Abstract
Radical cure of Plasmodium vivax infection applies blood schizontocidal therapy against the acute attack and hypnozoitocidal therapy against later relapse. Chloroquine and primaquine have been used for 60 years in this manner. Resistance to chloroquine by the parasite now requires partnering other blood schizontocides with primaquine. However, the safety and efficacy of primaquine against relapse when combined with other drugs have not been demonstrated. This randomized, open-label, and relapse-controlled trial estimated the efficacy of primaquine against relapse when administered with quinine or dihydroartemisinin-piperaquine for treatment of the acute infection. Among 650 soldiers who had returned to their malaria-free base in Java, Indonesia, after 12 months in malarious Papua, Indonesia, 143 with acute P. vivax malaria were eligible for study. One hundred sixteen enrolled subjects were randomized to these treatments: artesunate (200-mg dose followed by 100 mg/day for 6 days), quinine (1.8 g/day for 7 days) plus concurrent primaquine (30 mg/day for 14 days), or dihydroartemisinin (120 mg) plus piperaquine (960 mg) daily for 3 days followed 25 days later by primaquine (30 mg/day for 14 days). Follow-up was for 12 months. One hundred thirteen subjects were analyzable. Relapse occurred in 32 of 41 (78%) subjects administered artesunate alone (2.71 attacks/person-year), 7 of 36 (19%) administered quinine plus primaquine (0.23 attack/person-year), and 2 of 36 (6%) administered dihydroartemisinin-piperaquine plus primaquine (0.06 attack/person-year). The efficacy of primaquine against relapse was 92% (95% confidence interval [CI] = 81% to 96%) for quinine plus primaquine and 98% (95% CI = 91% to 99%) for dihydroartemisinin-piperaquine plus primaquine. Antirelapse therapy with primaquine begun a month after treatment of the acute attack with dihydroartemisinin-piperaquine proved safe and highly efficacious against relapse by P. vivax acquired in Papua, Indonesia.
INTRODUCTION
Malaria caused by Plasmodium vivax threatens severe illness in travelers and the several billion people living at risk in zones of endemicity (1–4). Despite the long-standing dogma of benign identity for this parasite, it is pernicious and often provokes life-threatening illness (5). Unlike the other important cause of malaria, Plasmodium falciparum, this parasite places dormant forms in the liver (hypnozoites) that cause repeated attacks called relapses (6). The only licensed therapy against relapse is primaquine (PQ) used in combination with chloroquine (CQ) for the primary attack, together called radical cure. Worsening CQ resistance threatens this 60-year-old treatment (7), and PQ efficacy against relapse has only rarely been estimated since its development in experimental challenge of American prisoner volunteers. The failing efficacy of CQ and the uncertain efficacy of PQ raise doubts regarding successful chemotherapeutic management of this dangerous infection.
Emerging CQ-resistant P. vivax compels consideration of dihydroartemisinin (DHA) plus piperaquine (PP) as a blood schizontocide with PQ (8). Although good evidence shows the safety and efficacy of DHA-PP against acute vivax malaria (9, 10), no evidence yet demonstrates the safety and efficacy of PQ against relapse when partnered with DHA-PP. This is a critical gap because PQ efficacy against relapse depends on an appropriate companion blood schizontocide: PQ administered following rather than concurrently with quinine (QN) failed to prevent relapse of the Chesson strain of P. vivax (11). These findings and others indicate that replacing CQ in radical cure requires assessment of the safety and efficacy of PQ against relapse when partnered with each new blood schizontocidal therapy (12, 13).
Hypnozoites impose analytical ambiguities in estimating PQ efficacy. Recurrent parasitemia following primary attack may originate from failed therapy against asexual blood stages (recrudescence), biting infectious anopheline mosquitoes (reinfection), or hypnozoites (relapse). Parasitemia arising from relapse may be genetically homologous, like recrudescence, or heterologous, like reinfection (14). Discriminating these events using molecular technologies is thus not yet possible, and this imposes limitations in estimating PQ efficacy against relapse. Experimental challenge in zones of nonendemicity largely solves this problem but imposes logistical, fiscal, and ethical burdens. Recruitment of naturally infected travelers who have returned to areas of nonendemicity (and given effective blood schizontocidal therapy) can be used to rule out reinfection and recrudescence, and recurrent parasitemias could arise only from activated hypnozoites.
Variability in the timing and risk of relapse imposes another analytical problem. The risk of relapse without PQ therapy ranges from about 10% to 90%, and the first relapse may occur at anytime between 3 and 52 weeks (15). Thus, a 10% relapse rate following PQ may represent 0% to 90% efficacy, depending on natural relapse behaviors (16). Reliably estimating PQ efficacy thus requires knowing the natural timing and risk of relapse, and a relapse control arm, i.e., subjects receiving no PQ, achieves this (17, 18).
The current study leveraged heavily exposed travelers who had returned to an area of nonendemicity, together with a relapse control arm, to derive relatively unambiguous estimates of PQ efficacy. After a year of duty in malarious Papua, Indonesia, Indonesian soldiers with vivax malaria were randomized to radical cure with DHA-PP or QN and PQ or treatment with artesunate (AS) without PQ and followed for 12 months in an area free from risk of reinfection at their base in Java, Indonesia. The QN treatment arm essentially served as a surrogate CQ arm in terms of a proven partner drug with PQ, i.e., a positive control for PQ efficacy against relapse. Prevalent CQ-resistant P. vivax parasites where these infections were acquired (7) precluded a CQ treatment arm in this study. The study thus aimed principally at estimating the efficacy of PQ when partnered with DHA-PP.
MATERIALS AND METHODS
Study design and oversight.
This randomized, open-label, relapse-controlled trial estimated PQ efficacy against relapse when partnered with QN or DHA-PP. Patients randomized to relapse control received AS. Subjects were followed until they had recurrent P. vivax or for 12 months. Prior to recruitment, the research team undertook structured seminars aimed at familiarizing soldiers and commanders with informed consent to participate in medical research. Institutional ethics review boards of the Faculty of Medicine, University of Indonesia, Jakarta, Indonesia, and the Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom, reviewed and approved a protocol detailing the conduct of this work. Authorized food and drug regulators for Indonesia (Badan Pengawas Obat dan Makanan, Jakarta, Indonesia) reviewed, approved, and later audited the trial in progress. An appointed data safety monitoring board (DSMB) reviewed periodic summaries of adverse events and reports of severe adverse events within several days of their occurrence. The trial was registered with ISRCTN as trial no. 38290380 after commencement of enrollment as a consequence of administrative error. The editor of this journal reviewed the approved protocol for this trial as a condition of acceptance of the manuscript for publication.
Study sites and subjects.
The Indonesian army base at Lumajang, East Java, Indonesia, served as the study site. The surrounding district of Lumajang (1,053,000 residents) had an average population density of 588/km2. Twenty-five health care delivery centers and five hospitals performed 9,015 blood film examinations for malaria in 2010, with none being positive. During 2011, authorities reported 10 cases of malaria, all classified as imported. A mass blood survey of 1,155 schoolchildren conducted in February 2012 in areas considered to be at a relatively high risk of malaria transmission uncovered no cases. The subjects of the current study were thus considered free of risk of reinfection during the posttreatment period of follow-up (19, 20).
Six hundred fifty soldiers based at Lumajang deployed for duties in northeastern Papua in October 2009. Previous studies of soldiers in the same area of that deployment showed P. vivax infection rates of 2/person-year (21, 22). The soldiers departed Papua on 1 November 2010, and on 8 November 2010 they disembarked from a ship at Surabaya, East Java. They remained at port for several days for routine health screening prior to their repatriation to Lumajang. After the soldiers resettled at their base and the study was explained to troops and commanders, screening by mass blood surveys commenced on 30 November 2010 and continued on a rolling basis through April 2011, when enrollment closed. The research team operated an all-hours clinic and encouraged soldiers with illness to visit at any time.
Soldiers positive for vivax malaria by microscopy were invited for counseling, to provide informed consent, and clinical assessment. Laboratory screening of venous blood included a complete blood count (Coulter Ac-T diff analyzer; Beckman Coulter, Brea, CA), analysis of liver and renal function (BS-120 chemical analyzer; Mindray, Guangdong, China), electrolyte concentrations (Ilyte electrolyte analyzer; Medica Corporation, MA), and glucose-6-phosphate dehydrogenase (G6PD) deficiency (NADPH qualitative spot test; kit 203A; Trinity Biotech, Wicklow, Ireland). Physical examination included an electrocardiogram (BTL-08; BTL Industries Inc., MA) that used 12 leads with a paper speed of 50 mm/s and that was calibrated to 10 mm/mV. Exclusion criteria included refusal to provide informed consent, any condition requiring hospitalization, a history or evidence of any disorder incompatible with subject safety, definite plans for absence from the site within 28 days, G6PD deficiency, liver transferase levels >2.5-fold above the upper limit of normal, a Fridericia-corrected QT interval of >450 ms, or anemia (hemoglobin level, <8 g/dl).
Drug therapy.
A randomized list of study numbers that were block allocated was generated by varying the blocking number at random (23). An envelope revealing the assigned therapy was opened after informed consent. Assignments were as follows: for AS, 200 mg AS (Arsuamoon; a 50-mg artesunate tablet packaged with an amodiaquine hydrochloride tablet [discarded]; Guilin Pharmaceuticals Co. Ltd., Shanghai, China) was administered, followed by a daily dose of 100 mg daily for 6 days. For QN plus PQ (QN+PQ), 200-mg quinine base tablets (Q; Kimia Pharma, Bandung, Indonesia) were administered 3 times/day for 7 days at 10 mg/kg of body weight per dose and were given concurrently with a single daily dose of two tablets each containing 15 mg primaquine base (Malafree; Shin Poon Pharmaceuticals, Seoul, South Korea) for 14 days. Subjects weighing >70 kg received a 45-mg PQ daily dose. For DHA-PP plus PQ (DHA-PP+PQ), a single daily dose of 3 tablets, each containing 40 mg dihydroartemisinin base and 320 mg piperaquine base (Eurartesim; Sigma Tau, Italy), was administered for 3 days. Primaquine was administered precisely as detailed above, except that dosing commenced on day 28 following enrollment.
The WHO recommends a daily adult primaquine dose of 15 mg, 22.5 mg, or 30 mg for 14 days, depending on geographic origin; i.e., in Southeast Asia or Oceania, the higher dose is required (24). This study applied the 30-mg daily dose or a 45-mg daily dose to subjects weighting >70 kg (25). PQ dosing commenced on day 28 after starting DHA-PP because the safety of concurrent administration remains unknown.
Clinical procedures.
A clinical team member witnessed and signature affirmed all doses, as did the subject. Subjects were offered and encouraged to consume a nonfatty, carbohydrate snack prior to consuming medication. Subjects were observed for 1 h, and emesis prompted repeated dosing. On days 3, 7, 14, 28, 41, and 84, venous blood was analyzed by the methods detailed for screening. Additionally, a noninvasive oximeter (Masimo set; Masimo Corp., CA) recorded peripheral blood oxy- and methemoglobin (metHb) levels. Subjects were queried for adverse events during the same clinical visits. Blood for routine blood films was collected on days 3, 7, 14, 21, 28, 35, 41, 56, 63, 70, 84, 126, 140, 180, and 365. All visits to the clinic with illness prompted blood film examination, regardless of the day of study.
A positive diagnosis of malaria confirmed by a second microscopist prompted immediate rescue therapy according to national treatment guidelines (8), except that a 0.5-mg/kg daily primaquine dose was applied (in lieu of 0.25 mg/kg). Blots of blood were collected onto Whatman FTA Classic card filter paper when subjects had malaria. DNA extracted from these was analyzed using a multiplex sphere-based PCR assay (26).
Statistical analysis.
Data were double entered into an Access database and validated using EpiInfo (version 3.5.1) software. The statistical analysis used the Stata (version 11) program. Descriptive statistics were performed to determine the proportions for categorical data and the summary statistics for continuous data. Chi-square and Fisher's exact tests compared proportions. The Kruskal-Wallis test was used for nonparametric comparisons. The primary endpoint was the percent therapeutic efficacy of the treatment groups, calculated using the incidence density (ID) of recurrent P. vivax, as follows: [(IDAS − IDQN or IDDHA-PP)/IDAS] × 100.
The 95% confidence interval (CI) for the estimate was calculated from the binomial distribution. Incidence density was the number of P. vivax recurrences divided by the sum of person-years at risk at the end of the study. Cumulative incidence was estimated using life table calculations with application of weekly intervals (27). The study was powered for a primary assessment of the noninferiority of primaquine against relapse when partnered with QN versus DHA-PP for radical cure. Per protocol, this required 60 subjects per treatment arm, a number not realized in recruitment. We thus defaulted to the secondary objective, precision in individual estimates of PQ efficacy in each treatment arm, as reported here.
RESULTS
Screening and enrollment.
Figure 1 summarizes the patient and subject flow during the study. Malaria occurred in 152 soldiers, with 9 cases being P. falciparum malaria. Among 143 eligible patients diagnosed with acute P. vivax infection, 27 were not enrolled: 4 were G6PD deficient, 6 were clinically unfit (because of recent antimalarial therapy [n = 2], elevated liver enzymes [n = 2], or acute hepatitis [n = 2]), 6 refused consent, 2 had planned absences, and 9 declined to specify a reason. These 36 patients were promptly treated according to national guidelines and monitored until they fully recovered. One hundred sixteen soldiers with vivax malaria were randomized to AS (n = 41), QN+PQ (n = 39), or DHA-PP+PQ (n = 36). One subject assigned to QN+PQ withdrew informed consent after enrollment but prior to initiating treatment, leaving 38 subjects in this arm. PCR diagnostics later affirmed the enrollment diagnosis of P. vivax infection in 114 subjects; 2 discordant PCR diagnoses were positive for Plasmodium falciparum and Plasmodium malariae, respectively. Both of those subjects had been assigned to QN+PQ and were excluded from analysis, leaving 36 evaluable subjects in that arm. In all, 113 subjects were evaluable at the close of the study.
Fig 1.
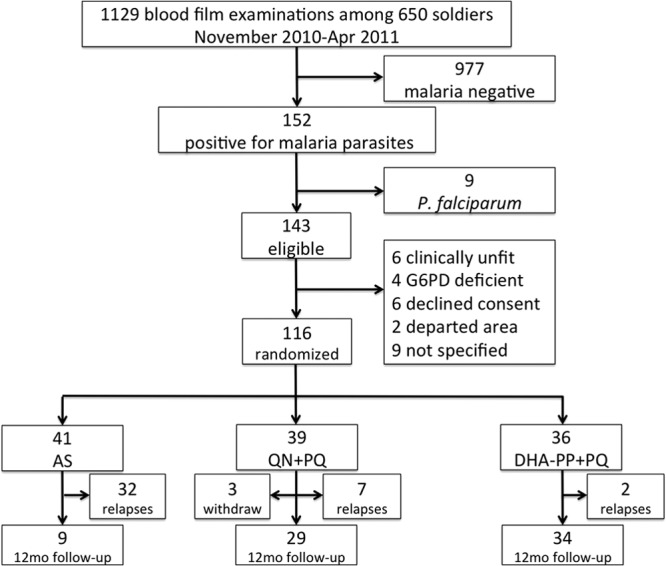
Screening, enrollment, randomization, and follow-up of subject patients.
Figure 2 illustrates the timing and means of case detection during the period of enrollment (30 November 2010 to 26 April 2011). The first mass screening of soldiers for asymptomatic malaria occurred during the first 2 weeks of subject recruitment, during which 45 soldiers were found to be positive for vivax malaria by screening and 3 soldiers reported to the clinic complaining of illness. Despite further mass blood film screening up to week 21 of recruitment, no soldiers were found to be positive. The remaining 88 soldiers found to be positive all came to the study clinic complaining of illness throughout the recruitment period.
Fig 2.
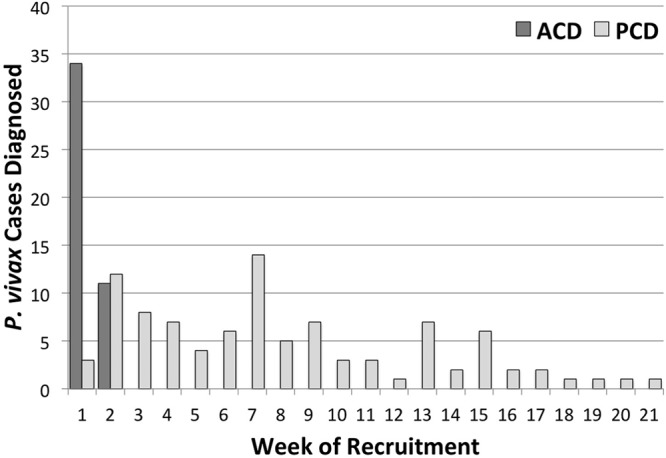
Case detection means and timing during subject recruitment in the study. Active case detection (ACD) versus passive case detection (PCD) findings of acute vivax malaria among soldiers reporting to the study clinic complaining of illness are illustrated.
Table 1 lists the baseline demographic, laboratory, and clinical features among the treatment groups. Young men (mean age, 27 to 28 years; age range, 22 to 42 years; P = 0.89) of similar weights (mean weight, 65 to 67 kg; weight range, 52 to 90 kg; P = 0.90) having similar parasitemias (median, 1,304 to 2,264 parasites/μl blood; range, 32 to 15,248 parasites/μl blood; P = 0.83) composed the study population. Fever (>37.5°C) occurred in 17% to 33% of subjects among the groups (P = 0.17). No significant differences in any mean vital sign or laboratory measurement of blood appeared. The incidence of complaints of illness among the groups at enrollment was essentially similar, with headache (>0.7 complaint/person-day), fever (>0.6 complaint/person-day), muscle ache (>0.4 complaint/person-day), nausea (>0.2 complaint/person-day), cough (>0.05 complaint/person-day), and vomiting (>0.05 complaint/person-day) being the dominant complaints.
Table 1.
Characteristics of subject patients among assigned treatment arms at enrollment
| Characteristic | Result by treatment assignment |
P value | ||
|---|---|---|---|---|
| AS | QN+PQ | DHA-PP+PQ | ||
| No. (%) of subjects | 41 (35.3) | 39 (33.6) | 36 (31.0) | 0.78 |
| Age (yr) | ||||
| Mean | 27.7 | 27.1 | 27.8 | 0.89 |
| Range | 21.5–38.8 | 22.1–31.5 | 23.6–42.1 | |
| Wt (kg) | ||||
| Mean | 66.5 | 65.4 | 65.0 | 0.90 |
| Range | 52–90 | 52–90 | 55–78 | |
| Mean tympanic temp (°C) | 36.7 | 36.7 | 36.9 | 0.67 |
| No. (%) of febrile patients | 7 (17.1) | 7 (18.0) | 12 (33.3) | 0.17 |
| Parasite count (no. of parasites/μl blood) | ||||
| Median | 1,408 | 1,304 | 2,264 | 0.83 |
| Range | 32–15,248 | 32–8,560 | 48–10,112 | |
| Mean hemoglobin concn (g/dl) | 14.2 | 14.0 | 13.5 | 0.06 |
| Mean blood glucose concn (mg/dl) | 108 | 107 | 110 | 0.82 |
| Mean total bilirubin level (mg/dl) | 1.95 | 1.69 | 1.97 | 0.23 |
| Mean platelet count (no. of platelets [103]/ml) | 167 | 162 | 156 | 0.64 |
| Mean white blood cell count (no. of cells/μl) | 7,700 | 7,500 | 7,700 | 0.83 |
Tolerability and safety.
The dominant complaints of illness and many minority complaints fell sharply during treatment and resolved by the end of treatment. Only two exceptions occurred: no one complained of tinnitus or hearing loss at enrollment, but individuals in the QN+PQ group did so during treatment (0.2 and 0.1 complaint/person-day, respectively). Four subjects were hospitalized during the study and were recorded as having serious adverse events, but the DSMB considered none to be related to either acute malaria or study drugs (appendicitis, colitis, and trauma [n = 2]).
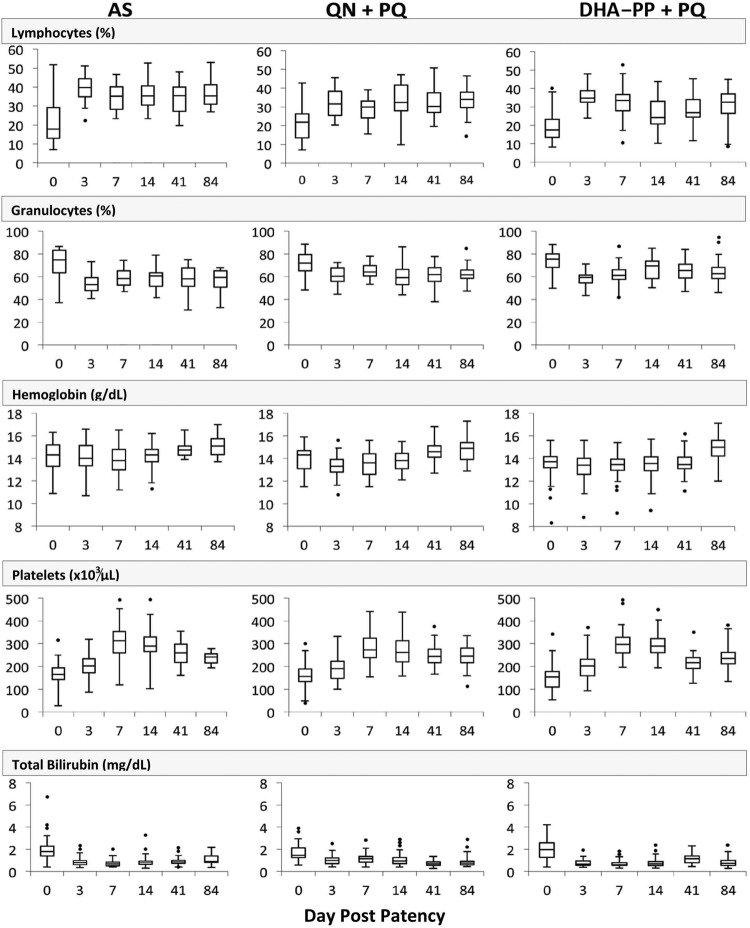
Most laboratory findings during the first 3 months of study among the groups remained within normal ranges before, during, and after treatment. These included white blood cell counts; percent monocytes; red blood cell count; mean corpuscular volume; mean corpuscular hemoglobin; mean corpuscular hemoglobin concentration; percent red cell distribution width; and glucose, urea, creatinine, serum glutamic oxalacetic transaminase, serum glutamic pyruvic transaminase, total protein, albumin, alkaline phosphatase, gamma glutathione, lactate dehydrogenase, creatine kinase, sodium, potassium, and chloride concentrations. Some values did change (Fig. 3), however: initially depressed lymphocytes and platelets (approximately 20% and 180,000/μl, respectively) recovered to normal within 3 to 7 days (approximately 35% and 200,000/μl, respectively) of treatment in all groups, and elevated granulocytes and total bilirubin (approximately 75% and 2 mg/dl, respectively) returned to normal levels (approximately 60% and <1 mg/dl, respectively) within 3 days of treatment. All groups showed a slight drop in mean hemoglobin concentration (<0.5 g/dl to approximately 13 to 14 g/dl) within 3 days of starting treatment, followed by incremental increases up to day 84 (to approximately 15 g/dl). One soldier randomized to DHA-PP had just 8.3 g/dl hemoglobin at enrollment, and his recovery to normal levels may be seen in Fig. 3 as the lowest outlier data point up to day 41. This borderline anemic soldier did not experience a posttherapy drop in hemoglobin but saw steady increases on days 3, 7, 14, 41, and 84.
Fig 3.
Summary of blood laboratory findings with changes during the course of treatment and convalescence to day 84 showing the median (horizontal lines), interquartile range (boxes), 1.5-fold the quartile range (vertical lines), and outlying observations (points).
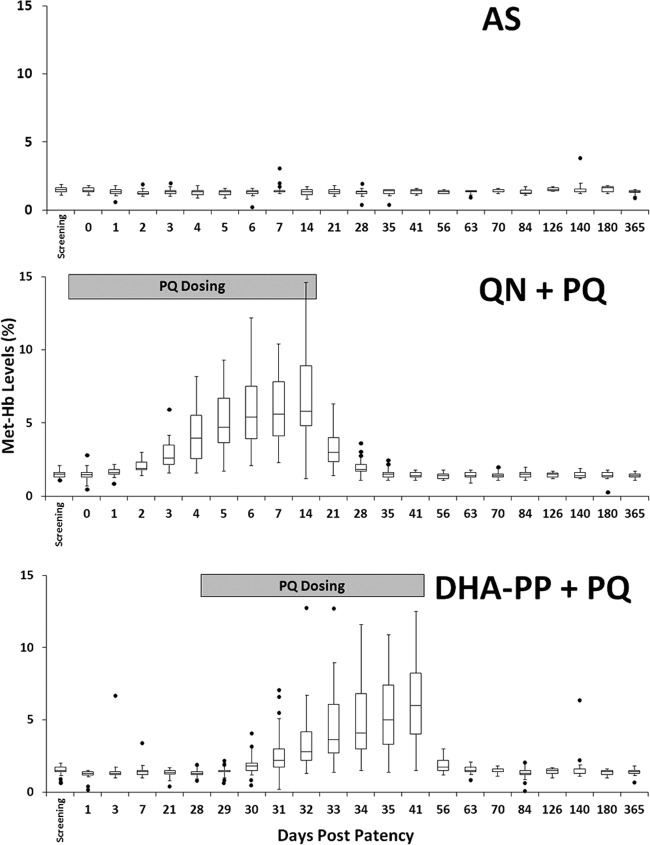
Figure 4 illustrates metHb levels sharply rising among groups dosed with PQ, peaking at about day 7 postpatency and remaining at that level until the cessation of dosing a week later. The mean metHb level peaked at approximately 6%, with interquartile ranges not exceeding 10%. A single measurement in one subject (QN+PQ group) was 17% (not shown in Fig. 3). metHb returned to normal levels in all subjects within 2 weeks of completing PQ.
Fig 4.
metHb levels (percent for each y axis) among subjects showing the median (horizontal lines), interquartile range (boxes), 1.5-fold the quartile range (vertical lines), and outlying observations (points) relative to the days of daily primaquine dosing (gray boxes).
Therapeutic response.
All except 3 subjects (1 in the QN+PQ group and 2 in the DHA-PP group) were negative for blood-stage parasites within 3 days, and none of these subjects later had a recurrence. No subjects were positive at day 7 or day 14. The earliest recurrence appeared at day 17 in a subject in the AS group, and he was followed in rapid succession by most other subjects in the AS arm; 32 of 41 (78%) had recurrences, and 25 of these 32 (78%) appeared on or before day 28 postpatency. The seven other recurrences appeared between days 29 and 163; day 21 was the median day of recurrence in this group. All recurrences in all three treatment arms were identified by microscopy and confirmed with PCR diagnostics to be P. vivax monoinfections. All 41 recurrences among the subjects in the three arms were classified as relapses (see Discussion).
Figure 5 illustrates the cumulative incidence of relapse among treatment arms at weekly intervals. Among QN+PQ subjects, seven relapses occurred between days 36 and 194. Two subjects that received DHA-PP+PQ relapsed on days 82 and 126. The nine study subjects who relapsed following primaquine therapy were not heavier than subjects given successful primaquine therapy (P > 0.6). All relapses were treated with DHA-PP+PQ as per study treatment, and none of these relapsed again up to the closure of the study and withdrawal of the study team from Lumajang in May 2012. In November 2012, the battalion medical team at Lumajang reported no cases of malaria since closure of the study.
Fig 5.
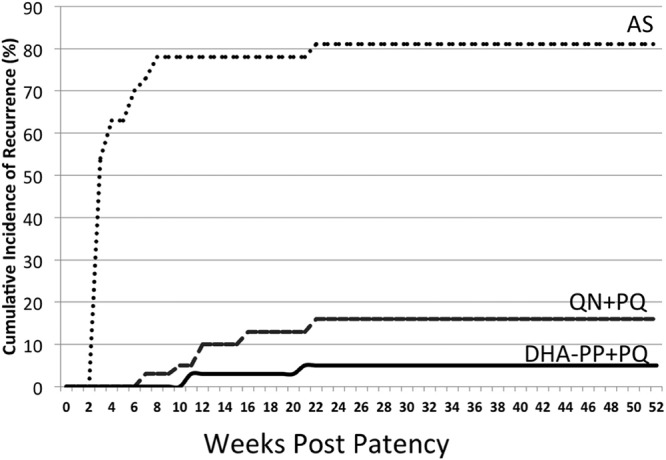
Cumulative incidence of recurrent parasitemia among treatment groups over 1 year of observation. The first relapse occurred on day 17 postpatency (AS), and the last occurred on day 194 (QN+PQ).
Table 2 summarizes estimates of the relapse-controlled therapeutic efficacy of PQ on the basis of the incidence density of recurrent parasitemia. These were 0.226 and 0.058 relapses/person-year for QN+PQ and DHA-PP+PQ, respectively. The denominator for the estimate of PQ efficacy was the incidence density of relapse among subjects receiving AS alone, i.e., 2.71 relapses/person-year. The efficacy of QN+PQ against relapse was 92% (95% CI = 81 to 96%), and for DHA-PP+PQ it was 98% (95% CI = 91 to 99%). Efficacy calculated by cumulative incidence is also shown in Table 2: 75% (95% CI = 52 to 87%) for QN+PQ and 93% (95% CI = 72 to 98%) for DHA-PP+PQ.
Table 2.
Estimates of therapeutic efficacy of radical cure of vivax malaria with PQ and QN or DHA-PP
| Characteristic | Result by treatment assignment |
||
|---|---|---|---|
| AS | QN+PQ | DHA-PP+PQ | |
| No. of subjects | 41 | 39 | 36 |
| No. of withdrawals | 0 | 3 | 0 |
| No. of relapses | 32 | 7 | 2 |
| Incidence density | |||
| No. of person-wk at risk | 615 | 1,613 | 1,803 |
| Incidence density (no. of incidents/person-yr) | 2.707 | 0.226 | 0.058 |
| Efficacy (%) against relapse (95% CI) | 92 (81–96) | 98 (91–99) | |
| Cumulative incidence | |||
| 12-mo cumulative incidence (%) | 80.48 | 19.44 | 5.56 |
| Efficacy against relapse (95% CI) | 75.4 (52–87) | 92.9 (72–98) | |
DISCUSSION
This study found good safety, tolerability, and efficacy of PQ against relapse in G6PD-normal men when administered after DHA-PP for acute vivax malaria. The efficacy of PQ (by incidence density) with QN was not significantly lower than that of PQ with DHA-PP (P = 0.19), but QN therapy came with side effects typical of cinchonism, along with less convenient dosing than DHA-PP. These findings show excellent PQ efficacy when partnered with this new artemisinin-combined therapy (ACT) (28) for radical cure of vivax malaria.
The relatively low estimate of efficacy of QN+PQ by cumulative incidence (75%) versus incidence density (92%) calculations derives from the effect of mathematical management of the respective denominators and the relatively long period of follow-up. Similar effects have been observed in trials of malaria chemoprophylaxis also involving prolonged follow-up (22, 29). The primary distinction between the two approaches is a denominator that does not increase as time passes versus one that does, i.e., cumulative incidence versus incidence density, respectively. As more time passes, the difference expands. The mathematics of this phenomenon have been explained (30, 31). Like those statisticians, we consider incidence density to be the preferred analytical approach in estimating primaquine efficacy because, like trials of chemoprophylaxis, inclusion of person-time at risk offers more meaningful estimates of efficacy against intrinsically prolonged events like relapse. The informative visual representation of timing of relapse represents the primary utility of the cumulative incidence analysis in this study.
Evidence of Lumajang being free of malaria transmission permitted assignment of a very low probability to reinfection confounding in this study. Recrudescence must likewise be carefully considered. If AS, QN, or DHA-PP failed to kill all asexual blood stages, recrudescence would confound estimates of PQ efficacy against hypnozoites. However, late recurrences (Fig. 5) and good evidence of efficacy for the applied regimens of QN and DHA-PP (9, 10, 32–34) also render recrudescence an improbable confounder. The rapid recurrences following AS therapy warrant greater caution, but evidence supports these being classified as relapses. First, the doses of AS applied have been demonstrated to be completely efficacious against asexual blood stages of P. vivax (32, 35, 36). Second, the rapidity and risk of relapse seen in the AS group (Fig. 5) precisely mirrored the natural rate of relapse for the Chesson strain of P. vivax (37) from an American soldier infected in New Guinea in 1944 (38). This high rate of relapse also occurred among Thai patients observed as inpatients in a hospital in Bangkok, Thailand (32). The Chesson strain from New Guinea, where the infections treated in this study were acquired, has historically been considered PQ tolerant (7, 39) but typically responsive to the 420-mg total dose delivered in this study (14).
Alving et al. (11) demonstrated that QN or CQ, despite having no effect on relapse without PQ, strongly potentiated the hypnozoitocidal activity of PQ when administered concurrently. Recent findings of the activity of a PQ-like drug against relapse of Plasmodium cynomolgi in rhesus macaques also point to potentiation: the dose minimally effective against relapse decreased 10-fold when administered with blood schizontocides (40). DHA-PP may also have potentiated PQ against relapse, despite a 25-day interval before PQ administration. If potentiation occurred, PP seems to be the agent more likely responsible because DHA is rapidly excreted (elimination half-life, <1 h), whereas the elimination half-life of PP is 3 to 4 weeks (41). PP thus would have been present through PQ dosing in weeks 5 and 6, but the relationship between those levels and the possible potentiation of the therapeutic activity of PQ remains unknown.
The timing of post-PQ relapse demonstrates an important factor in estimating the efficacy of PQ, especially in the context of a rapidly versus a slowly eliminated blood schizontocide. Despite the high risk of relapse by day 28 (as in the AS group; Fig. 5), PQ failure (relapse) did not occur during that period but occurred later and continued up to 28 weeks. Similarly, subjects with candidate 8-aminoquinoline failures against Chesson strain relapse in experimental challenge trials also relapsed later than 28 days (42). The therapeutic efficacy of PQ may not be ascertained with a follow-up of less than 3 months even for the rapidly relapsing strains from Southeast Asia and Oceania; subjects infected with strains having much longer relapse intervals (6) would likely require 8 to 12 months of follow-up. In the current study, no first relapses occurred in the latter half of the 12-month observation period; a 6-month follow up may suffice in the future.
Suppression of early relapse by slowly excreted blood schizontocides should also be considered in the timing of relapse in trials of PQ efficacy. In this study, the QN treatment arm demonstrated PQ killing of the hypnozoites responsible for the early relapses evident in the AS treatment arm (Fig. 5). Conversely, in the DHA-PP treatment arm, many relapses would have occurred before the first dose of PQ on day 28, but these emergent blood stages were almost certainly killed by the therapeutic levels of the PP blood schizontocide lingering for about 1 month (9). It is doubtful, however, that such activity explains the apparently good efficacy of PQ against hypnozoites with this partner drug; multiple relapses are the rule for strains of P. vivax from New Guinea (6). In earlier studies (43), a 55% relapse rate was detected between days 38 and 63 posttreatment with slowly eliminated CQ, whereas the relapse rate from days 17 to 28 was 60% among subjects treated with rapidly eliminated QN (no PQ either group). This slight difference reflects the very high probability of relapse, despite suppression of the earliest relapses by a slowly eliminated blood schizontocide.
All of the acute vivax malaria cases treated in this study were very likely relapses rather than primary attacks. Most subjects recruited were initially negative for malaria at mass screening and only later developed acute vivax malaria (Fig. 2). Even the 45 soldiers found to be positive in the first mass screening within 2 weeks of commencing enrollment were almost certainly not experiencing primary attacks: at that point they had not been exposed to biting infectious anopheline mosquitoes for over a month. All of these soldiers probably experienced one or more primary attacks during their 1 year of duties in the high-transmission area (21, 22). Chemoprophylaxis is not medical doctrine of the Indonesian army, and it is not routinely practiced. The battalion medical team reported treating many soldiers while deployed, but records were not maintained. Soldiers were treated with stocks of various ACTs provided by the army health authorities in the region. At no time did the army medical team administer primaquine therapy for acute malaria in the field, as they lacked a capacity for screening for G6PD deficiency and feared causing serious harm. First exposure to primaquine by the infections acquired by the study subjects occurred with the therapies administered in this study.
The findings of this study provide clinical evidence of the good safety, tolerability, and efficacy of DHA-PP+PQ for radical cure of vivax malaria. This option may be applied in the face of emerging CQ-resistant P. vivax. The safety of the more convenient concurrent administration of DHA-PP+PQ remains to be evaluated. Despite this significant progress in chemotherapeutics for vivax malaria, the problem of PQ toxicity in G6PD-deficient patients and the prohibition of its use in pregnant women and very young children remain very significant obstacles to effective PQ therapy against relapse in zones of endemicity. Practical point-of-care diagnostics for G6PD deficiency constitute an urgent requirement in support of broader application of this therapy against relapse in malaria control and elimination. As the data in Fig. 5 starkly illustrate, failure to attack the hypnozoite reservoir in zones of endemicity poses a conspicuously serious threat to the health of several billion people.
ACKNOWLEDGMENTS
We express our sincere gratitude to the many soldiers, officers, and commanders of the Army of the Republic of Indonesia who actively and enthusiastically supported the efforts represented in this report. We are especially indebted to Lt. Col. Trio Tangkas, Lt. Muhammad Sya'ir, Lt. Winarso, Sgt. Desantu Harwono, and Sgt. Dharma Indarto Yogo, who each contributed throughout the 15 months of study operations in their battalion. We also owe debts of gratitude to members of the clinical research team: Winarni Arimanunggal, Yulia Widya Santy, Decy Subekti, Sofyan Masbar, Baitur Rohmah, Sunardi, Asep Sutisna, Saraswati Soebianto, Ungke Antonjaya, Fitria Wulandari, and Ratno Nugroho. Gratitude is likewise owed to the Data Safety Monitoring Board: Steve Wignall (Bali), Paul Tambayah (Singapore), and Elizabeth Ashley (Bangkok, Thailand). We are indebted to the officers of the Medicines for Malaria Venture (MMV; Geneva, Switzerland) and the ALERTAsia Foundation (Jakarta, Indonesia) for their active support of the work, especially Stephan Duparc at MMV and Claudia Surjadjaja and Shanti Gayatri at the ALERTAsia Foundation.
This study was funded in part by a clinical trials capacity-building award from the Medicines for Malaria Venture (Geneva, Switzerland) to the ALERTAsia Foundation (Jakarta, Indonesia). J.K.B. is supported by grant B9RJIXO from the Wellcome Trust.
Sigma Tau (Italy) graciously provided the DHA-PP used in this study and helped with the documentation necessary for its importation into Indonesia.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64(1–2 Suppl):97–106 [DOI] [PubMed] [Google Scholar]
- 2. Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77(6 Suppl):79–87 [PMC free article] [PubMed] [Google Scholar]
- 3. Baird JK. 2007. Neglect of Plasmodium vivax malaria. Trends Parasitol. 23:533–539 [DOI] [PubMed] [Google Scholar]
- 4. Guerra CA, Howes RE, Patil PA, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4:e774 doi:10.1371/journal.pntd.0000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baird JK. 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin. Microbiol. Rev. 26:36–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White NJ. 2011. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 10:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baird JK. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 22:508–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Setiawan B. 2010. Ministry of Health Indonesia: current malaria management guideline 2009. Acta Med. Indones. 42:258–261 [PubMed] [Google Scholar]
- 9. Hasugian AR, Purba HL, Kenangalem E, Wuwung RM, Ebsworth EP, Maristela R, Penttinen PM, Laihad F, Anstey NM, Tjitra E, Price RN. 2007. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and post-treatment prophylaxis against multidrug resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin. Infect. Dis. 44:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karunajeewa HA, Mueller I, Senn M, Lin E, Law I, Gomorrai PS, Oa O, Griffin S, Kotab K, Suano P, Tarongka N, Ura A, Lautu D, Page-Sharp M, Wong R, Salman S, Siba P, Ilett KF, Davis TM. 2008. A trial of combination therapies in children from Papua New Guinea. N. Engl. J. Med. 359:2545–2557 [DOI] [PubMed] [Google Scholar]
- 11. Alving AS, Arnold J, Hockwald RS, Clayman CB, Dern RJ, Beutler E, Flanagan CL. 1955. Potentiation of the curative action of primaquine in vivax malaria by quinine and chloroquine. J. Lab. Clin. Med. 46:301–306 [PubMed] [Google Scholar]
- 12. Baird JK. 2011. Resistance to chloroquine unhinges vivax malaria therapeutics. Antimicrob. Agents Chemother. 55:1827–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baird JK. 2008. Real world therapies and the problem of vivax malaria. N. Engl. J. Med. 359:2601–2603 [DOI] [PubMed] [Google Scholar]
- 14. Imwong M, Boel ME, Pagornrat W, Pimanpanarak M, McGready R, Day NP, Nosten F, White NJ. 2012. The first Plasmodium vivax relapses of life are usually genetically homologous. J. Infect. Dis. 205:680–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baird JK, Hoffman SL. 2003. Primaquine therapy for malaria. Clin. Infect. Dis. 37:1659–1667 [DOI] [PubMed] [Google Scholar]
- 16. Baird JK. 1998. Primaquine as anti-relapse therapy for Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 92:687. [DOI] [PubMed] [Google Scholar]
- 17. Gogtay NJ, Desai S, Kamtekar KD, Kadam KS, Dalvi SS, Kshirsagar NA. 1999. Efficacies of the 5- and 14-day regimens in the prevention of relapses in Plasmodium vivax infections. Ann. Trop. Med. Parasitol. 93:809–812 [DOI] [PubMed] [Google Scholar]
- 18. Yadav RS, Ghash SK. 2002. Radical curative efficacy for five-day regimen of primaquine for treatment of Plasmodium vivax malaria in India. J. Parasitol. 88:1042–1044 [DOI] [PubMed] [Google Scholar]
- 19. Elyazar IR, Gething PW, Patil AP, Rogayah H, Kusriastuti R, Wismarini DM, Tarmizi SN, Baird JK, Hay SI. 2011. Plasmodium falciparum endemicity in Indonesia in 2010. PLoS One 6:e21315 doi:10.1371/journal.pone.0021315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elyazar IR, Gething PW, Patil AP, Rogayah H, Sariwati E, Palupi NW, Tarmizi SN, Kusriastuti R, Baird JK, Hay SI. 2012. Plasmodium vivax endemicity in Indonesia in 2010. PLoS One 7:e37325 doi:10.1371/journal.pone.0037325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohrt C, Richie TL, Widjaja H, Shanks GD, Fitriadi J, Fryauff DJ, Handschin J, Tang D, Sandjaja B, Tjitra E, Hadiarso L, Watt G, Wignall FS. 1997. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 126:963–972 [DOI] [PubMed] [Google Scholar]
- 22. Taylor WR, Richie TL, Fryauff DJ, Picarima H, Ohrt C, Tang D, Braitman D, Murphy GS, Widjaja H, Tjitra E, Ganjar A, Jones TR, Basri H, Berman J. 1999. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin. Infect. Dis. 28:74–81 [DOI] [PubMed] [Google Scholar]
- 23. Tsiatis AA, Zang D. 2009. Statistical principles of clinical trials. Department of Statistics, North Carolina State University, Raleigh, NC [Google Scholar]
- 24. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed, p 166–187 World Health Organization, Geneva, Switzerland [Google Scholar]
- 25. Schwartz E, Regev-Yochay G, Kurnik D. 2000. Short report: a consideration of primaquine dose adjustment for radical cure of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 62:393–395 [DOI] [PubMed] [Google Scholar]
- 26. McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. 2006. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am. J. Trop. Med. Hyg. 74:413–421 [PMC free article] [PubMed] [Google Scholar]
- 27. Baird JK, Wiady I, Fryrauff DJ, Sutanihardja MA, Leksana B, Widjaya H, Kysdarmanto Subianto B. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627–631 [DOI] [PubMed] [Google Scholar]
- 28. Myint HY, Ashely EA, Day NP, Nosten F, White NJ. 2007. Efficacy and safety of dihydroartemisinin-piperaquine. Trans. R. Soc. Trop. Med. Hyg. 101:858–866 [DOI] [PubMed] [Google Scholar]
- 29. Hale BR, Owusu-Agyei S, Fryauff DJ, Koram KA, Adjuik M, Oduro AR, Prescott WR, Baird JK, Nkrumah F, Richie TL, Franke ED, Binka FN, Horton J, Hoffman SL. 2003. A randomized double-blind placebo-controlled dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparum. Clin. Infect. Dis. 36:541–549 [DOI] [PubMed] [Google Scholar]
- 30. Morgenstern H, Kleinbaum DG, Kupper LL. 1980. Measures of disease incidence in epidemiological research. Int. J. Epidemiol. 9:97–104 [DOI] [PubMed] [Google Scholar]
- 31. Tapia Granados JA. 1997. On the terminology and dimensions of incidence. J. Clin. Epidemiol. 50:891–897 [DOI] [PubMed] [Google Scholar]
- 32. Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, White NJ. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tjitra E, Hasugian A, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, Purnamasari T, Driyah S, Salwati E, Nurhayati Yuwarni E, Januar L, Labora J, Wijayanto B, Amansyah F, Dedang TA, Purnama A, Trihono 2012. Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia. Malar. J. 11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phyo AP, Lwin KM, Price RN, Ashley EA, Russell B, Sriprawat K, Lindegardh N, Singhasivanon P, White NJ, Nosten F. 2011. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin. Infect. Dis. 53:977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamedi Y, Safa O, Zare S, Tan-ariya P, Kojima S, Looareesuwan S. 2004. Therapeutic efficacy of artesunate in Plasmodium vivax malaria in Thailand. Southeast Asian J. Trop. Med. Public Health 35:570–574 [PubMed] [Google Scholar]
- 36. Krudsood S, Tangpukdee N, Wilairatana P, Phophak N, Baird JK, Brittenham GM, Looareesuwan S. 2008. High-dose primaquine regimens against relapse of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 78:736–740 [PMC free article] [PubMed] [Google Scholar]
- 37. Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi, Wignall FS, Hoffman SL. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621–626 [DOI] [PubMed] [Google Scholar]
- 38. Ehrman FC, Ellis JM, Young MD. 1945. Plasmodium vivax Chesson strain. Science 101:377. [DOI] [PubMed] [Google Scholar]
- 39. Collins WE, Jeffery GM. 1996. Primaquine resistance in Plasmodium vivax. Am. J. Trop. Med. Hyg. 55:243–249 [DOI] [PubMed] [Google Scholar]
- 40. Dow GS, Gettayacamin M, Hasukjariya P, Imbersin R, Komcharoen S, Sattabangkot J, Kyle D, Milhous W, Cozens S, Kenworthy D, Miller A, Veazey J, Ohrt C. 2011. Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi-infected rhesus monkeys (Macaca mulatta). Malar. J. 10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tarning J, Rijken MJ, McGready R, Phyo AP, Hanpithakpong W, Day NP, White NJ, Nosten F, Lindegardh N. 2012. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob. Agents Chemother. 56:1997–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooper WC, Myatt AV, Hernandez T, Jeffery GM, Coatney GR. 1953. Studies in human malaria. XXXI. Comparison of primaquine, isopentaquine, SN-3883, and pamaquine as curative agents against Chesson strain vivax malaria. Am. J. Trop. Med. Hyg. 2:949–957 [PubMed] [Google Scholar]
- 43. Baird JK. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]