical biomechanics of the spine. Lippincott, Philadelphia
- 102.White Bone Joint Surg Am. 1973;5:525. [PubMed] [Google Scholar]
- 103.Williams J Bone Joint Surg Am. 1968;50:277. doi: 10.2106/00004623-196850020-00006. [DOI] [PubMed] [Google Scholar]
- 104.Yonenobu Eur Spine J. 2000;9:1. doi: 10.1007/s005860050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yonenobu Spine. 1985;10:710. doi: 10.1097/00007632-198510000-00004. [DOI] [PubMed] [Google Scholar]
- 106.Zdeblick J Bone Joint Surg Am. 1989;71:170. [PubMed] [Google Scholar]
- 107.Zdeblick Spine. 1991;16:726. doi: 10.1097/00007632-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 108.Zdeblick Spine. 1993;14:1974. doi: 10.1097/00007632-199310001-00009. [DOI] [PubMed] [Google Scholar]
- 109.Zoega Acta Orthop Scand. 1998;69:363. doi: 10.3109/17453679808999048. [DOI] [PubMed] [Google Scholar]
- 110.Zoega Eur Spine J. 1998;7:302. doi: 10.1007/s005860050079. [DOI] [PMC free article] [PubMed] [Google Scholar]


