Abstract
Recent evidence indicates that immunomodulation by antibiotics may enhance their clinical efficacy. Specifically, drug-induced leukocyte apoptosis and macrophage efferocytosis have been shown to promote the resolution of inflammation in a variety of disease settings. Tulathromycin is a new macrolide antibiotic for the treatment of bovine respiratory disease. The direct antimicrobial effects of the drug alone do not fully justify its superior clinical efficacy, and we hypothesize that tulathromycin may have immunomodulating properties. We recently reported that tulathromycin promotes apoptosis and inhibits proinflammatory NF-κB signaling in bovine neutrophils. In this study, we investigated the direct and indirect anti-inflammatory effects of tulathromycin in bovine macrophages. The findings indicate that bovine monocyte-derived macrophages and alveolar macrophages readily phagocytose tulathromycin-induced apoptotic neutrophils both in vitro and in the airways of Mannheimia haemolytica-infected calves. Moreover, tulathromycin promotes delayed, concentration-dependent apoptosis, but not necrosis, in bovine macrophages in vitro. Activation of caspase-3 and detection of mono- and oligonucleosomes in bovine monocyte-derived macrophages treated with tulathromycin was observed 12 h posttreatment; pretreatment with a pan-caspase inhibitor (ZVAD) blocked the proapoptotic effects of the drug. Lastly, tulathromycin inhibited the secretion of proinflammatory CXCL-8 in lipopolysaccharide (LPS)-stimulated bovine macrophages; this effect was independent of caspase activation or programmed cell death. Taken together, these immunomodulating effects observed in bovine macrophages help further elucidate the mechanisms through which tulathromycin confers anti-inflammatory and proresolution benefits. Furthermore, these findings offer novel insights on how antibiotics may offer anti-inflammatory benefits by modulating macrophage-mediated events that play a key role in inflammation.
INTRODUCTION
Macrophages play a central role in immune surveillance, inflammatory responses, and tissue remodeling as well as provide a bridge between innate and adaptive immunity (1). They are distributed throughout various tissues in the body, performing a variety of functions that are dependent on their state of differentiation and the specific microenvironmental factors they are exposed to (2). Lung macrophages, derived from circulating monocytes, are compartmentalized in the airways, interstitium, and intravascular spaces. In healthy lungs, resident alveolar macrophages are the primary phagocytes of the innate immune system (2). Upon recognition and phagocytosis of foreign pathogens, resident macrophages produce a variety of proinflammatory mediatory mediators, including CXCL-8 and leukotriene B4 (LTB4), that recruit other cells, primarily neutrophils, to the site of injury (1). Recent evidence also suggests that autophagy is an important innate immune mechanism in macrophages to protect the host from intracellular bacterial or viral pathogens (3). Aside from contributing to the initial inflammatory response, resident macrophages and recruited monocyte-derived macrophages resolve inflammation by ingesting apoptotic cells and clearing them from the tissues, a process known as efferocytosis (4).
Efferocytosis plays a fundamental role in a variety of biological processes (5–7) but most notably in the resolution of inflammation (4). Clearance of apoptotic neutrophils by macrophages prevents the release of cytotoxic granules and inflammatory mediators into the local environment (8). Furthermore, monocyte-derived macrophages recruited to the site of injury have always been thought to exit the tissues via the draining lymph nodes (9, 10); however, increasing evidence suggests that recruited macrophages undergo apoptosis and are removed by resident phagocytes, thus further helping resolve inflammation (11, 12). Moreover, macrophages that ingest apoptotic cells develop an anti-inflammatory phenotype, whereby they secrete anti-inflammatory mediators such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β) and stop producing proinflammatory products (13, 14). Under chronic inflammatory conditions, the exaggerated recruitment of inflammatory cells into the tissues can overwhelm the normal phagocytic functions of macrophages. Apoptotic cells die by secondary necrosis if not ingested, which releases harmful intracellular contents, further amplifying the inflammatory response and causing tissue damage. In the lung, this self-perpetuating inflammatory response ultimately leads to pulmonary failure and death of the host.
Bacterial pneumonia is a classic example whereby exaggerated innate inflammatory responses play a central role in disease pathogenesis. Similarly to human streptococcal pneumonia, bovine respiratory disease, caused by opportunistic pathogens such as Mannheimia haemolytica (15–18), is characterized by the exacerbated infiltration and activation of neutrophils into the lower airways, by excessive accumulation of proinflammatory mediators, such as CXCL-8 and LTB4, and by uncontrolled cell necrosis (19, 20). Leukotoxins (LKT) produced by M. haemolytica lyse macrophages and neutrophils, which impairs their antibacterial defenses and further amplifies the inflammatory response (21). Hence, the combination of host products and bacterial virulence factors leads to the development of uncontrolled self-perpetuating inflammation. Therefore, therapeutic interventions for this multifactorial disease should target both the invading pathogen and the host inflammatory response.
Macrolides have been shown to reduce the recruitment of neutrophils (22–24) and their release of histotoxic compounds (22) and to inhibit the accumulation of proinflammatory cytokines (24–26). A growing body of evidence has also demonstrated that some macrolides such as tilmicosin, tulathromycin, and erythromycin deliver anti-inflammatory benefits by promoting apoptosis in neutrophils (27–30). However, the effects of macrolides on macrophage survival and cell death in the context of inflammation remain incompletely understood.
Tulathromycin, a new triamilide macrolide used in the treatment of bovine respiratory disease, displays superior clinical efficacy for reasons not fully explained by its antimicrobial actions. We recently reported that tulathromycin induces caspase-3-dependent apoptosis in bovine neutrophils and inhibits NF-κB signaling and LTB4 synthesis (30). The present study investigated the immunomodulating effects of tulathromycin in bovine macrophages, in hopes to further elucidate the anti-inflammatory mechanisms of this drug in a clinically relevant model system. The findings indicate for the first time that tulathromycin has direct anti-inflammatory actions in macrophages via the inhibition of proinflammatory CXCL-8 signaling, the induction of delayed macrophage apoptosis, and enhanced efferocytosis.
MATERIALS AND METHODS
Bacteria.
M. haemolytica biotype A serotype 1 (strain B122) was used for the in vivo studies as previously described (29, 30). Briefly, M. haemolytica was streaked out onto Columbia blood agar plates from a stock solution and grown at 37°C overnight. McFarland nephelometry and enumeration of CFU on Columbia blood agar plates were used to prepare the bacterial inoculants at a concentration of 2 × 107 CFU/ml in endotoxin-free Hanks balanced salt solution (HBSS).
Animals.
For in vitro experiments, peripheral blood was drawn from the jugular vein of healthy donor cattle (1 to 4 years of age) into Vacutainers containing 1.5 ml anticoagulant acid citrate dextrose (ACD solution A; Becton, Dickinson). Animals were housed outdoors at the University of Calgary's Veterinary Sciences Research Station (Calgary, AB).
In vivo studies were performed as previously reported (29, 30). Calves were housed indoors at the Veterinary Sciences Research Station, fed antibiotic-free milk replaced 2 times a day, and given access to water ad libitum. Following 7 days of acclimation, healthy male Holstein calves (2 to 3 weeks old, 47 to 53 kg) were challenged intratracheally with 10 ml of 2 × 108 cells of live Mannheimia haemolytica biotype A serotype 1 (strain B122) or the endotoxin-free HBSS vehicle (with NaHCO3, without phenol red, calcium chloride, or magnesium sulfate) in conjunction with a subcutaneous injection of 2.5 mg/kg of body weight tulathromycin (Draxxin Injectable Solution; Pfizer Animal Health, Kalamazoo, MI) or 25% propylene glycol vehicle. Rectal temperatures, respiratory rates, and heart rates were measured daily and were not significantly different among any of the calves prior to the onset of experimentation. Photoperiods were 12:12 h, and the temperature was 20 ± 3°C, with 40% humidity.
Care and experimental practices for both in vitro and in vivo studies were conducted under the standards of the Canadian Council on Animal Care and approved by the University of Calgary Life and Environmental Science Animal Care Committee.
Bacterial challenge and BAL fluid collection.
The protocols for challenging calves intratracheally with M. haemolytica were conducted as previously described (29, 30). Local anesthetic was applied using lidocaine (lidocaine HCl 2% and epinephrine injection; Biomeda MTC, Animal Health Inc.) at the site of tracheal insertion of a sterile trochar into each calf. A sterile catheter was inserted through the trochar, and 10 ml of M. haemolytica or HBSS was injected into the lungs at the site of the tracheal bifurfaction. Bronchoalveolar lavage (BAL) fluid was collected at 3 h and 24 h postinfection using three 20-ml washes of endotoxin-free HBSS. Samples of BAL fluid (100 μl) were centrifuged onto a microslide using a Shandon Cytospin 4 Cytocentrifuge (Thermo Electron Corporation, Pittsburgh, PA). Slides were fixed with a Diff-Quik stain (Baxter Healthcare Corp., Miami, FL) for cell analysis.
Monocyte isolation and macrophage differentiation.
Fresh peripheral bovine blood was pooled into 50-ml polypropylene tubes and subjected to centrifugation at 1,200 × g for 20 min in a Beckman J-6B centrifuge (Beckman) at 4°C without braking. The buffy coat layer was isolated, diluted 1:1 with filter-sterilized 0.9% NaCl, layered onto a polysucrose and sodium diatrixoate gradient (Histopaque-1077; Sigma), and centrifuged at 1,500 × g for 40 min. Contaminating erythrocytes were removed from the cell suspension by hypotonic lysis (10 ml of cold sterile double-distilled water for 30 s; 20 ml of cold filter-sterilized 2× HBSS for restoration of isotonicity) and resuspended in Iscove's modified Dulbecco's medium (IMDM; Sigma) containing 10% heat-inactivated fetal bovine serum (HI-FBS). Mononuclear cells (2 × 105 cells/well) were plated onto tissue culture flasks or 48-well plates (Costar, Cambridge, MA) and incubated at 37°C and 5% CO2 in IMDM containing 10% HI-FBS, 100 U/ml penicillin, 100 U/ml streptomycin, and 80 μg/ml tylosin (all from Sigma) for 7 days to allow for macrophage differentiation. For experiments, IMDM containing only 10% HI-FBS was used on the cells.
Neutrophil purification.
Whole blood was collected, pooled, and centrifuged in the same manner as described in the previous section. Following the removal of the plasma and buffy coat, the remaining layer of cells were washed with endotoxin-free HBSS and spun at 1,200 × g for 10 min at 4°C. Contaminating erythrocytes were removed with 20 ml of an ice-cold hypotonic lysis solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4). Isotonicity was restored with the addition of a hypertonic solution (10 ml; 10.6 mM Na2HPO4, 2.7 mM NaH2PO4, 462 mM NaCl). Cells were resuspended in prewarmed (37°C) endotoxin-free HBSS containing 10% HI-FBS. Cell viability was assessed by 0.1% trypan blue exclusion, and differential cell counts were performed on cytospin preparations stained with Diff-Quik. Neutrophil populations were >90% pure and >90% viable for all experiments.
In vitro assessments of macrophage efferocytosis.
Bovine neutrophils treated with tulathromycin (2 mg/ml) or HBSS (control) for 0.5 h at 37°C were centrifuged (500 × g for 5 min), washed with HBSS, and resuspended in HBSS containing 10% HI-FBS. The pretreated neutrophils were subsequently added to macrophages (isolated from the same animal) at an approximate ratio of either 10:1 or 50:1. Cells were coincubated at 37°C, 5% CO2 for 0.5 h, 1.0 h, or 2.0 h. Following incubation, slides were washed with HBSS, Diff-Quik stained, and observed under light microscopy. The percentage of macrophages containing one or more phagocytosed neutrophil was determined. A minimum of 100 cells were counted in each replicate for all treatment groups.
For myeloperoxidase (MPO) detection of phagocytosed neutrophils, adherent cells (macrophages) were analyzed for MPO activity using a commercially available myeloperoxidase detection kit (Cell Technology, Mountain View, CA), according to the manufacturer's instructions.
TEM.
Detection of apoptotic neutrophils and macrophage efferocytosis was confirmed using transmission electron microscopy (TEM), as previously described (28, 29). Flasks of bovine macrophages incubated with tulathromycin (2 mg/ml)-, staurosporine (1 μM)-, or HBSS-treated neutrophils for 2.0 h were washed and fixed with 5% glutaraldehyde (Electron Microscopy Services, Fort Washington, PA) in 0.1 M sodium cacodylate (Caco) buffer overnight at 4°C. Cells were subsequently transferred into BEEM capsules (JBS Supplies, JB EM Services Inc., Dorval, Quebec, Canada) and postfixed in 1% osmium tetroxide (Polysciences Inc., Warrington, PA) for 2 h at room temperature. Following fixation, cells were washed in 0.1 M Caco buffer, dehydrated using a series of acetone-H2O washes (30%, 50%, 70%, 90%, and 100%, vol/vol), and embedded in Spurr low-viscosity medium (Polysciences). Thin sections (90 nm) were stained with saturated uranyl acetate in 50% aqueous ethanol and 0.04% (wt/vol) lead citrate. Photomicrographs of apoptotic neutrophils and macrophages containing phagocytosed (apoptotic) neutrophils were obtained with a Hitachi H-7650 transmission electron microscope at an acceleration voltage of 80 kV.
Detection of apoptotic mono- and oligonucleosomes.
Monocyte-derived macrophages (1 × 106 cells) were incubated in medium alone or with tulathromycin (0.1 and 1.0 mg/ml) at 37°C and 5% CO2 for various time points (2 to 48 h). Cells with staurosporine (1 μM) served as a positive control. Following incubation, adherent cells were washed with HBSS, and macrophage apoptosis was subsequently assessed using a cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Biochemicals, Laval, Quebec, Canada) according to the manufacturer's instructions. Absorbance readings were taken at 405 nm using a THERMOmax microplate reader (Molecular Devices Corp., Menlo Park, CA).
Assessment of cytotoxicity/necrosis.
Release of lactate dehydrogenase (LDH) into the medium is indicative of cell membrane damage and necrosis. LDH release from neutrophils or monocyte-derived macrophages treated with medium alone (control), tulathromycin (0.1 mg/ml to 2.0 mg/ml), or staurosporine (1 μM) was determined using a cytotoxicity detection kit (Roche) according to the manufacturer's instructions. Supernatant levels of LDH were measured in parallel with cell apoptosis and represented as the absorbance ratio versus untreated control. Total LDH was determined by exposing cells to 2% Triton-X in media. Colorimetric changes were measured using a SpectraMax M2e microplate reader (Molecular Devices) at 492 nm.
Caspase-3 activity assay.
Proapoptotic caspase-3 activity in monocyte-derived macrophages treated with medium alone (control) or tulathromycin (0.1, 1.0 mg/ml) was measured using a caspase-3 fluorescent activity assay (Calbiochem, La Jolla, CA), as per the manufacturer's instructions.
Detection of secreted CXCL-8.
Mature, 7-day-old bovine macrophages were stimulated with LPS (1 μg/ml) and treated with medium alone (control) or tulathromycin (0.1 mg/ml or 1 mg/ml) for various time points (2 h to 18 h). As previously published (31), secreted levels of bovine CXCL-8 were determined colorimetrically at 405 nm on a SpectraMax M2e microplate reader (Molecular Devices), using a Quantikine Human CXCL-8/IL-8 immunoassay (R&D Systems, Minneapolis, MN) as per the manufacturer's instructions. The specificity of the assay is 100% for CXCL-8 with a detection limit of 7 pg/ml.
Statistical analysis.
Numeric values were expressed as means ± standard errors of the means (SEM). Statistical analyses of the data were performed using the Prism 5 software. Comparisons where made using Student's t test or one-way analysis of variance (ANOVA) where appropriate. Multicomparison post hoc analysis was performed with the Tukey's test. For analysis of in vivo data, the nonparametric Kruskal-Wallis statistical test was performed. Statistical significance was established at P values of <0.05 using multiple replicates of a minimum of 3 separate independent experiments.
RESULTS
Tulathromycin-treatment in calves challenged intratracheally with live M. haemolytica is associated with increased macrophage efferocytosis.
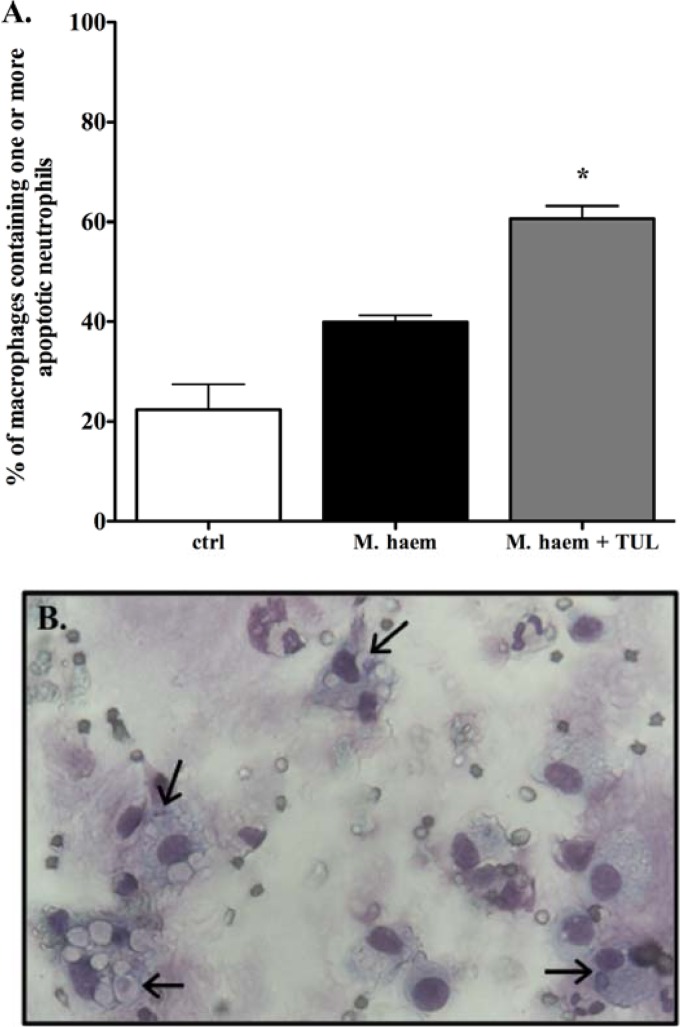
As clearance of apoptotic neutrophils by phagocytes is key to the resolution of acute inflammation (32), and in view of the apparent proapoptotic effects of tulathromycin in M. haemolytica-challenged calves (30), we investigated whether this phenomenon was associated with an increase in macrophage efferocytosis. Using light microscopy and Diff-Quik staining, we identified alveolar macrophages present in the BAL fluid and noted that the number of macrophages containing one or more phagocytosed neutrophils was significantly elevated in the BAL fluid isolated from tulathromycin-treated calves (60.6% ± 2.6%) compared to the sham-treated infected calves (39.9% ± 1.4%) and uninfected control calves (22.4% ± 5.1%) 3 h postinfection (Fig. 1).
Fig 1.
Alveolar macrophages readily phagocytose apoptotic neutrophils in tulathromycin-treated calves challenged with live M. haemolytica. Counts (A) and representative light microscopy image (B) of cytospin preparations of BAL fluid samples isolated from uninfected control calves (ctrl), sham-treated M. haemolytica-challenged calves (M. haem), and tulathromycin-treated M. haemolytica-challenged calves (M. haem + TUL) 3 h postinfection, stained with Diff-Quik and analyzed using light microscopy. Macrophages containing one of more apoptotic neutrophils were enumerated. Values were expressed as a percentage of total macrophages on each slide preparation. Values are means ± SEM. n = 3 to 7/group. *, P < 0.001 versus the control group. A minimum of 100 macrophages were counted in each sample for all treatment groups.
Monocyte-derived macrophages phagocytose tulathromycin-induced apoptotic neutrophils with more avidity than untreated neutrophils.
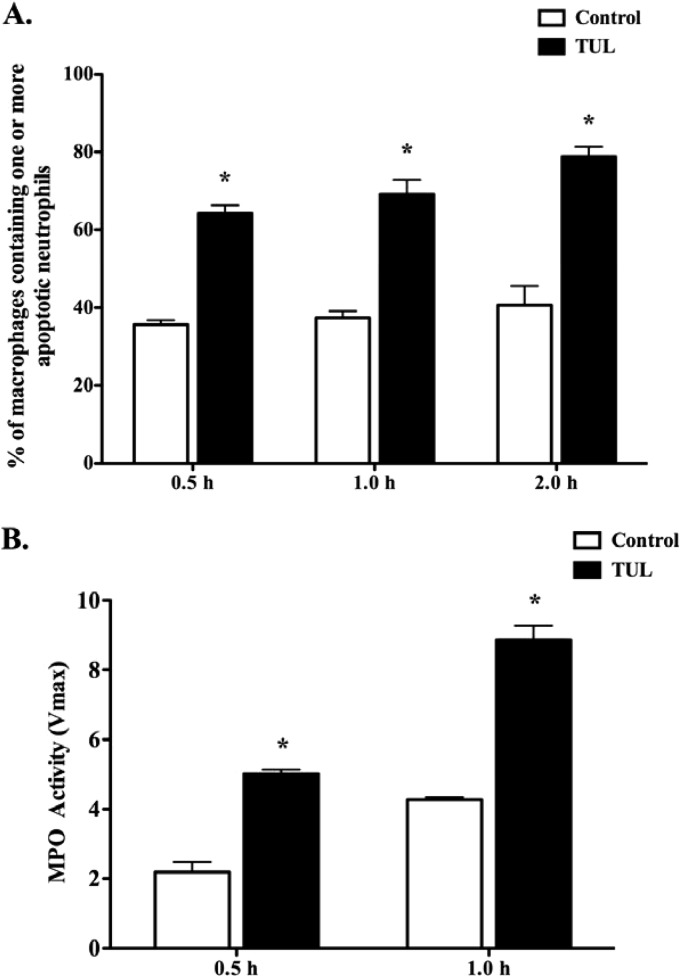
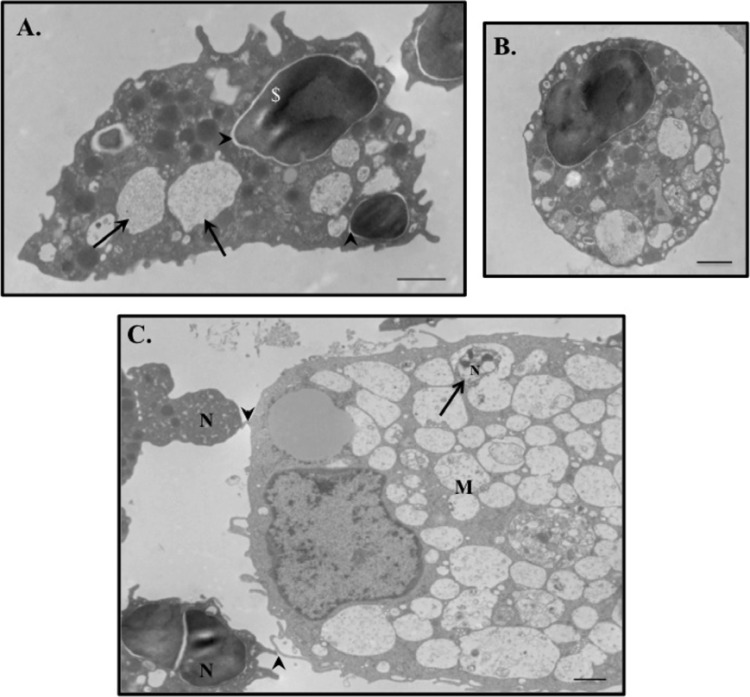
To further investigate the mechanisms of tulathromycin-induced macrophage efferocytosis, bovine neutrophils pretreated with HBSS (control) or tulathromycin (2 mg/ml) for 0.5 h at 37°C were added to bovine macrophages isolated from the same animal. Using Diff-Quik staining (Fig. 2A) and myeloperoxidase detection (Fig. 2B), we observed a significant increase in the number of macrophages containing one or more tulathromycin-treated neutrophils than control-treated bovine neutrophils at 0.5 h, 1.0 h, and 2.0 h. Following a 2.0-h coincubation, cells were prepared for transmission electron microscopy (TEM) of the complete phagocytic ingestion of apoptotic bodies. Analysis of TEM micrographs confirmed that tulathromycin-treated neutrophils, showing characteristic signs of apoptosis (Fig. 3A and B), either were found within phagocytic vacuoles of bovine macrophages or had their plasma membranes in close apposition to that of an adjacent macrophage (Fig. 3C). Neutrophils pretreated with staurosporine (1 μM), serving as a positive proapoptotic control for all efferocytosis experiments, revealed similar processes (data not shown).
Fig 2.
Tulathromycin-induced apoptotic bovine neutrophils are readily phagocytosed by bovine macrophages in vitro. Circulating bovine neutrophils pretreated with HBSS (control) or tulathromycin (TUL; 2 mg/ml) for 0.5 h at 37°C were incubated with bovine macrophages for 0.5 h, 1.0 h, and 2.0 h. (A) Light microscopy counts of Diff-Quik-stained macrophages containing one or more phagocytosed apoptotic neutrophils. A minimum of 300 macrophages were counted per treatment group in triplicate. (B) Detection of myeloperoxidase (MPO; used as a neutrophil marker) in macrophages. Values are means ± SEM. n = 3/group. *, P < 0.001.
Fig 3.
Bovine macrophages readily phagocytose tulathromycin-induced apoptotic bovine neutrophils in vitro. Representative transmission electron micrographs of tulathromycin-treated bovine neutrophils (N), displaying ultrastructural features characteristic of apoptosis, including intact plasma membrane with blebbing, nuclear membrane delamination (arrowheads), chromatin condensation, intact cytoplasmic organelles and granules, and vacuolization of the cytoplasm (arrows), in close apposition to (arrowheads) (C) and/or within phagocytic vacuoles (large arrow) of a bovine macrophage (M) (A and C). Bovine neutrophils pretreated with HBSS (control) or tulathromycin (TUL; 2 mg/ml) for 0.5 h at 37°C were incubated with bovine macrophages for 2.0 h at 37°C. TEM images were taken at a magnification of ×4,000 at an acceleration voltage of 80.0 kV. Bar, 1 μm.
Tulathromycin induces apoptosis, but not necrosis, in bovine monocyte-derived macrophages in both a time- and a concentration-dependent manner.
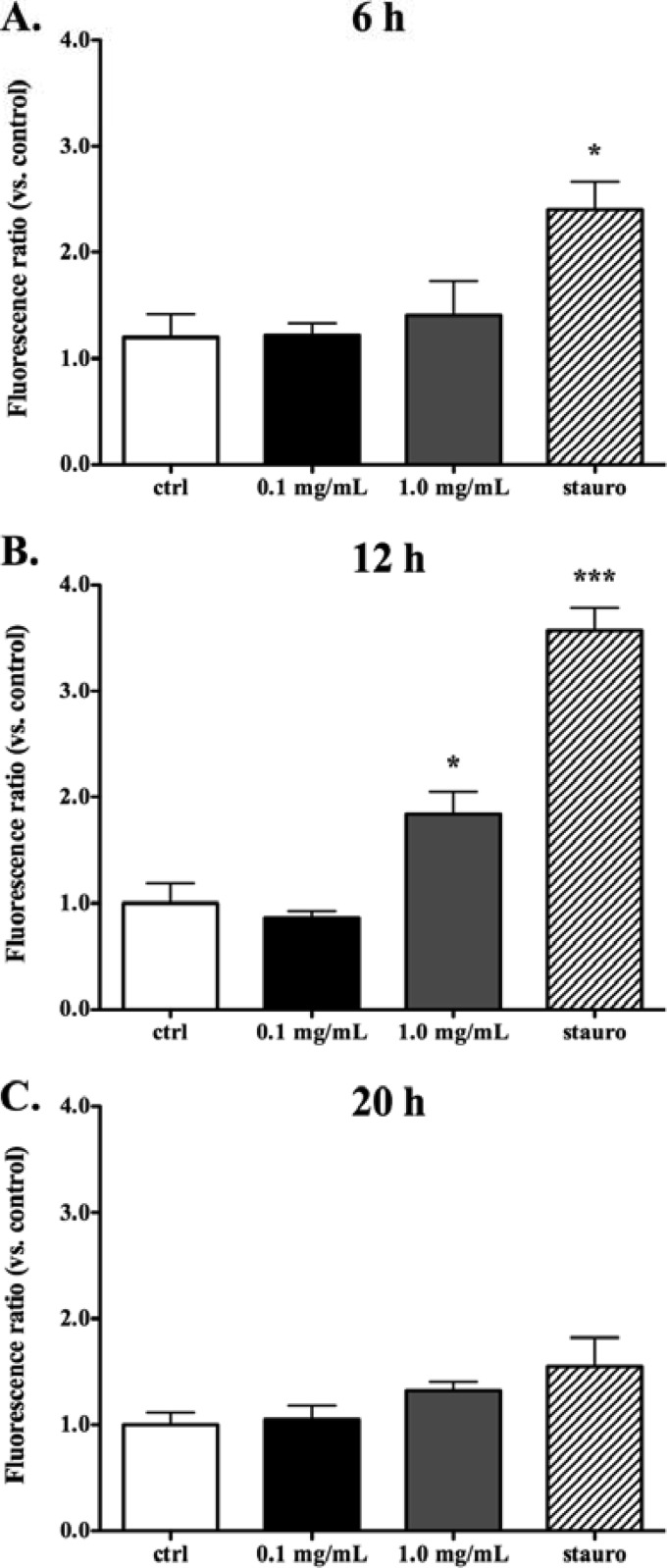
In view of the proapoptotic effects of tulathromycin in bovine neutrophils, we investigated whether tulathromycin-induced cell death would also occur in bovine monocyte-derived macrophages. Detection of mono- and oligonucleosomes in bovine macrophages revealed that treatment with 1 mg/ml tulathromycin induced apoptosis in these cells following 12 h and 20 h of incubation, but not after 2 h or 6 h of treatment (Fig. 4A). Induction of apoptosis by a lower tulathromycin concentration of 0.1 mg/ml was also detected after 48 h of treatment, consistent with a dose-dependent effect (Fig. 4C). Necrosis, as measured by secreted levels of lactate dehydrogenase, was only significantly higher in bovine macrophages treated with 1.0 mg/ml of tulathromycin or staurosporine for 20 h or 48 h, and at no time point sooner (Fig. 4B and D).
Fig 4.
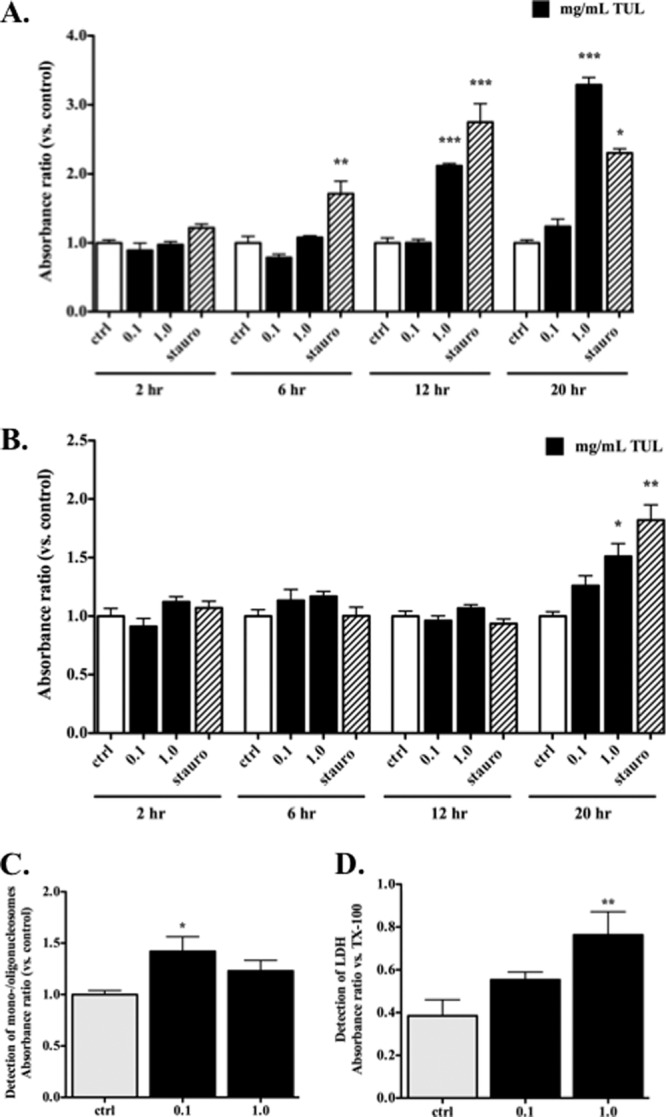
Tulathromycin induces apoptosis, but not necrosis, in bovine macrophages in vitro in a time- and concentration-dependent manner. Apoptotic mono-and oligonucleosomes (A) and secreted lactate dehydrogenase (LDH) (B) were measured in bovine macrophages incubated with IMDM alone (control [ctrl]) or treated with tulathromycin (0.1 mg/ml or 1.0 mg/ml) for 2 h, 6 h, 12 h, or 20 h at 37°C. A separate set of experiments then measured mono-/oligonucleosomes (C) and LDH release (D) in bovine macrophages incubated under similar conditions, but for a longer period of 48 h. Values were calculated as absorbance ratios versus values measured in control macrophages arbitrarily set to 1.0. Staurosporine (stauro; 1 μM) served as a positive apoptotic control. Cells exposed to 2% Triton-X (TX-100) served as a total LDH control. Values are means ± SEM. n = 6/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control at each time point.
The proapoptotic effect of tulathromycin in monocyte-derived macrophages is caspase dependent.
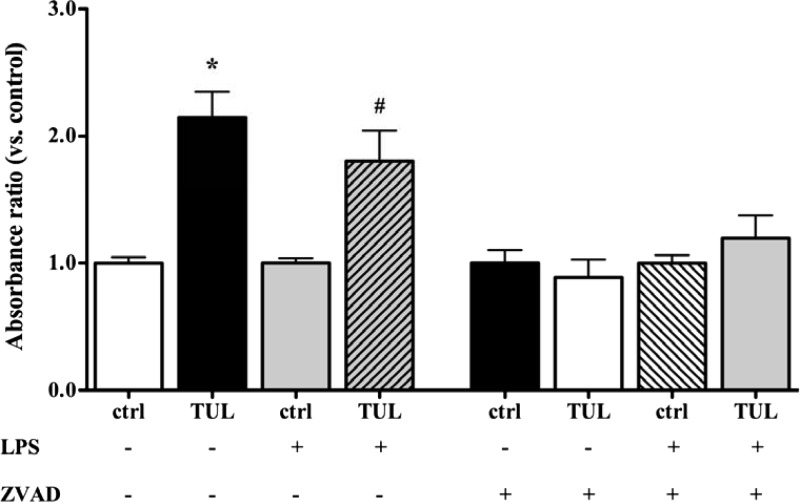
Activated caspases are well known to induce apoptosis in a variety of cell types, including neutrophils and macrophages (33). In view of the caspase-3-dependent proapoptotic effects of tulathromycin in bovine neutrophils (30), we investigated whether caspase-3 was involved in tulathromycin-induced apoptosis in bovine macrophages. Bovine macrophages treated with tulathromycin (1 mg/ml, but not 0.1 mg/ml) exhibited increased caspase-3 activity following 12 h of treatment (Fig. 5B), but not after 6 h or 20 h of treatment (Fig. 5A and C, respectively). To determine whether the proapoptotic effects of tulathromycin in bovine macrophages were also caspase dependent, cells were incubated with a pan-caspase inhibitor (ZVAD; 50 μM) or a dimethyl sulfoxide (DMSO) vehicle control for 1.0 h prior to treatment. Inhibition of caspases by ZVAD blocked the proapoptotic effects of tulathromycin observed in unstimulated and LPS (1 μg/ml)-stimulated macrophages (Fig. 6).
Fig 5.
Tulathromycin activates caspase-3 in bovine macrophages in vitro in a time- and concentration-dependent manner. Activity of caspase-3 in macrophages treated with IMDM alone (control [ctrl]) or tulathromycin (0.1 mg/ml or 1.0 mg/ml) for 6 h (A), 12 h (B), and 20 h (C) at 37°C. Values were not significantly different at 20 h. Staurosporine (stauro; 1 μM) served as a positive apoptotic control. Values were calculated as fluorescence ratios versus values measured in control macrophages arbitrarily set to 1.0. Values are means ± SEM. n = 6/group. *, P < 0.001; ***, P < 0.0001 versus control.
Fig 6.
The proapoptotic effect of tulathromycin in bovine macrophages in vitro is caspase dependent. Levels of mono- and oligonucleosomes in bovine macrophages incubated with IMDM alone (control [ctrl]) or treated with tulathromycin (TUL; 1 mg/ml) for 12 h at 37°C in the absence or presence of LPS (1 μg/ml). Cells were pretreated with a 50 μM concentration of a pan-caspase inhibitor (ZVAD) or a DMSO vehicle control for 1.0 h at 37°C prior to treatment. There was no significant difference between any of the groups treated with ZVAD. Values were calculated as absorbance ratios versus values measured in control macrophages arbitrarily set to 1.0. Values are means ± SEM. n = 6 to 8/group. *, P < 0.001 versus unstimulated control; #, P < 0.05 versus LPS-stimulated control.
Tulathromycin directly inhibits secretion of CXCL-8 in LPS-stimulated bovine monocyte-derived macrophages in a caspase-independent manner.
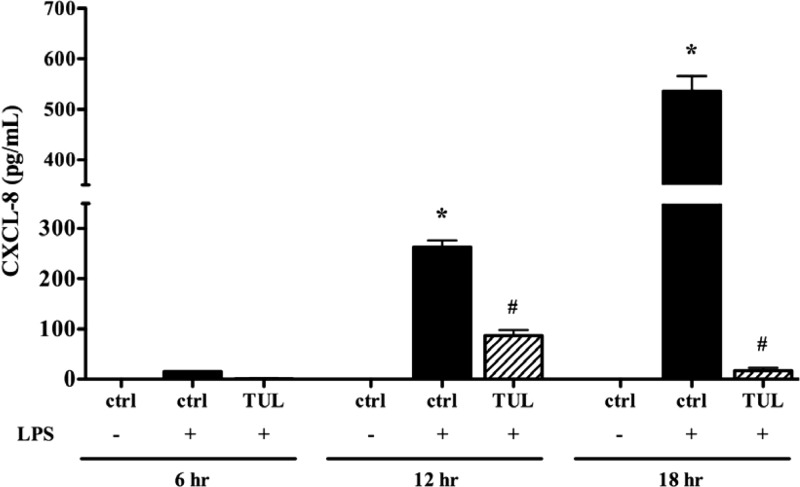
Tulathromycin has been shown to have direct anti-inflammatory effects in neutrophils (30). Another set of experiments assessed the effects of tulathromycin-induced macrophage apoptosis on the synthesis of proinflammatory CXCL-8. Following 12 h and 20 h of incubation, there was a significant reduction in the secreted levels of CXCL-8 in LPS (1 μg/ml)-stimulated bovine macrophages treated with 1.0 mg/ml of tulathromycin (Fig. 7).
Fig 7.
Tulathromycin inhibits secretion of proinflammatory CXCL-8 in LPS-stimulated bovine macrophages in vitro. Levels of secreted CXCL-8 in macrophages treated with IMDM alone (control [ctrl]) or tulathromycin (1.0 mg/ml) for 6 h, 12 h, or 20 h at 37°C in the presence or absence of LPS (1 μg/ml). There were no detectable levels of CXCL-8 in unstimulated macrophages treated with tulathromycin (data not shown). Values are means ± SEM. n = 6 to 9/group. *, P < 0.05 versus control at each time point; #, P < 0.05 versus LPS-stimulated control at each time point.
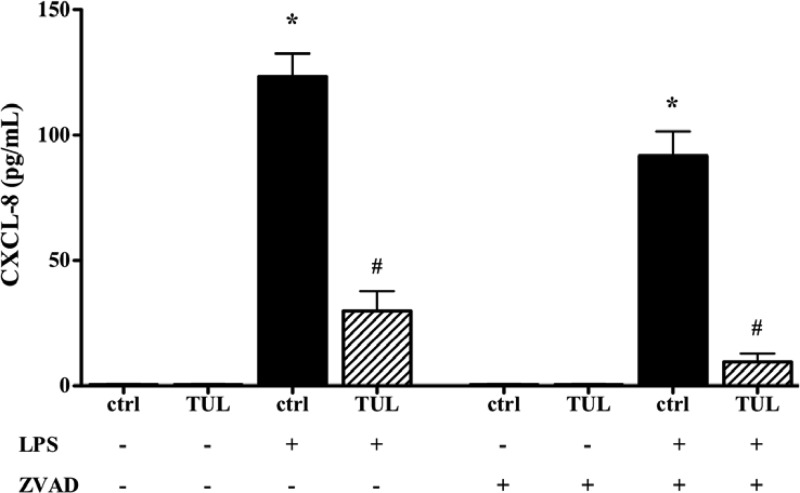
Given that tulathromycin (1 mg/ml) induced apoptosis in bovine macrophages after 12 h, we investigated whether an increase in apoptotic cell death was responsible for the inhibition of CXCL-8 production by tulathromycin. In the presence of a pan-caspase inhibitor (ZVAD; 50 μM), treatment with tulathromycin (1 mg/ml) significantly reduced CXCL-8 production in LPS-stimulated bovine macrophages (Fig. 8).
Fig 8.
Tulathromycin inhibits CXCL-8 secretion in vitro in a caspase-independent manner. Levels of secreted CXCL-8 in macrophages treated with IMDM alone (control [ctrl]) or tulathromycin (TUL; 1.0 mg/ml) for 12 h at 37°C in the presence or absence of LPS (1 μg/ml). Cells were pretreated with a 50 μM concentration of a pan-caspase inhibitor (ZVAD) or a DMSO vehicle control for 1.0 h at 37°C prior to treatment. Values are means ± SEM. n = 6 to 9/group. *, P < 0.05 versus unstimulated DMSO or ZVAD control; #, P < 0.05 versus LPS-stimulated DMSO or ZVAD control.
DISCUSSION
Some macrolide antibiotics are capable of delivering dual antimicrobial and anti-inflammatory benefits (19, 34). Mannheimia haemolytica serotype S1, one of the primary causative agents of bovine respiratory disease, produces leukotoxins (LKT) that stimulate the release of inflammatory cytokines and lipid mediators, such as CXCL-8, TNF-α, and LTB4, from alveolar macrophages and neutrophils and cause cell necrosis (15–17, 20, 21, 36, 37). We recently reported that a new triamilide, tulathromycin, both promotes caspase-3-dependent apoptosis in bovine neutrophils in vitro and inhibits proinflammatory NF-κB signaling and LTB4 production in circulating neutrophils, using a clinically relevant infection model of bovine respiratory disease (30). In the present study, we focused our attention on the effects of tulathromycin in bovine monocyte-derived macrophages. Associated with the significant increase in apoptotic leukocytes (neutrophils) in the BAL fluid in tulathromycin-treated calves challenged with M. haemolytica, we observed a significantly greater number of macrophages containing apoptotic neutrophils in these animals 3 h postinfection. Further examination of this effect in vitro revealed that monocyte-derived macrophages phagocytose tulathromycin-induced apoptotic neutrophils with more avidity than untreated neutrophils. The findings also revealed that tulathromycin (1 mg/ml) induces apoptosis, but not necrosis, in resting and LPS-stimulated bovine macrophages following 12 h or 20 h of treatment. This effect was time and concentration dependent (0.1 mg/ml tulathromycin-induced apoptosis was detected at 48 h but not earlier) and occurs via the activation of caspase-3. In order to maintain optimal cell viability conditions to perform mechanistic studies, experiments were performed using the higher and quicker inducer concentration of 1.0 mg/ml. Finally, we found that tulathromycin directly inhibits the secretion of proinflammatory CXCL-8 in LPS-stimulated bovine macrophages after 12 h, an effect independent of caspase activation and apoptosis. The findings from this study collectively demonstrate that, in addition to its selective immunomodulatory effects in neutrophils, tulathromycin has anti-inflammatory and proresolving effects in macrophages.
Apoptosis of inflammatory cells and their subsequent clearance by phagocytes are two key processes involved in the resolution phase of inflammation (32). Apoptotic cells send out soluble “find me” signals and present cell surface “eat me” markers that facilitate recognition and engulfment by phagocytes (38). Receptors associated with the phagocytosis of apoptotic cells have included a receptor for phosphatidylserine (PS), the integrins αvβ3 and αvβ5, certain lectins, CD36, CD14, and CD68 (38, 39). These recognition and engulfment processes not only help remove potentially histotoxic inflammatory cells from the tissues but also activate an anti-inflammatory phenotype in macrophages that causes them to release proresolution TGF-β and IL-10 (13, 14). We previously reported that tulathromycin increases surface expression of PS in bovine neutrophils (30); in this study, we demonstrate that bovine macrophages readily phagocytose tulathromycin-induced apoptotic neutrophils. Similarly, azithromycin promotes the phagocytic clearance of apoptotic neutrophils and bronchial epithelial cells by macrophages, an effect that is partially dependent on PS receptor binding (40). Moreover, azithromycin was shown to modify activation of macrophages toward the M2 anti-inflammatory phenotype (41, 42), possibly via inhibition of Toll-like receptor 4 (TLR4)-mediated NF-κB signaling (43). Erythromycin and clarithromycin also promote PS receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages in vitro (44). Interestingly, this effect was observed only following 72 h of treatment but not at any of the earlier time points (44). In contrast, our studies indicate that tulathromycin-treated bovine neutrophils are rapidly phagocytosed by macrophages.
It was believed that monocyte macrophages recruited to a site of inflammation leave the inflamed tissue by lymphatic drainage following the resolution of inflammation and migrate to the lymphoid organs for antigen presentation, with only a small population of these cells undergoing apoptosis in the tissues (9, 10, 45). Recently, however, Kolaczkowska et al. (12) characterized the kinetics of apoptotic death in inflammatory cells recruited to the tissues. The findings revealed that following an early increase in neutrophil apoptosis, heightened apoptosis of monocyte-derived macrophages coincides with the final resolution phase of the inflammatory response (12). Since tulathromycin accumulates intracellularly in bovine macrophages, we hypothesized that the drug could potentially induce apoptosis in these cells at a later time point. Indeed, treatment with tulathromycin for 12 h, but not earlier time points, resulted in a significant increase in the levels of apoptotic bovine macrophages. The effects were also found to be caspase-3 dependent. The ketolide telithromycin was recently shown to promote delayed apoptosis in LPS-stimulated RAW 264.7 macrophages (46). The authors of this study postulated that this effect was beneficial in promoting the resolution of inflammation by enhancing apoptosis in macrophages in the later stages of inflammatory responses. Indeed, Fas-induced apoptosis of recruited macrophages was found to be essential for the resolution of acute lung injury in vivo (11). Moreover, the phagocytosis of apoptotic macrophages was important for downregulating the production of inflammatory mediators (TNF-α) in a murine model of pneumococcal infection (41). In addition, apoptosis of alveolar macrophages appears to be important for intracellular killing of phagocytosed microbes (47) and prevention of bacterial proliferation during a pneumococcal infection (35, 41). Similarly, the induction of apoptosis in alveolar macrophages during a Mycobacterium tuberculosis infection is postulated to be an important host defense mechanism for containing mycobacterial survival and growth (48, 49). Taken together, these findings suggest that promoting a delayed induction of apoptosis in macrophages may limit lung injury and contribute to the resolution of inflammation. Future studies need to assess the involvement of the extrinsic apoptotic pathway and/or the cell mitochondria in tulathromycin-induced apoptosis in bovine macrophages.
CXCL-8, a potent neutrophil chemoattractant, is a major determinant in the accumulation of neutrophils at the site of an infection. Leukotoxins produced by M. haemolytica activate NF-κB signaling and stimulate the production of CXCL-8 in bovine alveolar macrophages, leading to the self-amplifying inflammatory response associated with full-blown bovine respiratory disease (20, 21, 36). Our results indicate, for the first time, that tulathromycin directly inhibits secretion of this proinflammatory mediator in LPS-stimulated bovine macrophages. Interestingly, although caspases have been shown to block NF-κB signaling during apoptosis (50–52), we did not find the inhibitory effect of tulathromycin on CXCL-8 production to be dependent on caspase activity or cell death, suggesting that this indeed represents a hitherto unrecognized direct anti-inflammatory effect of this antibiotic. Future studies will elucidate the mechanism through which tulathromycin blocks CXCL-8 production. We previously reported that tulathromycin blocks NF-κB signaling in bovine neutrophils by preventing phosphorylation of IκB-α and subsequent translocation of p65 into the nucleus (30). The impact of other macrolides on NF-κB signaling remains controversial, and the effects seem to be dependent on cell type and drug concentration. Clarithromycin was found to suppress NF-κB activation in human peripheral mononuclear cells (53); in contrast, other anti-inflammatory macrolides, such as tilmicosin, appear to have no effect on the transcription of CXCL-8 (54). Further studies will determine the mechanism through which tulathromycin inhibits CXCL-8 production in macrophages as well as explore the effects of this antibiotic on proinflammatory signaling in other cell types and the production of other inflammatory cytokines generated by NF-κB.
Taken together, the data presented in the present study illustrate that tulathromycin has immunomodulating benefits that extend beyond its previously observed effects in bovine neutrophils (30). Indeed, the results demonstrate that tulathromycin inhibits secretion of proinflammatory CXCL-8 and induces delayed, caspase-3-dependent apoptosis in bovine macrophages. Furthermore, tulathromycin-induced apoptotic neutrophils are readily phagocytosed by bovine macrophages, which represents a vital process for resolving inflammation. A therapeutic with the potential to limit an overt and uncontrolled immune cell accumulation and retention at the site of infection can provide numerous benefits against the development of chronic inflammatory diseases. Herein, we provide further explanation of the ability of a new triamilide macrolide to exert superior clinical efficacy against a respiratory infectious disease. These mechanisms may shed light on new targets for future therapeutic developments.
Footnotes
Published ahead of print 7 January 2013
REFERENCES
- 1. Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11:723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. 1976. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 192:1016–1018 [DOI] [PubMed] [Google Scholar]
- 3. Xu Y, Eissa NT. 2010. Autophagy in innate and adaptive immunity. Proc. Am. Thorac Soc. 7:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savill J, Dransfield I, Gregory C, Haslett C. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975 [DOI] [PubMed] [Google Scholar]
- 5. Cohen JJ. 1991. Programmed cell death in the immune system. Adv. Immunol. 50:55–85 [DOI] [PubMed] [Google Scholar]
- 6. Han H, Iwanaga T, Uchiyama Y, Fujita T. 1993. Aggregation of macrophages in the tips of intestinal villi in guinea pigs: their possible role in the phagocytosis of effete epithelial cells. Cell Tissue Res. 271:407–416 [DOI] [PubMed] [Google Scholar]
- 7. Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. 1994. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J. Cell Sci. 107(Part 5):1159–1167 [DOI] [PubMed] [Google Scholar]
- 8. Maianski NA, Maianski AN, Kuijpers TW, Roos D. 2004. Apoptosis of neutrophils. Acta Haematol. 111:56–66 [DOI] [PubMed] [Google Scholar]
- 9. Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. 1996. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157:2577–2585 [PubMed] [Google Scholar]
- 10. Cao C, Lawrence DA, Strickland DK, Zhang L. 2005. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106:3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. 2011. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 184:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolaczkowska E, Koziol A, Plytycz B, Arnold B. 2010. Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology 215:492–504 [DOI] [PubMed] [Google Scholar]
- 13. Huynh ML, Fadok VA, Henson PM. 2002. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 109:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. 1997. Immunosuppressive effects of apoptotic cells. Nature 390:350–351 [DOI] [PubMed] [Google Scholar]
- 15. Clinkenbeard KD, Clarke CR, Hague CM, Clinkenbeard P, Srikumaran S, Morton RJ. 1994. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J. Leukoc. Biol. 56:644–649 [DOI] [PubMed] [Google Scholar]
- 16. Henricks PA, Binkhorst GJ, Drijver AA, Nijkamp FP. 1992. Pasteurella haemolytica leukotoxin enhances production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect. Immun. 60:3238–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shewen PE, Wilkie BN. 1982. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect. Immun. 35:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker RD, Hopkins FM, Schultz TW, McCracken MD, Moore RN. 1985. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am. J. Vet. Res. 46:2429–2433 [PubMed] [Google Scholar]
- 19. Buret AG. 2010. Immuno-modulation and anti-inflammatory benefits of antibiotics: the example of tilmicosin. Can. J. Vet. Res. 74:1–10 [PMC free article] [PubMed] [Google Scholar]
- 20. Hsuan SL, Kannan MS, Jeyaseelan S, Prakash YS, Malazdrewich C, Abrahamsen MS, Sieck GC, Maheswaran SK. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Microb. Pathog. 26:263–273 [DOI] [PubMed] [Google Scholar]
- 21. Singh K, Ritchey JW, Confer AW. 2011. Mannheimia haemolytica: bacterial-host interactions in bovine pneumonia. Vet. Pathol. 48:338–348 [DOI] [PubMed] [Google Scholar]
- 22. Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, Yano T, Oizumi K. 1992. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am. Rev. Respir. Dis. 146:196–203 [DOI] [PubMed] [Google Scholar]
- 23. Lee WD, Flynn AN, LeBlanc JM, Merrill JK, Dick P, Morck DW, Buret AG. 2004. Tilmicosin-induced bovine neutrophil apoptosis is cell-specific and downregulates spontaneous LTB4 synthesis without increasing Fas expression. Vet. Res. 35:213–224 [DOI] [PubMed] [Google Scholar]
- 24. Mikasa K, Kita E, Sawaki M, Kunimatsu M, Hamada K, Konishi M, Kashiba S, Narita N. 1992. The anti-inflammatory effect of erythromycin in zymosan-induced peritonitis of mice. J. Antimicrob. Chemother. 30:339–348 [DOI] [PubMed] [Google Scholar]
- 25. Takeshita K, Yamagishi I, Harada M, Otomo S, Nakagawa T, Mizushima Y. 1989. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs Exp. Clin. Res. 15:527–533 [PubMed] [Google Scholar]
- 26. Tsuchihashi Y, Oishi K, Yoshimine H, Suzuki S, Kumatori A, Sunazuka T, Omura S, Matsushima K, Nagatake T. 2002. Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils. Antimicrob. Agents Chemother. 46:1101–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aoshiba K, Nagai A, Konno K. 1995. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 39:872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chin AC, Lee WD, Murrin KA, Morck DW, Merrill JK, Dick P, Buret AG. 2000. Tilmicosin induces apoptosis in bovine peripheral neutrophils in the presence or in the absence of Pasteurella haemolytica and promotes neutrophil phagocytosis by macrophages. Antimicrob. Agents Chemother. 44:2465–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chin AC, Morck DW, Merrill JK, Ceri H, Olson ME, Read RR, Dick P, Buret AG. 1998. Anti-inflammatory benefits of tilmicosin in calves with Pasteurella haemolytica-infected lungs. Am. J. Vet. Res. 59:765–771 [PubMed] [Google Scholar]
- 30. Fischer CD, Beatty JK, Zvaigzne CG, Morck DW, Lucas MJ, Buret AG. 2011. Anti-inflammatory benefits of antibiotic-induced neutrophil apoptosis: tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-kappaB signaling and CXCL8 transcription. Antimicrob. Agents Chemother. 55:338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shuster DE, Kehrli ME, Jr, Rainard P, Paape M. 1997. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 65:3286–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG. 2010. Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol. Rev. 236:28–40 [DOI] [PubMed] [Google Scholar]
- 33. Crawford ED, Wells JA. 2011. Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 80:1055–1087 [DOI] [PubMed] [Google Scholar]
- 34. Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. 2011. Immunomodulatory effects of macrolide antibiotics—part 1: biological mechanisms. Respiration 81:67–74 [DOI] [PubMed] [Google Scholar]
- 35. Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380–5388 [DOI] [PubMed] [Google Scholar]
- 36. Singh K, Confer AW, Hope JC, Rizzi T, Wyckoff JH, III, Weng HY, Ritchey JW. 2011. Cytotoxicity and cytokine production by bovine alveolar macrophages challenged with wild type and leukotoxin-deficient Mannheimia haemolytica. Vet. J. 188:221–227 [DOI] [PubMed] [Google Scholar]
- 37. Singh K, Confer AW, Step DL, Rizzi T, Wyckoff JH, III, Weng HY, Ritchey JW. 2012. Cytokine expression by pulmonary leukocytes from calves challenged with wild-type and leukotoxin-deficient Mannheimia haemolytica. Vet. J. 192:112–119 [DOI] [PubMed] [Google Scholar]
- 38. Ravichandran KS. 2011. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity 35:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hart SP, Dransfield I, Rossi AG. 2008. Phagocytosis of apoptotic cells. Methods 44:280–285 [DOI] [PubMed] [Google Scholar]
- 40. Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN. 2006. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 28:486–495 [DOI] [PubMed] [Google Scholar]
- 41. Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. 2006. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 177:6480–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy BS, Sundareshan V, Cory TJ, Hayes D, Jr, Anstead MI, Feola DJ. 2008. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 61:554–560 [DOI] [PubMed] [Google Scholar]
- 43. Vrancic M, Banjanac M, Nujic K, Bosnar M, Murati T, Munic V, Polancec DS, Belamaric D, Parnham MJ, Haber VE. 2012. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br. J. Pharmacol. 165:1348–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T. 2003. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob. Agents Chemother. 47:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. 2007. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leiva M, Ruiz-Bravo A, Jimenez-Valera M. 2008. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest 134:20–29 [DOI] [PubMed] [Google Scholar]
- 47. Dockrell DH, Lee M, Lynch DH, Read RC. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713–722 [DOI] [PubMed] [Google Scholar]
- 48. Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161:2636–2641 [PubMed] [Google Scholar]
- 49. Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barkett M, Xue D, Horvitz HR, Gilmore TD. 1997. Phosphorylation of IkappaB-alpha inhibits its cleavage by caspase CPP32 in vitro. J. Biol. Chem. 272:29419–29422 [DOI] [PubMed] [Google Scholar]
- 51. Levkau B, Scatena M, Giachelli CM, Ross R, Raines EW. 1999. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat. Cell Biol. 1:227–233 [DOI] [PubMed] [Google Scholar]
- 52. Reuther JY, Baldwin AS., Jr 1999. Apoptosis promotes a caspase-induced amino-terminal truncation of IkappaBalpha that functions as a stable inhibitor of NF-kappaB. J. Biol. Chem. 274:20664–20670 [DOI] [PubMed] [Google Scholar]
- 53. Ichiyama T, Nishikawa M, Yoshitomi T, Hasegawa S, Matsubara T, Hayashi T, Furukawa S. 2001. Clarithromycin inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob. Agents Chemother. 45:44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goubau S, Morck DW, Buret A. 2000. Tilmicosin does not inhibit interleukin-8 gene expression in the bovine lung experimentally infected with Mannheimia (Pasteurella) haemolytica. Can. J. Vet. Res. 64:238–242 [PMC free article] [PubMed] [Google Scholar]