Abstract
Bacterial infections are known to cause severe health-threatening conditions, including sepsis. All attempts to get this disease under control failed in the past, and especially in times of increasing antibiotic resistance, this leads to one of the most urgent medical challenges of our times. We designed a peptide to bind with high affinity to endotoxins, one of the most potent pathogenicity factors involved in triggering sepsis. The peptide Pep19-2.5 reveals high endotoxin neutralization efficiency in vitro, and here, we demonstrate its antiseptic/anti-inflammatory effects in vivo in the mouse models of endotoxemia, bacteremia, and cecal ligation and puncture, as well as in an ex vivo model of human tissue. Furthermore, we show that Pep19-2.5 can bind and neutralize not only endotoxins but also other bacterial pathogenicity factors, such as those from the Gram-positive bacterium Staphylococcus aureus. This broad neutralization efficiency and the additive action of the peptide with common antibiotics makes it an exceptionally appropriate drug candidate against bacterial sepsis and also offers multiple other medication opportunities.
INTRODUCTION
Bacterial diseases are one of the major threats for human health, still claiming millions of lives each year and causing incalculable economic losses. In particular, a pathology known as sepsis that arises subsequent to microbial infections is a prominent and as-yet-unresolved challenge to modern medicine. Sepsis is rated as one of the leading causes of death worldwide (1, 2), despite many efforts in the last decades to improve the therapeutic regimen (3–5). The disease is the result of an unbalanced immune reaction against a microbial infection and is associated with a dysregulation of cytokines leading to the so-called systemic inflammatory response syndrome (SIRS). There are two major challenges in sepsis therapy. One is the variety of different bacteria able to spread systemically and induce the inflammatory reaction. The other is the rapidly initiated host response to a given infection, which involves massive release of different cytokines. To a large extent, this explains why there is no specific therapy for this pathology yet. Due to the rapid and often fatal course of sepsis, the current treatment for suspected septic patients begins with the immediate administration of broad-spectrum antibiotics combined with goal-directed resuscitation (6). Since these medical practices are performed before the etiology of the disease or its causative agent are known, the initial antimicrobial treatments are always empirical and the modulation of the systemic inflammatory response cannot be performed in a selective way (7, 8).
In sepsis caused by Gram-negative organisms, endotoxin (lipopolysaccharide [LPS]) is clearly the major and most potent immune stimulatory factor. It is released from the bacterial cell wall, reaching pronounced concentrations particularly when bacteria are destroyed through the activity of the immune system or antibiotic treatment. In contrast, the stimuli of Gram-positive organisms are less well defined but include lipoproteins and other cell wall components (9). The released microbial stimuli are recognized by cell surface receptors of the innate immune system, such as CD14 and the Toll-like receptors (TLR) (TLR4 for endotoxin and TLR2 for lipoprotein) (10, 11). Receptor binding activates intracellular signaling cascades that lead to the release of potent inflammatory mediators (12). These include interleukin-1 (IL-1), IL-8, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), macrophage inflammatory protein-2 (MIP-2), prostaglandin E2 (PGE2), and reactive oxygen species (ROS). These factors cause cell activation and migration into tissues, endothelial leakage, coagulation, and other profound physiological alterations that, when combined, lead to tissue hypoperfusion and organ failure (13–15).
The approach to prevent this fatal inflammatory cascade by neutralizing bacterial LPS was also the aim of former studies, where peptides derived from the LPS-binding domain of the Limulus anti-LPS factor (cyclic LALF) were investigated (16–18). These investigations, performed with cyclic peptides corresponding to the LPS-binding domain (comprised of amino acids 31 to 52 [LALF31-52]) and with derivatives thereof, gave some new information about the neutralization mechanisms but did not lead to the development of antisepsis drugs. The reason was apparently the insufficient affinity of the peptides for LPS, which must be higher than that of the binding of LPS to human receptors, such as CD14 and TLR4.
We have taken the LALF protein as the template, but we rationally designed a library with linear peptides, in which sequences were improved on the basis of their ability to bind and neutralize the hydrophobic moiety of LPS, the lipid A, which is responsible for the pathophysiological effects of the molecule (19–21).
In this article, we demonstrate that Pep19-2.5 (44) neutralizes the inflammatory responses triggered by both Gram-negative (Salmonella enterica) and Gram-positive (Staphylococcus aureus) bacteria. We also show that this peptide greatly reduces levels of inflammatory cytokines in a model of human tissue and in the murine sepsis models of endotoxemia, bacteremia, and induction by cecal ligation and puncture. Furthermore, the additive action of Pep19-2.5 with commonly applied antibiotics, which led to a survival advantage of mice receiving the combinational therapy, is of crucial importance.
MATERIALS AND METHODS
Pathogenicity factors, chemicals, and peptides.
Lipopolysaccharide from Salmonella enterica Minnesota R60 was extracted from bacteria by the PCP (aqueous phenol, chloroform, and petroleum ether) method (22) and analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF).
The lung-pathogenic methicillin-resistant Staphylococcus aureus (MRSA) strain t008 was provided by J. Knobloch (University of Lübeck). To yield heat-killed bacteria, the MRSA strain and S. enterica strain R60 were grown for 16 h at 37°C in LB medium, boiled for 15 min, and centrifuged for 5 min at 13,000 × g. The pellet was suspended in 0.9% NaCl and centrifuged again for 5 min. Subsequently, the supernatant was removed and the pellet was dried.
The antibiotics used were amoxicillin, amikacin, ceftriaxone, erythromycin, imipenem, tetracycline, polymyxin B (Sigma-Aldrich, Deisenhofen or Madrid), and ciprofloxacin (Fluka, Madrid). The synthesis and purification of Pep19-2.5 was described previously (20); the batch used here was produced (good manufacturing practice [GMP] conditions) by Bachem (Bubendorf, Switzerland). The amino acid sequence of this 20-mer is GCKKYRRFRWKFKGKFWFWG, containing a C-terminal amidation. Peptides 19-4 (GKKYRRFRWKFKGKWFWFG) and 19-8 (GRRYKKFRWKFKGRWFWFG) were synthesized in the Research Center Borstel.
Calorimetric binding studies.
The interaction of peptide with heat-killed bacteria was analyzed by microcalorimetric measurements in the ITC200 (GE Healthcare, Munich) as recently described (23). For this, 2 mg/ml Pep19-2.5 in 5% glucose solution was titrated into 1 mg/ml heat-killed bacteria (S. enterica or MRSA) in 5% glucose solution and the measured enthalpy changes (ΔH) were recorded versus time and the concentration ratio of peptide to bacteria.
Stimulation of human tissue.
Ex vivo experiments were performed as previously described (24). Human lung tissues were prepared as 0.5-cm3 pieces 1 to 4 h after the lung resection operation. The tissues were incubated for 4 h at 37°C in 24-well plates containing 2 ml of RPMI 1640 and the concentrations of purified LPS R60, heat-killed methicillin-resistant S. aureus, and Pep19-2.5 given below. Subsequently, the supernatants were collected and stored at −20°C. The immunological determination of TNF-α was performed as described in the manufacturer's protocol (OptEIA; BD, Heidelberg, Germany).
Antimicrobial susceptibility testing.
The MICs of the peptides were determined in Mueller-Hinton medium (Difco Laboratories, Detroit, MI) with the broth microdilution assay (25).
In vitro antimicrobial synergy between Pep19-2.5 and antibiotics was assessed with the checkerboard assay (26, 27). The fractional inhibitory concentration (FIC) index was calculated using the following formula: FIC index = (MICcombination/MICantibiotic alone) + (MICcombination/MICpeptide alone).
According to their FIC indices, combinations were classified as synergistic (values of ≤0.5), additive or indifferent (indices from 0.5 to 2), and antagonist (index of ≥2).
Mouse model of cecal ligation and puncture.
All mice (male NMRI mice, n = 65, body weight of 38 ± 3 g [mean ± standard deviation]) underwent a catheterization procedure. Spontaneously breathing animals received general anesthesia (8 to 10% desflurane in oxygen-air mix with a fraction of inspired oxygen [FiO2] of 0.3) while fixed in prone position. The right neck vessels were exposed after local anesthesia with 0.2 ml of 2% lidocaine (Astra Zeneca, Wedel, Germany), and a central vein catheter (CVC; self-made using sterilized polyethylene tube with an outer diameter of 0.61 mm) was implanted 1 cm deep in the jugular vein, enabling continuous intravenous (i.v.) application of fluids and drugs. The CVC was tunneled to the back of each mouse and guided through a flexible plastic tube (Drainobag 40; B. Braun, Melsungen, Germany) to prevent bite damage. A 27-gauge cannula was inserted into the CVC to connect the CVC to the syringe pump. Subsequently, after further local infiltration with lidocaine, the neck was closed by single sutures and the mouse transferred back into the cage to rest for 48 h prior to sepsis by cecal ligation and puncture (CLP). To prevent hypothermia, animals were kept on a heating pad throughout the surgical procedure.
Under sterile conditions, a midline laparotomy of 1 cm was performed. Subtotal ligation of the cecum was performed approximately 1 cm away from its base, and afterwards, the cecum was twice perforated with a 18-gauge needle. Feces were protruded to ensure that the perforations were opened. Then, the cecum was replaced and the abdominal cavity was closed by single sutures. The animal was transferred to the cage and reconnected with the i.v. line to the syringe pump at a rate of 100 μl/h. All animals were killed after 24 h. Blood samples were drawn and processed by cytometer bead array (CBA) (BD Biosciences, Heidelberg) in a flow cytometer to determine the levels of TNF-α, IFN-γ, and IL-12p70.
The animals were randomly assigned to control group (control, sepsis plus vehicle infusion), polymyxin B (polymyxin, sepsis plus 0.12 μg polymyxin B/h), Pep19-2.5 (Pep19-2.5, sepsis plus 0.2 μg Pep19-2.5/h), Pep19-4 (Pep4, sepsis plus 0.2 μg Pep19-4/h), Pep19-8 (Pep8, sepsis plus 0.2 μg Pep19-8/h), or sham group (sham operation, vehicle infusion). All animals received a bolus of 200 μl of 0.9% NaCl upon transfer to the cage.
Animal models of infection and endotoxic shock.
Endotoxic shock in female BALB/c mice (n = 8; Harlan Interfauna Iberica S.A., Barcelona, Spain) was induced by the intraperitoneal coadministration of a lethal dose of LPS from S. enterica Minnesota R60 (50 ng/mouse) and galactosamine (18 mg/mouse) following the method of Galanos and collaborators (28). LPS stock solutions contained triethylamine (1 μl per 2 mg of LPS) to ensure the appropriate solubility of this rough-type LPS (final concentration, <25 μM). Antiendotoxic treatment consisted of 25 μg of Pep19-2.5 per mouse in 0.9% NaCl and was administered at a different site of the peritoneum immediately after the LPS challenge or 30 min before, 30 min after, or 60 min after LPS challenge. Animal mortality was monitored for 5 days.
The model of lethal bacteremia was based on that described by Bucklin and Morrison (29). Briefly, groups of mice (n = 8; n = 6 in one experiment [see Fig. 4]) received an intraperitoneal injection of S. enterica Minnesota (107 CFU) suspended in 200 μl of 0.9% NaCl and supplemented with 18 mg of galactosamine. Immediately after bacterial inoculation, the combination therapy (150 to 400 μg of Pep19-2.5 and 200 to 400 μg of antibiotic per mouse) was administered at a different site of the peritoneum. In control experiments, the peptide and the antibiotic were administered alone. Animal mortality was monitored for 7 days.
Fig 4.
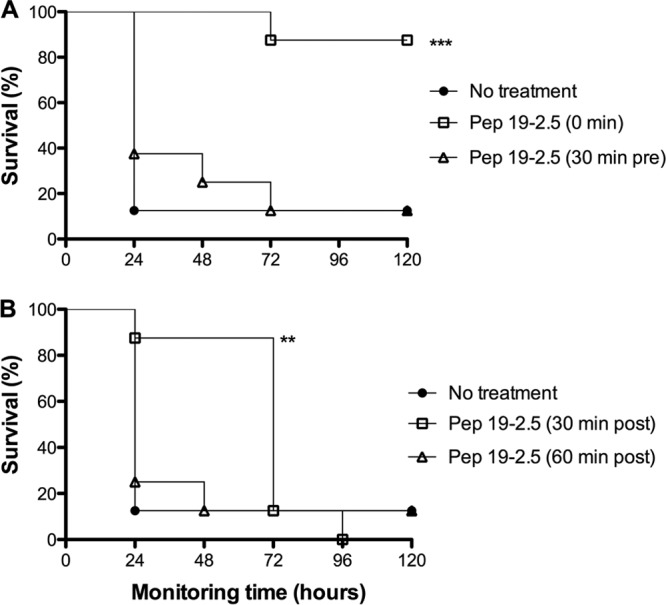
In vivo protection conferred by Pep19-2.5 when administered either before or after LPS challenge. (A and B) Groups of mice (n = 8) were intraperitoneally inoculated with S. enterica R60 LPS (50 ng/mouse plus 18 mg/mouse of galactosamine). Peptide (25 μg/mouse) was administered intraperitoneally at different time points to evaluate the efficacy of a prophylactic (A) or therapeutic treatment (B). Statistical differences were analyzed with the Kaplan-Meier survival test (**, P < 0.01; ***, P < 0.001).
Statistical analysis.
Statistical analysis was performed using Graph Pad Prism 5 software (GraphPad Software, La Jolla, CA). Statistical differences between groups (data for 6 or 8 mice per treatment or 3 or 5 independent ex vivo experiments) were determined by Mann-Whitney U Test or one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (*, P < 0.05; **, P < 0.01). All data are shown as the mean ± standard error of the mean (SEM).
The statistical differences in mouse survival experiments were analyzed using the Kaplan-Meier survival test (**, P < 0.01; ***, P < 0.001). When the survival plots were parallel, data were compared by the log-rank test, whereas for those plots that intersected, the Breslow-Gehan-Wilcoxon test was applied. P values were always obtained by comparing data from the same experiment (mortality in treated versus untreated groups).
RESULTS
Binding to heat-killed bacteria.
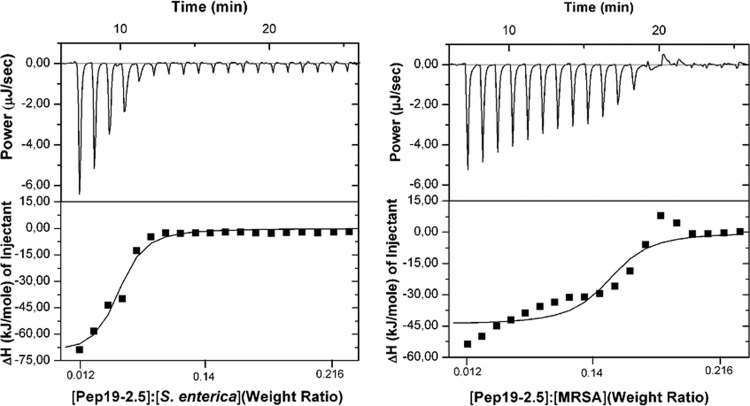
To study the interaction of Pep19-2.5 with Gram-negative and Gram-positive bacteria, isothermal titration calorimetric (ITC) measurements were applied. Using ITC, the enthalpy change of the peptide-bacterium interaction can be measured, which leads to an exothermic signal (peaks downwards) in the case of a Coulomb interaction and to an endothermic signal (peaks upwards) in the case of a pure entropic interaction. Clearly, the observed enthalpy changes (ΔH) plotted versus the weight ratios [Pep19-2.5]/[bacteria] exhibit an exothermic reaction followed by saturation of binding for both the Gram-negative (S. enterica) (Fig. 1, left) and Gram-positive (MRSA) (Fig. 1, right) bacteria, which can be explained by the attractive forces between the positively charged amino acids (K and R) of the peptides and the negative charges of the bacterial envelope.
Fig 1.
Calorimetric analysis of the binding of Pep19-2.5 to heat-killed bacteria. Determination of the interaction of Pep19-2.5 with either heat-killed S. enterica Minnesota R60 or heat-killed methicillin-resistant Staphylococcus aureus (MRSA) by isothermal titration calorimetry. The peptide (2 mg/ml) was titrated to the bacteria (2 mg/ml) in 1.5-μl portions.
Decreased TNF-α release by human tissue.
As shown in initial in vitro experiments, Pep19-2.5 had a strong anti-inflammatory effect on the LPS-induced production of cytokines and inflammatory mediators in different immune cell types for both the murine and the human system. MIP-2 in the murine and TNF-α and reactive oxygen species (ROS) in the human system (see Fig. S1 in the supplemental material) were markedly reduced by simultaneous administration of Pep19-2.5 with LPS or other bacterial cell wall components (see Table S1 in the supplemental material). To further investigate the potential of Pep19-2.5 to inhibit both the LPS- and bacterium-induced stimulation of cytokines in a complex human tissue system, we used surgically removed lung tissue samples of five different donors and tested the potential to inhibit the immune response. It could be shown thereby that the production of TNF-α was markedly reduced by the addition of Pep19-2.5 in a molar ratio of LPS to peptide of 1:10 and statistically significantly reduced by a molar ratio of 1:100 (Fig. 2, left). Also, the addition of Pep19-2.5 to heat-killed MRSA cells resulted in a significant drop in TNF-α release by the lung cells (Fig. 2, right). With both LPS and MRSA cells, the inhibitory effect of Pep19-2.5 was strongly dependent on the two concentrations applied and revealed unambiguously the high neutralization potential of the peptide.
Fig 2.
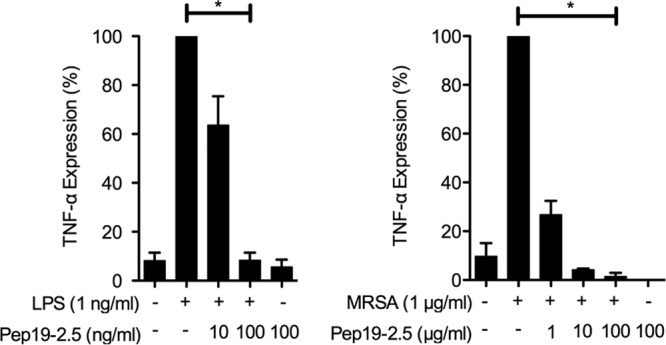
Pep19-2.5 mediated protection against the LPS- or bacterium-induced inflammation of human lung tissue. TNF-α expression levels from human lung tissue were determined after incubation with S. enterica LPS or heat-killed MRSA combined with different concentrations of Pep19-2.5. Depicted are the means ± SEM of 5 (LPS) or 3 (MRSA) independent experiments. Assuming parametric distribution, data were log-transformed and groups statistically compared by use of one-way repeated-measure ANOVA (*, P < 0.05; **, P < 0.01).
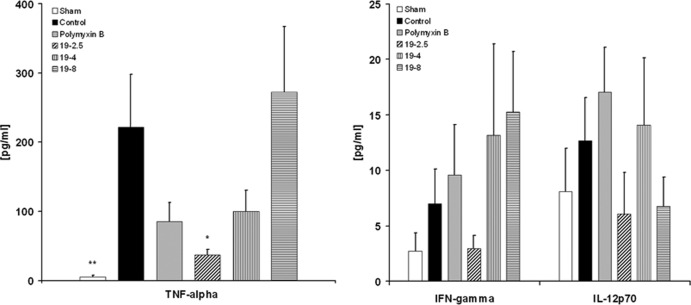
Reduction of inflammatory cytokines in the mouse model of cecal ligation and puncture.
Sepsis was induced in mice by cecal ligation and puncture and, subsequently, the animals were treated by continuous infusion with Pep19-2.5 intravenously for 24 h. To compare efficacy, further groups were treated with polymyxin B or with Pep19-4 or Pep19-8. The latter two peptides were selected since they have been shown to represent compounds with medium and weak efficacy in the inhibition of the LPS-induced cytokine secretion of human mononuclear cells (20, 21).
To evaluate the influence of the experimental procedures (i.e., surgery) on the sepsis model, a sham-operated group (free from sepsis) was also analyzed. The data in Fig. 3 (left) demonstrate significant reduction of TNF-α levels after the 24-h treatment period in the Pep19-2.5-treated group compared to the levels in an untreated control group (37 ± 8 pg/ml [mean ± SEM] versus 222 ± 75 pg/ml; P = 0.020). Sham-treated mice revealed low TNF-α levels after 24 h compared to the levels in untreated sepsis controls, thus excluding any influence of the operating procedure in cytokine induction (5 ± 2 pg/ml; P = 0.005 versus the results for the controls). Mice treated with peptides Pep19-4 and Pep19-8 showed no significant reduction of TNF-α. IFN-γ and IL-12p70 levels were lower in the Pep19-2.5-treated animals than in the animals treated with other peptides (Fig. 3, right).
Fig 3.
Effects of peptides on mice after cecal ligation and puncture. TNF-α, IFN-γ, and IL-12p70 concentrations in the blood were determined after 24 h of sepsis caused by cecal ligation and puncture (CLP). Data are presented as means ± SEM (*, P < 0.05 versus the results for control; **, P < 0.01 versus the results for control).
Protection against endotoxicity and bacteremia.
To investigate the therapeutic potential of Pep19-2.5, we evaluated the ability of the peptide to protect mice against lethal endotoxemia. Peptide was administered at different time points, 30 min before LPS challenge (Fig. 4A), immediately after LPS administration (0 min), or with a 30-min or 60-min delay with respect to LPS injection (Fig. 4B).
As shown by the results in Fig. 4A, when administered prior to LPS challenge, the treatment was unable to protect the animals. In contrast, almost 100% of the mice were protected during the entire monitoring period when the treatment was administered immediately after the LPS challenge. When mice received Pep19-2.5 with a delay of 30 min after LPS administration, the protection was highly significant during the first 48 h. However, the majority of the animals died at later time points (Fig. 4B). An additional 30-min delay (60 min postchallenge) totally abrogated the protective activity of the peptide (Fig. 4B).
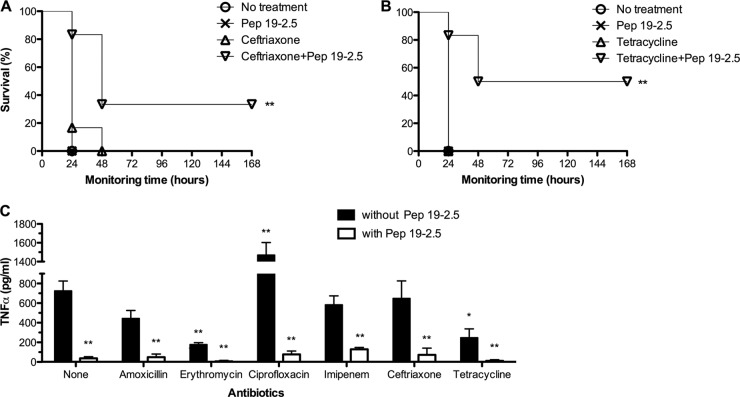
The efficacy of Pep19-2.5 combined with an antibiotic treatment to control endotoxemia and bacteremia was tested. For these experiments, we used the parental smooth strain of S. enterica Minnesota HL63, to avoid the attenuation of virulence that is characteristic of rough mutants.
Prior to the in vivo assays, we studied the antibiotic susceptibility profile of the test strain. In vitro assays demonstrated that S. enterica was highly susceptible to most of the antibiotics used, whose MIC values were as follows: amoxicillin (0.25 μg/ml), amikacin (1 μg/ml), ciprofloxacin (0.032 μg/ml), ceftriaxone (0.002 μg/ml), erythromycin (32 μg/ml), imipenem (0.5 μg/ml), and tetracycline (0.25 μg/ml). In contrast, Pep19-2.5 displayed a modest antibacterial activity on the same strain (MIC = 128 μg/ml). To quantify the existence of synergy in vitro between antibiotics and Pep19-2.5, mixtures of the peptide and the antibiotics, with both at subinhibitory concentration, were tested on S. enterica. These assays revealed that even at the highest concentration used, the peptide was unable to reduce the MIC of any antibiotic, thus indicating that Pep19-2.5 does not act in synergy with those antibiotics in respect to the antibacterial activity in vitro. Interestingly, the fractional inhibitory concentration (FIC) indices of the peptide-antibiotic combinations tested were lower than 2, implying that the effect of the peptide in the presence of the antibiotic is not antagonistic but additive (data not shown).
When a single dose of the combination therapy was used, none of the following antibiotics in combination with Pep19-2.5 conferred significant protection: amoxicillin, erythromycin, ciprofloxacin, and imipenem (data not shown). However, a two-dose regimen greatly improved the efficacy of Pep19-2.5 in combination with amikacin (Fig. 5). In addition, the three treatment regimens whose results are shown in Fig. 5, as well as the peptide by itself, markedly reduced TNF-α concentrations in serum, to basal levels, 90 min after bacterial inoculation. Specifically, the levels of TNF-α in untreated and peptide-treated groups were 529 ± 63 pg/ml and 41 ± 15 pg/ml, respectively. To study the in vivo antimicrobial activity of the peptide-antibiotic combinations, blood samples were obtained 6 h after the challenge and cultured to determine bacterial viability. At this time point, no bacteria were recovered from any of the three groups of treated mice (n = 5 per treatment), whereas untreated mice were bacteremic (approximately 3 × 104 CFU/ml of blood).
Fig 5.
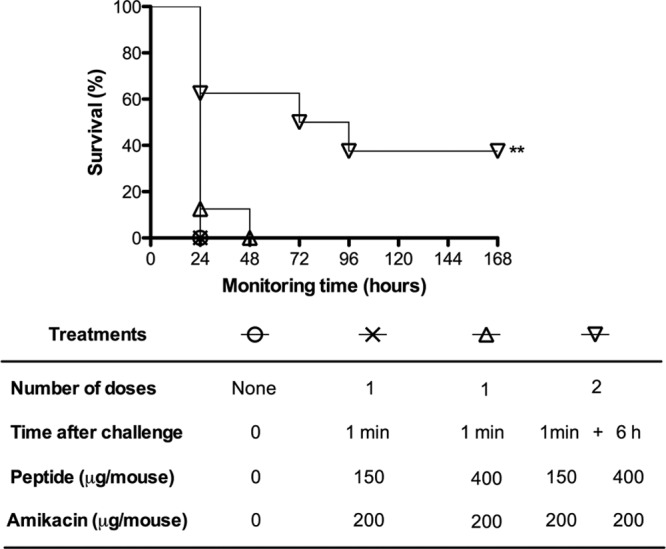
Therapeutic efficacy of Pep19-2.5 in combination with amikacin. To control endotoxemia and bacteremia caused by intraperitoneally inoculated S. enterica live cells, bacteria (107 CFU/mouse) were coinoculated with galactosamine, and then the mice (n = 8) immediately received an intraperitoneal injection of a combination of Pep19-2.5 and amikacin at different concentrations and mouse survival was monitored. Statistical differences were analyzed with the Kaplan-Meier survival test (**, P < 0.01).
Interestingly, the administration of only one dose of Pep19-2.5 afforded long-lasting protection to 40 to 50% of the animals when the peptide was combined with either ceftriaxone (Fig. 6A) or tetracycline (Fig. 6B). Analysis of TNF-α levels at 90 min postinfection revealed that the presence of Pep19-2.5 in the peptide-antibiotic combinations was sufficient to reduce the production of this cytokine (Fig. 6C). However, in the absence of the peptide, both tetracycline and erythromycin displayed a significant ability to decrease TNF-α, suggesting that these two antimicrobials have a certain immunomodulatory activity. Conversely, ciprofloxacin by itself caused a marked increase in TNF-α expression. This could be due to the LPS-releasing activity previously described for this antibiotic (30).
Fig 6.
Therapeutic efficacy of Pep19-2.5 combined with antibiotics to control endotoxemia and bacteremia caused by intraperitoneally inoculated S. enterica Minnesota live cells. Bacteria (107 CFU/mouse) were coinoculated with galactosamine (18 mg/mouse), and then mice (n = 6) immediately received an intraperitoneal injection of 200 μg antibiotic/mouse (except for erythromycin, at 400 μg/mouse) alone or in combination with 400 μg Pep19-2.5/mouse. (A and B) Survival was monitored at daily intervals after administration of ceftriaxone (A) or tetracycline (B) alone or in combination with the peptide. (C) TNF-α serum levels were determined in blood samples taken 90 min after the bacterial challenge. Statistical differences in mortality were analyzed by Scheffe's post hoc method and the Kaplan-Meier survival test. For the TNF-α analysis, the Mann-Whitney U test was performed by comparing the results for each treated group with the average values for the nontreated groups (*, P < 0.05; **, P < 0.01).
DISCUSSION
Despite continuous medical advances and the intense use of antibiotics, bacteria still lead to severe infections worldwide that cause high mortality. This is particularly true for the infection-associated systemic disease, sepsis, whose high death rates have remained almost unchanged for many years (31). Until recently, only one antisepsis agent, suitable for a minor cohort of patients, was available (activated protein C [Xigris], Ely Lilly). However, the drug was withdrawn in October 2011, because recent clinical studies failed to indicate a significant reduction in mortality but did report rather serious side effects, like severe bleeding (32). The main drawback of this and other therapeutic approaches, which failed in phase III studies (for example, see reference 33), may be that they are not aimed at eliminating the triggering factor of the septic syndrome but, rather, at controlling the resultant inflammatory reactions (e.g., anti-TNF-α antibodies [34]). Therapies targeting early steps of the inflammation cascade, such as the administration of monoclonal anti-LPS antibodies, were unsuccessful due to the lack of cross-reactivity against different LPS chemotypes. Furthermore, this strategy would be of no use to combat sepsis caused by Gram-positive pathogenicity factors (35).
Our strategy is intended to neutralize the bacterial molecules involved in eliciting inflammation. The general mechanism of LPS neutralization by Pep19-2.5 was described using purified LPS (20, 21, 36). Here, we demonstrate that Pep19-2.5 does not bind exclusively to LPS of Gram-negative bacteria but also exhibits binding to Gram-positive bacteria (Fig. 1). This peptide-bacterium interaction leads to a strong reduction of the inflammatory potential of bacterial pathogenicity factors, as shown in the complex cellular system of human tissue (Fig. 2). In contrast to other peptides, such as the human host defense peptide LL-37, for which the protective activity against Gram-negative and Gram-positive bacteria was described as an immunomodulatory function (37, 38), we could demonstrate a clear interaction with the different bacteria in binding studies (Fig. 1). It is not only the polycationic character of Pep19-2.5 that is decisive for the binding, since all polycationic compounds can be expected to bind to the bacterial envelope due to Coulomb interaction. A further important step is a hydrophobic interaction of the C-terminal end of the peptide with the acyl chain moiety of the membrane, as shown for the interaction with isolated LPS (20, 21). Beside the latter compound for Gram-negative bacteria, membrane-bound lipoproteins and/or lipoteichoic acids can be assumed as target structures of Gram-positive bacteria, and we have shown that Pep19-2.5 also binds with high affinity to these compounds (see the supplemental material).
These findings are supported by the significant protection by Pep19-2.5 in a neutropenic mouse model, where mice were intraperitoneally challenged with a lethal dose of viable MRSA; the protection correlated with a drop in IL-6 levels (data not shown).
Regarding the use of antimicrobial peptides to neutralize bacterial LPS, a great deal of work has been done (26, 36, 39, 40). None of these approaches led to the development of new antiseptic agents, mainly because the various peptides were primarily designed to bind to LPS on the bacterial surface rather than to its free form and were screened for their antimicrobial efficiency. In contrast to classical AMP (41, 42), Pep19-2.5 has a low antimicrobial effect, whereas it exhibits a potent antiendotoxic activity (20). The ability of Pep19-2.5 to neutralize the bacterial pathogenicity factors is here demonstrated in vivo using different mouse models of sepsis. More importantly, the combination of Pep19-2.5 with some classical antibiotics efficiently protected mice against lethal sepsis (Fig. 5 and 6). In this model, we demonstrated that the presence of the peptide was sufficient to reduce TNF-α to basal levels in the animals. Interestingly, one antibiotic (ciprofloxacin) even increased TNF-α levels (Fig. 6), a finding which is well in line with the observation that the application of certain antibiotics in septic patients may lead to a worsening of the disease status (43). Concerning the poor antimicrobial activity displayed by Pep19-2.5 in vitro, our results strongly suggest that Pep19-2.5 exerts an additive effect on the antibiotic activity in vivo. In these experiments, the antibiotic showed little or no antiendotoxic effect, whereas it was the major component responsible for the observed reduction in bacteremia. In contrast, the peptide was essential to neutralize the inflammatory activity of bacterial immune stimuli. Such a peptide-antibiotic combination would be the optimal treatment in Pep19-2.5-based antisepsis therapies.
The timing of Pep19-2.5 administration with respect to the administration of LPS had a direct impact on the LPS-induced septic shock in mice. The peptide protected the animals only when the bolus was administered immediately after LPS challenge but provided little or no protection when given significantly before or after LPS inoculation (Fig. 4). This observation is likely to be the result of peptide degradation. The stability of the peptide was determined in rat plasma, revealing a rapid loss of free peptide, presumably due to an attachment to plasma ingredients or degradation (unpublished data).
However, for the applicability of Pep19-2.5 in septic patients, this degradation process is not expected to compromise therapeutic efficacy, since the usual administration of drugs in critical care units is by continuous infusion rather than by bolus and can even be interrupted immediately if desired.
This means that, when the application of the combination of antibiotics and peptide as a bolus, as shown here, already leads to protection against sepsis, continuous administration would convey even more protection. Particularly worthy of note is the action of the peptide alone in cecal ligation and puncture experiments (Fig. 3), showing suppression of inflammatory cytokines even in the absence of antibiotic treatment. According to the results, the inflammatory activity of various bacteria is inhibited (summarized in Fig. 7). This inhibition mechanism may be understood by the ability of Pep19-2.5 to bind with high affinity to the pathogenicity factors LPS, from Gram-negative sources (21), and lipoproteins, such as SitC, from Gram-positive origins (unpublished data). In this way, a direct interaction of these pathogenicity factors with the corresponding cell surface receptors, TLR4 and -2, respectively, is inhibited.
Fig 7.
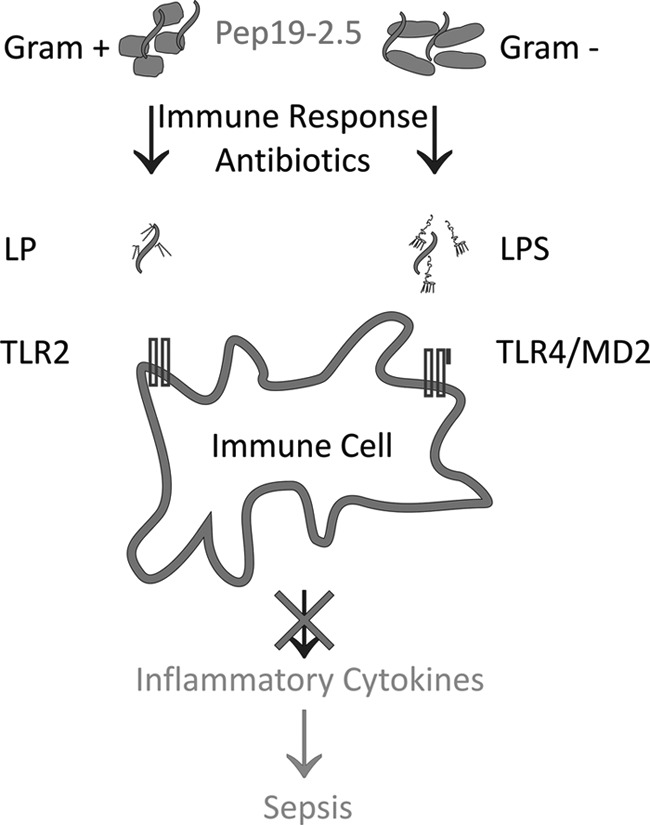
Scheme of interaction of Pep19-2.5 with bacteria and their pathogenicity factors. Peptide Pep19-2.5 exhibits binding to Gram-positive and Gram-negative bacteria and, moreover, to purified lipopolysaccharide (LPS) and lipoprotein (LP). In vivo models for bacteria and purified cell wall components revealed the remarkable anti-inflammatory effect of the peptide by reduction of the amount of inflammatory cytokines, along with reduced lethality to the mice.
Consequently, the results shown for Pep19-2.5 cover the key points for further development as an effective anti-infectious and antisepsis medicament: broad-spectrum activity, no inflammatory property of its own, and an additive action with the currently applied medication.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nina Hahlbrock for her excellent technical assistance, as well as Rainer Bartels and Volker Grote for peptide synthesis.
The use of human lung tissue was approved by the ethical committee of the University of Lübeck (reference number 03/158). All the animal protocols used in this study were approved by the University of Navarra Animal Research Committee (protocol numbers 069-09, E6-11, and E28-11), except for the use of the mice in the CLP model, which was approved by the local animal protection authority (LANUV, NRW, Germany, Az 8.87-50.10.35.09.044).
We are indebted to the German Ministry (Bundesministerium für Bildung und Forschung) BMBF, project 01GU0824, and the Else-Kröner-Fresenius-Stiftung, project 2011_A140, for financial help. G.M.D.T. and S.S.-G were also funded by a grant from Ministerio de Sanidad y Consumo (FIS-PI050768) and from Proyectos de Investigación Universidad de Navarra (PIUNA-P2008-11 and PIUNA-P2011-17), Spain.
Footnotes
Published ahead of print 14 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02066-12.
REFERENCES
- 1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 2. Wang HE, Shapiro NI, Angus DC, Yealy DM. 2007. National estimates of severe sepsis in United States emergency departments. Crit. Care Med. 35:1928–1936 [DOI] [PubMed] [Google Scholar]
- 3. Singer P, Cohen JD. 2011. The Surviving Sepsis Campaign guidelines: should we follow? Isr. Med. Assoc. J. IMAJ. 13:692–693 [PubMed] [Google Scholar]
- 4. Stone R. 1994. Search for sepsis drugs goes on despite past failures. Science 264:365–367 [DOI] [PubMed] [Google Scholar]
- 5. Suffredini AF, Munford RS. 2011. Novel therapies for septic shock over the past 4 decades. JAMA 306:194–199 [DOI] [PubMed] [Google Scholar]
- 6. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296–327 [DOI] [PubMed] [Google Scholar]
- 7. Cohen J. 2009. Non-antibiotic strategies for sepsis. Clin. Microbiol. Infect. 15:302–307 [DOI] [PubMed] [Google Scholar]
- 8. Nahra R, Dellinger RP. 2008. Targeting the lipopolysaccharides: still a matter of debate? Curr. Opin. Anaesthesiol. 21:98–104 [DOI] [PubMed] [Google Scholar]
- 9. Kurokawa K, Ryu KH, Ichikawa R, Masuda A, Kim MS, Lee H, Chae JH, Shimizu T, Saitoh T, Kuwano K, Akira S, Dohmae N, Nakayama H, Lee BL. 2012. Novel bacterial lipoprotein structures conserved in low-GC content Gram-positive bacteria are recognized by Toll-like receptor 2. J. Biol. Chem. 287:13170–13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez-Bojorquez LN, Dehesa AZ, Reyes-Teran G. 2004. Molecular mechanisms involved in the pathogenesis of septic shock. Arch. Med. Res. 35:465–479 [DOI] [PubMed] [Google Scholar]
- 11. Aderem A, Ulevitch RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782–787 [DOI] [PubMed] [Google Scholar]
- 12. Alexander C, Rietschel ET. 2001. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 7:167–202 [PubMed] [Google Scholar]
- 13. Annane D, Bellissant E, Cavaillon JM. 2005. Septic shock. Lancet 365:63–78 [DOI] [PubMed] [Google Scholar]
- 14. Cohen J. 2002. The immunopathogenesis of sepsis. Nature 420:885–891 [DOI] [PubMed] [Google Scholar]
- 15. Opal SM. 2007. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. 297:365–377 [DOI] [PubMed] [Google Scholar]
- 16. Andrä J, Howe J, Garidel P, Rossle M, Richter W, Leiva-Leon J, Moriyon I, Bartels R, Gutsmann T, Brandenburg K. 2007. Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis. Biochem. J. 406:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vallespi MG, Alvarez-Obregon JC, Rodriguez-Alonso I, Montero T, Garay H, Reyes O, Arana MJ. 2003. A Limulus anti-LPS factor-derived peptide modulates cytokine gene expression and promotes resolution of bacterial acute infection in mice. Int. Immunopharmacol. 3:247–256 [DOI] [PubMed] [Google Scholar]
- 18. Ren JD, Gu JS, Gao HF, Xia PY, Xiao GX. 2008. A synthetic cyclic peptide derived from Limulus anti-lipopolysaccharide factor neutralizes endotoxin in vitro and in vivo. Int. Immunopharmacol. 8:775–781 [DOI] [PubMed] [Google Scholar]
- 19. Zähringer U, Lindner B, Rietschel ET. 1994. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv. Carbohydr. Chem. Biochem. 50:211–276 [PubMed] [Google Scholar]
- 20. Gutsmann T, Razquin-Olazaran I, Kowalski I, Kaconis Y, Howe J, Bartels R, Hornef M, Schürholz T, Rossle M, Sanchez-Gomez S, Moriyon I, Martinez de Tejada G, Brandenburg K. 2010. New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob. Agents Chemother. 54:3817–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaconis Y, Kowalski I, Howe J, Brauser A, Richter W, Razquin-Olazaran I, Inigo-Pestana M, Garidel P, Rossle M, Martinez de Tejada G, Gutsmann T, Brandenburg K. 2011. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys. J. 100:2652–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galanos C, Lüderitz O, Westphal O. 1969. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 9:245–249 [DOI] [PubMed] [Google Scholar]
- 23. Howe J, Andrä J, Conde R, Iriarte M, Garidel P, Koch MH, Gutsmann T, Moriyon I, Brandenburg K. 2007. Thermodynamic analysis of the lipopolysaccharide-dependent resistance of gram-negative bacteria against polymyxin B. Biophys. J. 92:2796–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Droemann D, Rupp J, Goldmann T, Uhlig U, Branscheid D, Vollmer E, Kujath P, Zabel P, Dalhoff K. 2007. Disparate innate immune responses to persistent and acute Chlamydia pneumoniae infection in chronic obstructive pulmonary disease. Am. J. Resp. Crit. Care Med. 175:791–797 [DOI] [PubMed] [Google Scholar]
- 25. Thornsberry C, McDougal LK. 1983. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J. Clin. Microbiol. 18:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanchez-Gomez S, Lamata M, Leiva J, Blondelle SE, Jerala R, Andrä J, Brandenburg K, Lohner K, Moriyon I, Martinez-de-Tejada G. 2008. Comparative analysis of selected methods for the assessment of antimicrobial and membrane-permeabilizing activity: a case study for lactoferricin derived peptides. BMC Microbiol. 8:196 doi:10.1186/1471-2180-8-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eliopoulos GM, Moellering RCJ. 1996. Antimicrobial combinations, p 330–393 In Lorian V. (ed), Antibiotics in laboratory medicine, vol. 4 Williams & Wilkins, Baltimore, MD [Google Scholar]
- 28. Galanos C, Freudenberg MA, Reutter W. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. U. S. A. 76:5939–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bucklin SE, Morrison DC. 1995. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J. Infect. Dis. 172:1519–1527 [DOI] [PubMed] [Google Scholar]
- 30. Evans ME, Pollack M. 1993. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J. Infect. Dis. 167:1336–1343 [DOI] [PubMed] [Google Scholar]
- 31. Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. 2007. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 33:606–618 [DOI] [PubMed] [Google Scholar]
- 32. U.S. Food and Drug Administration 2011. FDA drug safety communication: voluntary market withdrawal of Xigris due to failure to show a survival benefit. U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 33. Sweeney DA, Danner RL, Eichacker PQ, Natanson C. 2008. Once is not enough: clinical trials in sepsis. Intensive Care Med. 34:1955–1960 [DOI] [PubMed] [Google Scholar]
- 34. Seam N, Suffredini AF. 2007. Mechanisms of sepsis and insights from clinical trials. Drug Discov. Today. Dis. Mech. 4:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, Graham DR, Dedhia HV, Homann S, MacIntyre N. 2000. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 283:1723–1730 [DOI] [PubMed] [Google Scholar]
- 36. Schuerholz T, Brandenburg K, Marx G. 2012. Antimicrobial peptides and their potential application in inflammation and sepsis. Crit. Care 16:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459 [DOI] [PubMed] [Google Scholar]
- 38. Bowdish DM, Davidson DJ, Hancock RE. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6:35–51 [DOI] [PubMed] [Google Scholar]
- 39. Andrä J, Koch MH, Bartels R, Brandenburg K. 2004. Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob. Agents Chemother. 48:1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giuliani A, Pirri G, Rinaldi AC. 2010. Antimicrobial peptides, p 137–154 In Giuliani A, Rinaldi AC. (ed), Methods in molecular biology. Springer Science+Business Media, New York, NY: [DOI] [PubMed] [Google Scholar]
- 41. Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11:37–51 [DOI] [PubMed] [Google Scholar]
- 42. Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 43. Periti P, Mazzei T. 1998. Antibiotic-induced release of bacterial cell wall components in the pathogenesis of sepsis and septic shock: a review. J. Chemother. 10:427–448 [DOI] [PubMed] [Google Scholar]
- 44. Brandenburg K. April 2009. Novel antimicrobial peptides. Patent no. PCT/EP2009/002565
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.