Abstract
Although smallpox has been eradicated, the United States government considers it a “material threat” and has funded the discovery and development of potential therapeutic compounds. As reported here, the human efficacious dose for one of these compounds, ST-246, was determined using efficacy studies in nonhuman primates (NHPs), together with pharmacokinetic and pharmacodynamic analysis that predicted the appropriate dose and exposure levels to provide therapeutic benefit in humans. The efficacy analysis combined the data from studies conducted at three separate facilities that evaluated treatment following infection with a closely related virus, monkeypox virus (MPXV), in a total of 96 NHPs. The effect of infection on ST-246 pharmacokinetics in NHPs was applied to humans using population pharmacokinetic models. Exposure at the selected human dose of 600 mg is more than 4-fold higher than the lowest efficacious dose in NHPs and is predicted to provide protection to more than 95% of the population.
INTRODUCTION
Smallpox is one of the most devastating diseases in human history. A concerted and sustained vaccination campaign eradicated the pathogen, a human-specific orthopoxvirus, from the environment. The World Health Organization declared smallpox eradicated in 1980 (1, 2). In spite of its eradication, the U.S. government has designated smallpox a “material threat” and has invested in the discovery and development of safer vaccine products, as well as therapeutics, for smallpox. Vaccination can prevent disease if given soon after exposure, but that would be prior to symptomatic disease and therefore would be useful only for a small fraction of those exposed to, but not those ill with, smallpox (3, 4). Thus, therapeutics would be needed to prevent mortality in the event of an inadvertent or deliberate release of variola virus, the etiological agent of smallpox.
The lack of evaluable orthopoxvirus illness in humans means that development of a therapeutic must be conducted under the auspices of the “animal rule” (Code of Federal Regulations, Title 21, Section 314.600). One critical element of this rule is the requirement that the pharmacokinetics and pharmacodynamics of the product be used to select an effective human dose. This requirement entails an understanding of the pharmacokinetics of the compound in a well-characterized animal model whose pharmacodynamic endpoints are clearly related to the desired endpoints in humans. In relating the pharmacokinetic properties to the human experience, an assumption must be made that any effect of infection on the pharmacokinetics in the animal model is equivalent to that in humans. The caveat with this assumption is that disease severity, which could affect the pharmacokinetics of a therapeutic, may be dependent upon the orthopoxvirus species, the host genetics, the route of infection, and the size of the virus inoculum. Therefore, the effect of infection on the pharmacokinetics should be evaluated in the most severe infection possible, so that the maximum likely impact can be assessed.
ST-246 is a small-molecule therapeutic that has shown efficacy in multiple in vivo studies of orthopoxvirus infection in mice, rabbits, ground squirrels, prairie dogs, and nonhuman primates (NHPs) (5–9). Orthopoxvirus strains used in the in vivo studies included vaccinia virus, cowpox virus, ectromelia virus, rabbitpox virus, and monkeypox virus (MPXV) (6–9), (5, 10). ST-246 increased survival in all of these studies in a dose-dependent manner (5–10). Although experimental infection of cynomolgus monkeys via intravenous injection of variola virus can induce disease symptoms that resemble human smallpox, the model does not provide consistent lethality, making the only existing variola animal model inadequate for definitive assessment of the efficacy of a smallpox treatment (11–13). Furthermore, the model must be conducted under biosafety level 4 (BSL-4) constraints, which prevent the acquisition of meaningful pharmacokinetic and pharmacodynamic (PK/PD) data. Models with well-defined endpoints that use surrogate orthopoxviruses will be required to provide these critical data for antiviral evaluation. The molecular target for ST-246 antiviral activity is an envelope protein required for the production of extracellular virus, which is highly conserved among all orthopoxviruses (9). Studies in vitro demonstrated similar susceptibilities to ST-246 for all species of orthopoxviruses in cell culture, confirming the utility of the surrogate viruses for efficacy models for the variola pathogen (14).
Models developed in cynomolgus monkeys infected with MPXV via intravenous injection produce a nearly uniformly lethal infection with high viremia and exanthematous disease resembling human smallpox (5, 15, 16). The disease caused by MPXV infection in these NHPs results in a median time to death of 14 days after intravenous (i.v.) infection, lesion onset between days 3 and 5 postinfection that serves as a useful therapeutic trigger, and disease progression (viremia and lesion formation) that resembles that of human smallpox (3, 17, 18). Given the nearly uniform lethality, survival serves as the primary endpoint in this model system. Because the pharmacokinetics and drug metabolism for ST-246 in NHPs and humans are very similar, this animal model appears to be the best available tool for assessment of ST-246 antiviral efficacy (19).
To identify the efficacious dose in humans, population pharmacokinetic (POP PK) models were developed in humans and in uninfected and MPXV-infected NHPs. The effect of orthopoxvirus infection on the pharmacokinetics of ST-246 in NHPs was determined and then applied to the human POP PK model to support selection of the appropriate human dose. Simulations were conducted to predict the exposure (area under the curve [AUC]) and maximum (Cmax) and minimum (Cmin) plasma concentrations for infected humans, and then the same parameters were simulated for infected NHPs over a range of doses. An overlay of the three parameters, AUC, Cmax, and Cmin, identified equivalent dose levels. A pharmacodynamic analysis of combined data from five studies of MPXV infection of NHPs was used to determine the lowest efficacious dose of ST-246. This process identified a human dose that should be efficacious for over 95% of the human population.
MATERIALS AND METHODS
Pharmacokinetic analysis software.
The population PK analysis was performed using Phoenix NLME software (version 6.2.0.416) with the first-order conditional estimation extended-least-squares method (FOCE-ELS). Data set preparation and exploration and visualization of the data were performed using Excel (Microsoft) or S-PLUS version 8.1 (Tibco, Seattle, WA).
Data set construction.
The preclinical pharmacokinetic data set combined all plasma ST-246 concentrations. The final data set included the actual times of dose administration and of blood collection, demographic data, and infection status.
The clinical data set included all data except visually identified outliers. Method M6, reported by Beal (20), was used to handle the concentration below the lower limit of assay quantification (BLQ). At the end of the elimination phase, the first BLQ concentration was set to half of the lower limit of quantification. If consecutive concentrations were BLQ, only the first BLQ value was retained in the population PK analysis.
Population PK model development.
The NHP POP PK model development was initiated by inspecting the individual concentration-time profiles of ST-246 visually to identify outliers (a possible switch between peak and trough or an error in the reported time). In the preclinical studies, 21 samples were excluded from the PK analysis out of a total of 1,579 non-BQL plasma concentrations available (1.3%). For the clinical study, some outlier concentrations were also excluded from the analysis. Samples excluded from use in the clinical-model development included subjects who displayed either high trough concentrations or double peaks at 24 h postdose. Subjects with less than two concentration-time data were excluded from the data set (3 subjects in total). The number of excluded concentrations was 1.5% of the total included in the analysis. Overall, the analysis included 1,511 plasma concentrations, including 116 BQL concentrations. In addition, preliminary structural PK models were also used to fit concentration-time data to detect potential outliers based on conditional weighted residuals (CWRES).
Structural PK model development.
Population PK analyses used nonlinear mixed-effect modeling with the program Phoenix NLME (version 6.2.0.416). A basic structural PK model was developed for fitting plasma-concentration-versus-time data for ST-246 obtained from preclinical studies. A 2-compartment model adequately fit the plasma concentration data of ST-246 and was evaluated using diagnostic plots of goodness of fit (GOF) and the related statistical estimators: (i) minimum value of objective function (MOF) and (ii) the difference between the MOF values of a reference PK model and a tested PK model (ΔMOF). The population PK model for healthy humans was developed similarly, using clinical-study concentration data. The effects of demographic covariates (age, race, and sex), size covariates (body weight [wt] and body mass index [BMI]), a treatment covariate (dose), and infection status on population PK parameters (absorption rate constant [Ka], clearance [CL], and central volume of distribution [Vc]) were evaluated by covariate analysis to identify sources of variability. Covariate effect analyses were conducted with the covariate models built upon visual inspection and tested by forward additive and backward elimination approaches. Model validation was performed using model diagnostic plots and related statistical calculations. The final population PK model included covariates of weight on CL and Vc, of infection on CL and Vc, and of dose on relative bioavailability (Frel), Ka, and Vc.
Individual post hoc PK parameters in NHPs.
Rich concentration-time profiles of ST-246 following the first and last doses were simulated with the population PK model based on individual post hoc PK parameters of ST-246 (apparent clearance [CL/F], apparent central volume of distribution [Vc/F], apparent peripheral clearance [Q/F], and apparent peripheral volume of distribution [Vp/F]). Cmin, Cmax, and AUC were calculated from the individual concentration-time profiles using mathematical expressions of concentration-time profiles; the AUC was derived from the trapezoid rule on the simulated rich concentration-time profiles of ST-246. The descriptive statistics (n, arithmetic mean, standard deviation [SD], median, minimum, and maximum) of PK parameters were calculated for each dosing regimen.
Prediction of ST-246 exposure in infected humans.
The infection effect on CL and Vc quantified in NHPs was applied to humans in order to simulate ST-246 PK profiles for infected humans. Based on these simulated ST-246 profiles, dose-normalized exposure parameters (AUC/dose, Cmax/dose, and Cmin/dose) on day 1 and at steady state (day 14) were estimated using noncompartmental analysis.
Prediction of equivalent NHP dose.
The final population PK model developed for NHPs was used to predict ST-246 exposure in infected NHPs receiving doses of ST-246 from 0.3 to 30 mg/kg of body weight (3.6 to 360 mg/m2). Fifty-three NHPs were included in each dose simulation data set, with 250 replicates performed. Noncompartmental analysis was performed on simulated profiles of ST-246 in infected NHPs to determine ST-246 exposure (Cmax, Cmin, and AUC) at day 1 and steady state (i.e., day 14) at each dose. These parameters were compared to the predicted exposure of a 400-mg or 600-mg ST-246 dose administered to infected humans to determine the NHP equivalent dose.
PK/PD survival analysis.
Survival analysis combined the results from studies of ST-246 treatment after MPXV infection of NHPs. The survival analysis included 96 NHPs, 23 of which were placebo treated, with the others treated with ST-246 doses ranging from 0.3 mg/kg to 300 mg/kg. The number of NHPs per treatment group varied from 3 (30 and 100 mg/kg) to 25 (10 mg/kg). Treatment was started on day 3, 4, or 5 after infection with a lethal dose of MPXV (except for three animals treated at 300 mg/kg on day 1 after infection). Most treated NHPs were male (n = 82). NHPs from studies with PK sampling were used to evaluate PK parameter correlation versus time of survival using nonparametric Kaplan-Meier survival analysis.
Kaplan-Meier survival analysis.
Dose-survival was explored using Kaplan-Meier analysis to estimate the probability of survival over time. The temporal survival probabilities were compared between dose groups. For each Kaplan-Meier curve, the proportional-hazard assumption was tested using the Cox proportional-hazard regression model.
ROC analysis.
Receiver operating characteristic (ROC) analysis was used to identify potential predictors of survival. The algorithm identified covariates with a stopping rule of a P value of <0.05. The highest predicting variable is used to divide the sample into two subsamples, and the next predicting variable divides the higher-risk subsample. The process continues until no covariate is significant or there are not enough cases. Variables associated with a P value of >0.05 are not included in the decision tree. As a result, signal detection determines the optimal cutoff point across values of a covariate and all tested covariates. The sensitivity and specificity were estimated as follows: sensitivity = true positive/total number of dead NHPs and specificity = true negative/total number of surviving NHPs.
ROC analysis was performed with equal emphasis placed on both false positive and false negative (r = 0.5) to determine the survival cutoff values of each exposure marker.
Bioanalysis methodology.
Blood samples were collected in tubes containing either sodium or lithium heparin. The validated bioanalytical liquid chromatography-tandem mass spectrometry (LC–MS-MS) method was described previously (21). Briefly, ST-246 concentrations in NHP and human plasma were measured using an LC–MS-MS method. Blank plasma for calibration curves and quality control samples was purchased from Bioreclamation, Inc. (Westbury, NY). Two different extraction methods were used over the course of these studies; direct methanol precipitation of protein was used for all samples infected with MPXV, while the liquid-liquid extraction (LLE) method was used for all uninfected samples. Both methods were validated following the FDA bioanalytical-validation guidelines (22). In one method, the extraction of ST-246 from plasma was carried out by simple protein precipitation by the addition of 9 parts methanol (450 μl) containing the isotopic internal standard to 1 part (50 μl) plasma sample. After high-speed centrifugation, 100 μl of supernatant was added to 200 μl of compensation solution (0.05% acetic acid in 0.05% ammonium hydroxide-methanol [36:55 {vol/vol}]) and directly injected onto the LC–MS-MS instrument. This method was used for all MPXV-infected samples, as methanol was used to inactivate the virus and had to be added prior to removal of the samples to a non-BSL-3 facility.
The second extraction method was an LLE method. Plasma samples were diluted 1:1 with methanol containing an internal standard, and 3 volumes of water was added. These mixtures were vortexed, and the entire volume was transferred to the extraction plate (Biotage SLE; 200 mg). Minimal vacuum was applied to load the samples and then allowed to stand for 5 min. Methyl tertiary-butyl ether was added to all wells (500 μl/well) and eluted with a minimal vacuum. The solvent was evaporated to dryness under nitrogen (set at 50°C and 30 to 40 liters/min). The samples were reconstituted (0.05% acetic acid and 0.05% ammonium hydroxide in methanol-water [65:35 {vol/vol}]) by gently vortexing the plate afterward.
The chromatographic separation was performed using a phenyl-hexyl column (50 by 2.0 mm; 5 μm; Phenomenex) with a Securityguard column, using 0.05% ammonium hydroxide and 0.05% acetic acid in MeOH-H2O ([65:35 {vol/vol}]) at a flow rate of 400 μl/min for the mobile phase. A 3,200 (or 4,000) Qtrap (AB Sciex) mass spectrometer was tuned to the multiple-reaction-monitoring (MRM) mode to monitor the m/z transitions, m/z 375.0/283.2 for ST-246 and m/z 341.1/248.8 for the internal standard, in negative-ion mode. The MS-MS response was 1/x2 weighted linearly over the concentration range from 5.00 to 2,000 ng/ml for NHPs and from 50.0 to 4,000 ng/ml for human samples. The accuracy and precision of the method were within the acceptable limits of ±20% at the lower limit (5.0 ng/ml or 50 ng/ml) of quantitation and ±15% at other concentrations.
In vivo study summaries. (i) NHP studies.
Multiple laboratories conducted NHP studies according to all federal, state, and local guidelines for the use of animals in research. In each case, the studies were reviewed and approved by the respective Institutional Animal Care and Use Committees (IACUC). The IACUC study approval identification (ID) number for study 1 was 1151-065. It was conducted in compliance with the Testing Facility Animal Welfare Assurance (A2181-01) filed with the National Institutes of Health. Study 2 had an IACUC approval ID of FY10-087 and was conducted in compliance with the Testing Facility Animal Welfare Assurance (A3083-01) filed with the National Institutes of Health. Studies 3 through 6 had IACUC study approval ID numbers of AP-06-021G, AP-09-026G, AP-06-021, and AP-06-021E6, respectively. They were all conducted in compliance with the Testing Facility Animal Welfare Assurance (A3473-01) filed with the National Institutes of Health.
Six NHP studies were conducted to determine the NHP pharmacokinetics and pharmacodynamics of ST-246. Of the six studies, five evaluated the efficacy of ST-246 treatment after infection with a lethal dose of MPXV. The dose levels, numbers of animals per dose, and days of treatment initiation varied, but the treatment duration was consistently 14 days in all studies. Infection was by i.v. inoculation with ∼5 × 107 PFU of MPXV/animal. The animals were screened prior to placement in the study utilizing a plaque reduction neutralization titer (PRNT) analysis to ensure all animals randomized into the study were free of detectable MPXV-neutralizing-antibody levels (previously exposed to MPXV). Survival to the end of the study was the primary efficacy endpoint for the studies of MPXV infection.
Study 1 was a good laboratory practice (GLP) pharmacokinetic study of uninfected NHPs (n = 6; 3 males and 3 females per dose group) administered oral doses of ST-246 once a day (QD) at 0.3, 3, 10, 20, and 30 mg/kg. Samples were collected from the femoral artery/vein predose and at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h postdose on days 1 and 7 and predose and at 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20, 24, 36, and 48 h postdose on day 14. The samples were placed in tubes containing sodium heparin anticoagulant. The blood samples were collected on wet ice and centrifuged under refrigeration to collect the plasma, which was then stored frozen at −70°C until analysis.
Study 2 was a GLP study to determine the effect of MPXV infection on ST-246 pharmacokinetics in NHPs (n = 6; 3 males and 3 females/dose group). ST-246 doses of 3, 10, or 20 mg/kg or vehicle was administered beginning 4 days following infection. Monkeys were administered a single dose of ST-246 10 days prior to infection to allow intra-animal comparison of the pharmacokinetics according to infection status. ST-246 was administered in the fed state. Blood was taken for ST-246 concentration determination on treatment days 1 and 7 at 1, 3, 4, 8, 12, 18, and 24 h postdose and on day 14, as well as day −10, at 1, 3, 4, 8, 12, 18, 24, 48, and 72 h postdose. Samples were collected in tubes containing lithium heparin as the anticoagulant. The blood samples were collected on wet ice and centrifuged under refrigeration to isolate the plasma, which was stored frozen at −70°C until analysis.
Study 3 was a GLP double-blind, randomized, placebo-controlled efficacy study of oral ST-246 in NHPs infected with MPXV. ST-246 was administered either 3 or 4 days postinfection at 10 or 20 mg/kg/day (n = 3 males/group). Sparse samples were taken for determination of ST-246 concentrations in the NHPs (21). ST-246 levels were determined at 4 h (peak) and before dosing or 24 h after dosing (trough) on study days 4, 9, and 16 after infection. (As the objective of the collection of these samples was to confirm exposure, the timing of the collection of the sparse samples was solely to minimize any impact on the primary objective of the study, which was survival.)
Study 4 was a GLP study in which treatment was initiated at lesion onset in each NHP. A series of descending doses were evaluated in order to determine the minimum dose at which ST-246 was protective, thus establishing the lowest protective dose for human use. Male monkeys were randomized into 5 treatment cohorts with doses at 0.3, 1, 3, and 10 mg/kg and a placebo group. Each dose cohort contained 5 animals except the placebo dose cohort, which contained 7 animals. A single-dose daily treatment started after the first appearance of lesions and continued for 14 consecutive days. Sparse sampling was done to determine the plasma ST-246 levels in animals on treatment days 1, 7, and 14 at both predose and 4 h postdose. The study duration was 42 days after inoculation.
Study 5 was a randomized, double-blind, vehicle-controlled (5), repeat-dose study of ST-246 monotherapy administered orally in parallel groups. The study varied the daily doses of ST-246 used and treatment onset times for ST-246. There were a total of 23 male monkeys infected in the study, which was conducted in two cohorts. The infected monkeys were randomized to receive one of 5 different oral daily doses of ST-246 (3, 10, 30, 100, and 300 mg/kg/day) or vehicle for 14 days. The vehicle group had 5 monkeys, whereas the 3-, 10-, 30-, and 100-mg/kg/day groups had 3 monkeys each and the 300-mg/kg/day group had 6 monkeys. ST-246 treatment began on day 3 postinfection for all dose groups, including vehicle treated, except that there were 3 monkeys in the 300-mg/kg/day group receiving ST-246 on day 1 postinfection. Sparse samples were collected for bioanalysis.
Study 6 was an observational, parallel-group, placebo-controlled study evaluating the efficacy of ST-246 at 10 mg/kg/day (n = 5, 3 males and 2 females) compared to vehicle (n = 1 male) in MPXV-infected NHPs. Treatment was started on day 5 postinfection.
(ii) Human clinical PK study.
The clinical study used for the development of the human population pharmacokinetic model was designed as a double-blind, randomized, placebo-controlled, multicenter trial in order to evaluate the safety, tolerability, and pharmacokinetics of ST-246 in humans (23). The study procedures were in accordance with the ethical standards of the Helsinki Declaration. Each Institutional Review Board approved all aspects of the study conduct. This clinical study has been registered at the National Library of Medicine (http://www.clinicaltrials.gov). Healthy subjects (107 volunteers) were randomized into three cohorts and received a single daily oral dose of ST-246 at 400 mg (45 subjects) or 600 mg (46 subjects) or placebo (16 subjects) for 14 days in the nonfasted state. The study included healthy volunteers from ages 19 to 74 and had approximately equal numbers of male and female subjects. Comprehensive blood samples were taken on day 1 and day 14, in addition to sparse samples on days 2, 5, 6, 8, 12, and 13 collectively, for complete characterization of the pharmacokinetics of the compound.
RESULTS
NHP POP PK model development.
Exploratory noncompartmental analyses were used initially to evaluate the PK parameters of single and multiple doses of ST-246 in uninfected and infected NHPs. After a single dose, no correlation was observed between the CL/F and dose in either uninfected or infected NHPs. However, the apparent clearance of ST-246 was slightly higher in infected NHPs than in uninfected NHPs. A t test was performed on the PK parameters after single doses of ST-246 showed that dose-normalized AUC values (but not Cmax or time to maximum concentration of drug in serum [Tmax]) were significantly different between uninfected and infected NHPs (1.15 versus 0.9 mg · h/liter/[mg/kg]; P value = 0.025; n = 18/group). However, at steady state, there were no statistically significant differences noted between infected and uninfected NHPs.
Iterative evaluation identified the best POP PK model as a 2-compartment model with correlation between CL/F and Vc/F and a lag time in absorption (Tlag). Body weight was used as an allometric factor [wt/(median wt)θeff] on CL/F, Vc/F, Q/F, and Vp/F, with θeff equal to 0.75 for clearance-related parameters and θeff equal to 1 for volume-related parameters (24). Overall, the proposed two-compartment model demonstrated intraoccasion variability (IOV) for infection status and infection effects on CL/F and on the Ka with covariates of wt on Q/F and Vp/F and weight-adjusted dose on Ka, CL/F, and Vc/F. The observed concentrations versus population and individual predicted plasma concentrations of ST-246 were well distributed around the line of identity. The CWRES values were homogeneously distributed around zero, suggesting no bias in the predictions of high and low concentrations of ST-246. Of the 1,558 plasma concentrations used in the POP PK analysis, no concentration presented |CWRES| values of >4.
Human POP PK model development.
The final human POP PK model was a two-compartment model with allometric factors on PK parameters using wt as a covariate (Table 1), similar to the NHP model. The only differences between the final NHP and human POP PK models were an effect of gender on Vc/F in humans and a modest effect of dose in NHPs. As with the NHP model, the observed concentrations versus population and individual predicted concentrations of ST-246 were well distributed around the line of identity. CWRES values were homogeneously distributed around zero, suggesting no bias in the predictions of high and low concentrations of ST-246. Of the 1,395 plasma concentrations of ST-246 used in the human population PK analysis, no concentration presented |CWRES| values of >4.
Table 1.
Population pharmacokinetic parameters for the 2-compartment model that best fit the pharmacokinetic data for both cynomolgus monkeys (NHPs) and humans in addition to interindividual variability for each parameter and intraoccasion variability for those parameters altered by the infected statea
| POP PK parameter | NHP PK parameter |
Human PK parameter |
||||
|---|---|---|---|---|---|---|
| Status | Population estimate | IIV (%) | IOV (%) | Population estimate | IIV (%) | |
| Ka (h−1) | Infected | 0.868 · (dose/10)0.160 | 11 | 32 | 1.06 | 41 |
| Uninfected | 0.586 · (dose/10)0.160 | |||||
| Tlag (h) | 0.302 | NA | 1.46 | 17 | ||
| CL/F (liters/h) | Infected | 2.809 · (wt/3.105)0.75 · (dose/10)0.093 | 31 | 14 | 41.15 · (wt/78.4)0.75 | 31 |
| Uninfected | 2.827 · (wt/3.105)0.75 · (dose/10)0.093 | |||||
| Vc/F (liters) | 20.054 · (wt/3.105)1 · (dose/10)0.623 | 47 | 281.51 · (wt/78.4)1 (female); 217.44 · (wt/78.4)1 (male) | 28 | ||
| Q/F (liters/h) | 3.244 · (wt/3.105)0.75 | 75 | 36.79 · (wt/78.4)0.75 | 54 | ||
| Vp/F (liters) | 13.34 · (wt/3.105) | 55 | 413.53 · (wt/78.4)1 | 54 | ||
| Error model | ||||||
| Additive (μg/liter) | 0.133 | NA | 10.92 | NA | ||
| Proportional (%) | 30 | NA | 27 | NA | ||
In both species, weight was a covariate, whereas in humans, gender was a covariate for Vc/F, but not in NHPs. Infection was a covariate for both the Ka and CL/F, but the overall change in exposure was small. Dose, dose level in mg/kg; IIV, interindividual variability; IOV, intraoccasion variability; NA, not available.
For a healthy human with a body weight equivalent to the mean weight from the clinical study used in development of the POP PK model, 78.4 kg, the typical values of CL/F would be 41.15 liters/h (987.6 liters/day), and Vc/F in females and males would be 281.51 liters and 217.44 liters, respectively (Table 1). The terminal elimination half-lives in male and female subjects were similar (16.7 h in males versus 17.4 h in females). Consequently, no clinically significant sex effect was predicted for ST-246 exposure or the terminal elimination half-life.
Bridging the effect of infection on ST-246 pharmacokinetics to humans.
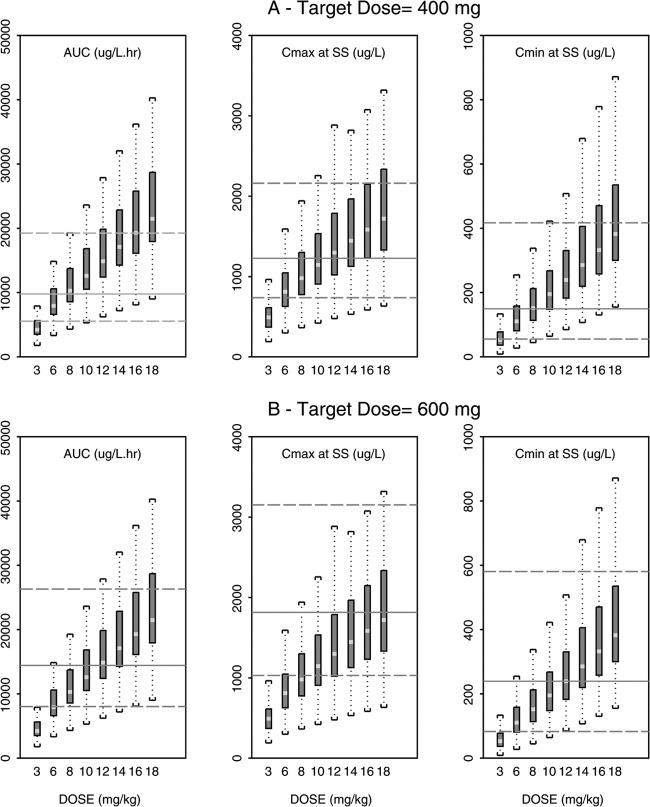
The observed effect of infection in NHPs was applied to the human POP PK model to predict pharmacokinetic parameters (AUC, Cmax, and Cmin) for orthopoxvirus-infected humans. Simulated PK parameters (AUC, Cmax, and Cmin) for infected NHPs over a range of discrete doses that encompassed the range used in efficacy evaluations (3.6 to 360 mg/m2, 0.3 to 30 mg/kg) were then overlaid using boxplots on the same parameters predicted for orthopoxvirus-infected humans after doses of either 400 or 600 mg daily (Fig. 1A and B). Doses of 96 to 120 mg/m2 (8 to 10 mg/kg) and 144 to 168 mg/m2 (12 to 14 mg/kg) administered to infected NHPs resulted in exposure (AUC) values for ST-246 equivalent to 400- and 600-mg doses predicted for infected humans, respectively. This was observed for day 1 and at steady state. The median predicted Cmax for infected humans was slightly higher than that observed in NHPs. The median predicted Cmin, however, was slightly lower in humans than in NHPs at exposure-equivalent doses on day 1 but equivalent at steady state (Fig. 1A and B). The NHP 3-mg/kg dose was predicted to be equivalent to a 100-mg dose in a 78.4-kg human, the mean weight from the clinical study used to develop the human POP PK model.
Fig 1.
Comparison of predicted AUC, Cmax, and Cmin for orthopoxvirus-infected humans and NHPs based on the population PK model of infected monkeys. (A and B) Steady-state values predicted for AUC, Cmax, and Cmin in infected humans after an ST-246 dose of 400 mg (A) or 600 mg (B). The solid line is the median predicted value for infected humans for each plot, while the dashed lines represent the 5th and 95th percentile values simulated for an infected human population. The box plots (bottom and top brackets are minimum and maximum values for each box plot, light line is median, and dark portion is the 25th to 75th percentage for simulated population) showing values that were simulated for NHP values at discrete doses from 3 mg/kg to 18 mg/kg ST-246 are overlaid on the corresponding human parameter values. The y-axis parameter and units are shown at the top of each individual plot.
Survival analysis after treatment with ST-246.
Among the 96 NHPs evaluated for the survival analysis, 73 received nonplacebo doses ranging from 0.3 to 300 mg/kg. Thirty-four NHPs died or were euthanized before the end of the study; most of these animals were administered placebo (n = 23; 100% death), 0.3 mg/kg ST-246 (n = 5; 80% death), and 1 mg/kg ST-246 (n = 5; 100% death). In one study, one animal died at a dose level of 3 mg/kg 5 days postinfection after having received one dose of ST-246. Overall, the survival rate for the 3-mg/kg treatment group was 92.9% (13 out of 14 animals). At the 10-mg/kg dose, one animal died 11 days after infection. The overall survival rate for the 10-mg/kg dose group was 96% (24 out of 25 animals). Although the histopathology report suggested that the two animals that had received doses of 3 mg/kg and 10 mg/kg did not have MPXV infection severe enough to be consistent with a cause of death (lack of necrotic lesions throughout the body), all animals were included in the initial dose-survival analysis.
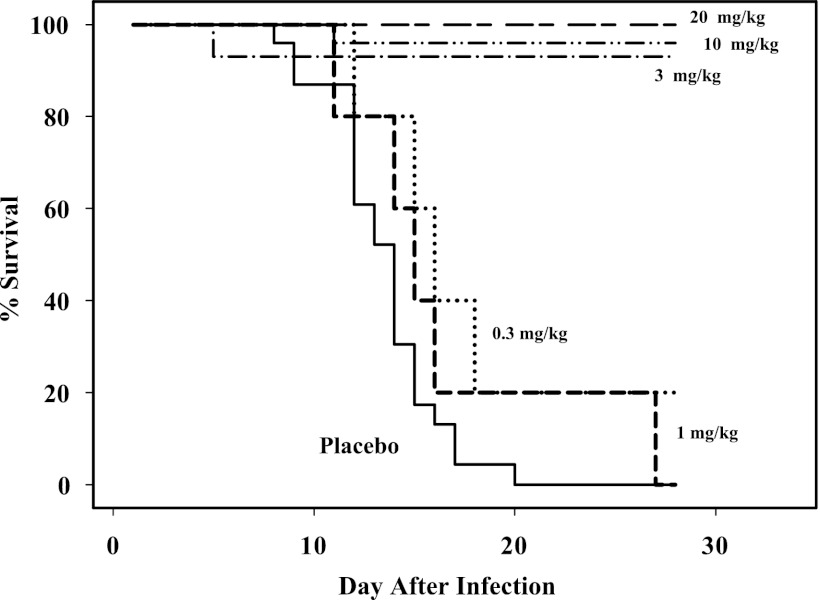
The dose-survival relationship was explored using the Kaplan-Meier approach (Fig. 2). For dose levels that were associated with death, 35 NHPs started their treatment on day 4, 9 started their treatment on day 3, and 5 NHPs started treatment on day 5. Due to small numbers of day 3 and 5 treatment initiation animals, no analysis could be done to explore the effect of “day of treatment start.” The exploratory Kaplan-Meier survival probability for doses of 1 mg/kg and lower demonstrated a large differential in the survival probability for doses of 1 mg/kg and lower compared to that for 3 mg/kg and higher. A Cox proportional-hazard model was then fitted to the data to conduct statistical tests. This Cox proportional-hazard assumption was tested with and without the single monkeys that died at each of the 3- and 10-mg/kg doses (25). Using the categorical dose variable analysis (doses grouped as ≥3 mg/kg and ≤1 mg/kg), there was evidence of nonproportional hazard at dose levels of 3 mg/kg and higher (P = 0.0016; n = 96) when all animals were included. However, the proportional-hazard assumption was not rejected when the lone two animals that died at higher doses were excluded from the analysis (P = 0.954; n = 94). Similar results were obtained with continuous dose variable analysis (0 to 300 mg/kg; all animals, P < 0.0000845, n = 96; excluding the same two animals, P = 0.806, n = 94).
Fig 2.
Kaplan-Meier survival curve for efficacy studies in NHPs using 14 days of ST-246 treatment. Shown is combined survival analysis of NHPs treated QD for 14 days at doses ranging from 0.3 to 20 mg/kg ST-246 starting either 3, 4, or 5 (10-mg/kg dose group only) days postinfection with a lethal dose of MPXV. The median time to death for placebo-treated animals (n = 23) was 14 days after infection. Doses above 20 mg/kg had 100% survival and are not shown.
A nonparametric Kaplan-Meier survival analysis using exposure levels (AUC/wt), as well as Cmin and Cminss levels, confirmed increased survival at levels that corresponded to doses at and above 3 mg/kg. When the two lone animals that died at doses above 3 mg/kg were excluded, the probability of survival was 100% for all animals with exposure above 0.5 mg · h/liters · kg and Cmin and Cmin at steady state (Cminss) values of 40 μg/liter.
ROC analysis.
Recursive ROC analysis was conducted in order to identify the potential cutoff points for dose, exposure level, or Cmin that best correlated with survival. This analysis identifies an optimal cutoff, determined based on statistical criteria, that optimizes both sensitivity (the probability of being a responder among those really responding; true positive/total number of dead NHPs) and specificity (the probability of being a nonresponder among true nonresponders; true negative/total number of surviving NHPs). In particular, both false positives and false negatives can be identified. In developing a compound under the animal rule that has a high potential for mortality, such as smallpox, a distinction between false positives and negatives is critical.
Selection of the efficacious dose from animal data.
The markers of exposure that were analyzed were dose, AUC/wt, Cmin after first dose (Cmin1), and Cmin at steady state (Cminss). The ROC analysis was first conducted using all animals from efficacy studies (n = 96) to evaluate the optimal cutoff point for dose and survival. The results confirmed that doses of 3 mg/kg and above were associated with survival while doses lower than 3 mg/kg did not provide any survival benefit over placebo-treated NHPs. The second ROC analysis was conducted with the subset of NHPs for which some concentration data were available. Using post hoc calculations to estimate PK parameters, these results were consistent with the Kaplan-Meier dose-survival analysis, showing that doses at and above 3 mg/kg, as well as the corresponding AUC/wt [0.5 mg · h/(liters · kg)] (Fig. 3), Cmin1 (21.63 μg/liter), and Cminss (21.22 μg/liter), were associated with maximum survival, whereas doses that resulted in corresponding lower values for AUC/wt, Cmin1, and Cminss had very low survival rates. The two animals that died at the 3- and 10-mg/kg doses were identified as false negatives by this ROC analysis.
Fig 3.
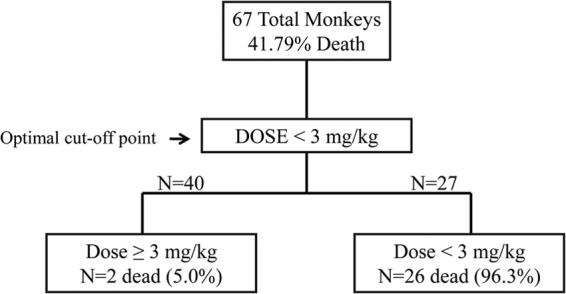
Receiver operator characteristic analysis of NHP ST-246 treatment using exposure levels as the cutoff parameter. ROC analysis of NHP treatments versus survival outcome versus the exposure determined that exposure equal to or greater than 0.50 mg · h/(liters/kg) was the cutoff value for survival for the 67 NHPs with sensitivity and specificity values of 92.86% and 97.44%, respectively. The ROC tree shows the number of NHPs in the analysis, along with the number of NHPs that died in the two groups.
Direct comparison of human and NHP dose levels.
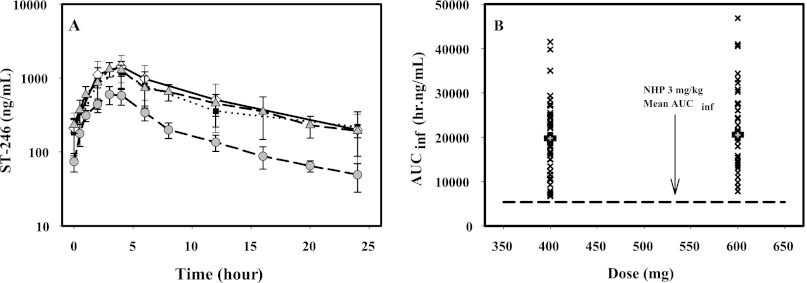
The POP PK model determined that the effect of MPXV infection on the pharmacokinetics of ST-246 in NHPs was limited. This established the validity of directly comparing uninfected human and NHP pharmacokinetic data. ST-246 plasma concentration-time curves at steady state for healthy humans after 400- and 600-mg doses (23) and 3 and 10 mg/kg in uninfected NHPs showed that the 400-mg human plasma concentration-time curve was essentially identical to the 10-mg/kg NHP curve, while that for the 600-mg dose was slightly higher (Fig. 4A). Concentrations at all time points for both human doses were substantially higher than those of the lowest efficacious dose for NHPs, 3 mg/kg. Examination of the area under the curve extrapolated to infinity (AUCinf) values for individuals at steady state for both the 400- and 600-mg doses in healthy volunteers showed that they were all above the mean value (5,370 h · ng/ml) for NHPs at the 3-mg/kg dose (Fig. 4B).
Fig 4.
(A) Plasma concentration-time curves for uninfected humans and NHPs over a range of doses relevant to efficacious-dose selection. Shown is a semilogarithmic graph of steady-state plasma concentration-time curves for 400-mg and 600-mg doses in healthy humans compared to 3-mg/kg and 10-mg/kg doses in uninfected NHPs. The means and standard deviations of the means are plotted for all curves. The NHP lines are dashed, while the lines for the human values are dotted (400 mg) and solid (600 mg). (B) Comparison of individual exposure levels in uninfected humans at two doses compared to the lowest efficacious dose in NHPs. Individual (×) and mean (solid squares) AUCinf values at steady state for 400- and 600-mg doses of ST-246 are shown. The black dashed line shows the AUCinf value at steady state for the 3-mg/kg dose in uninfected NHPs.
DISCUSSION
A key challenge in the development of medical countermeasures against smallpox is the selection of the human dose that would provide a high likelihood of survival. While allometric scaling is often used to select the initial human dose (26, 27), with efficacy doses based on subsequent human trials, the final efficacious dose for medical countermeasures against bioterrorism agents must be based on efficacy evaluation in animal models alongside human safety data (28, 29). Since smallpox is a uniquely human disease, surrogate animal models were used to establish the efficacy of antiviral therapeutics. Intravenous infection of NHPs with monkeypox virus produces a severe systemic infection that resembles many aspects of human smallpox. Unlike human smallpox, however, infection by this route is uniformly lethal, providing a primary endpoint (survival) relevant to the human disease.
A critical element in the “animal rule” requires that information on the pharmacokinetics and pharmacodynamics in animals and pharmacokinetics in humans allow selection of the human effective dose. For ST-246, the development of human and NHP POP PK models confirmed a high degree of similarity of PK between humans and NHPs. Neither the small gender effect in humans nor the effect of dose on NHP PK parameters had a substantial impact on the pharmacokinetics of ST-246 in humans or NHPs over the range of interest for efficacy evaluation.
Studies of the MPXV infection of NHPs require biosafety level 3 containment and, as such, limit the number of animals that can be evaluated. Because of these constraints, the primary PK determinant for efficacy of ST-246 in the treatment of orthopoxvirus diseases (5, 9) remains unknown, so predicted values for the AUC, Cmax, and Cmin were all compared to determine equivalence between human and NHP doses. Both simulated PK parameters for the infected state (Fig. 1A) and direct comparison of the plasma concentration-time curves (Fig. 4A and B) indicated equivalence of the 10-mg/kg NHP dose with the 400-mg dose of ST-246. Also, both methods showed that the lowest 5th percentile of the predicted or actual PK parameter in humans after oral administration of 400 mg was higher than the mean or median value of the lowest effective dose in NHPs, 3 mg/kg (Fig. 1A and 4B). The similarity of the uninfected plasma concentration time curves and the modest effect of infection on the PK of ST-246 provided confidence in this analysis to select an efficacious dose.
Combined pharmacodynamic analysis from five MPXV efficacy studies identified the minimum efficacious dose in NHPs as 3 mg/kg administered starting at 3 or 4 days after MPXV infection. This dose level was predicted to be equivalent to 100 mg administered to an orthopoxvirus-infected human having a mean weight of 78.4 kg. The selection of the final efficacious dose, however, must take into account additional factors. First, the human dose for smallpox will not be weight based and should therefore be determined to be effective for the entirety of the U.S. population (30). In addition, as smallpox infection had a high rate of mortality and morbidity, use of the minimum efficacious dose cannot be justified if the therapeutic index allows selection of a higher safe dose. Exposure to ST-246 in infected humans after administration of 400 or 600 mg was predicted to be higher for 95% of the population than the median exposure in infected NHPs after administration of the 3-mg/kg dose (Fig. 1A). In addition, comparisons of observed exposures demonstrate that all subjects administered either 400 or 600 mg had exposures above the mean exposure in NHPs after the 3-mg/kg dose (Fig. 4B). The 600-mg dose provided slightly higher exposure with apparently equivalent safety. Thus, the 600-mg dose may be most appropriate for the entire adult U.S. population. Since the 3-mg/kg dose was identified as the lowest efficacious dose using both Kaplan-Meier and ROC analyses, exposures higher than those obtained at this dose should be sufficient to protect more than 95% of the human population against disease due to orthopoxvirus infection.
ACKNOWLEDGMENTS
This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contracts no. HHSN27220800041C and HHSN266200600014C.
Shanthakumar R. Tyavanagimatt and Dennis E. Hruby are employees and stockholders of SIGA Technologies, and Rob Jordan is a former employee.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. Fenner F. 1980. The global eradication of smallpox. Med. J. Aust. 1:455. [DOI] [PubMed] [Google Scholar]
- 2. Fenner F, Henderson DA, Arista I, Jerzek Z, Ladnyi ID. 1988. The clinical features of smallpox. Smallpox and its eradication. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Dixon CW. 1962. Smallpox. J. & A. Churchill Ltd., London, United Kingdom [Google Scholar]
- 4. Hanna W, Baxby D. 2002. Studies in smallpox and vaccination. 1913. Rev. Med. Virol. 12:201–209 [DOI] [PubMed] [Google Scholar]
- 5. Jordan R, Goff A, Frimm A, Corrado ML, Hensley LE, Byrd CM, Mucker E, Shamblin J, Bolken TC, Wlazlowski C, Johnson W, Chapman J, Twenhafel N, Tyavanagimatt S, Amantana A, Chinsangaram J, Hruby DE, Huggins J. 2009. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 53:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nalca A, Hatkin JM, Garza NL, Nichols DK, Norris SW, Hruby DE, Jordan R. 2008. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antivir. Res. 79:121–127 [DOI] [PubMed] [Google Scholar]
- 7. Sbrana E, Jordan R, Hruby DE, Mateo RI, Xiao SY, Siirin M, Newman PC, Da Rosa AP, Tesh RB. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768–773 [PubMed] [Google Scholar]
- 8. Smith SK, Self J, Weiss S, Carroll D, Braden Z, Regnery RL, Davidson W, Jordan R, Hruby DE, Damon IK. 2011. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J. Virol. 85:9176–9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, DeClercq E, Jones K, Hruby D, Jordan R. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quenelle DC, Prichard MN, Keith KA, Hruby DE, Jordan R, Painter GR, Robertson A, Kern ER. 2007. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 51:4118–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huggins J, Goff A, Hensley L, Mucker E, Shamblin J, Wlazlowski C, Johnson W, Chapman J, Larsen T, Twenhafel N, Karem K, Damon IK, Byrd CM, Bolken TC, Jordan R, Hruby D. 2009. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53:2620–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jahrling PB, Hensley LE, Martinez MJ, Leduc JW, Rubins KH, Relman DA, Huggins JW. 2004. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. U. S. A. 101:15196–15200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahl-Jensen V, Cann JA, Rubins KH, Huggins JW, Fisher RW, Johnson AJ, de Kok-Mercado F, Larsen T, Raymond JL, Hensley LE, Jahrling PB. 2011. Progression of pathogenic events in cynomolgus macaques infected with variola virus. PLoS One 6:e24832 doi:10.1371/journal.pone.0024832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith SK, Olson VA, Karem KL, Jordan R, Hruby DE, Damon IK. 2009. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 53:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson RF, Yellayi S, Cann JA, Johnson A, Smith AL, Paragas J, Jahrling PB, Blaney JE. 2011. Cowpox virus infection of cynomolgus macaques as a model of hemorrhagic smallpox. Virology 418:102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marriott KA, Parkinson CV, Morefield SI, Davenport R, Nichols R, Monath TP. 2008. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine 26:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, Schmaljohn CS, Schmaljohn AL, Jahrling PB. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker S, Nuara A, Buller RM, Schultz DA. 2007. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2:17–34 [DOI] [PubMed] [Google Scholar]
- 19. Jordan R, Chinsangaram J, Bolken TC, Tyavanagimatt SR, Tien D, Jones KF, Frimm A, Corrado ML, Pickens M, Landis P, Clarke J, Marbury TC, Hruby DE. 2010. Safety and pharmacokinetics of the antiorthopoxvirus compound ST-246 following repeat oral dosing in healthy adult subjects. Antimicrob. Agents Chemother. 54:2560–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Amantana A, Tyavanagimatt SR, Zima D, Yan XS, Kasi G, Weeks M, Stone MA, Weimers WC, Samuel P, Tan Y, Jones KF, Lee DR, Kickner SS, Saville BM, Lauzon M, McIntyre A, Honeychurch KM, Jordan R, Hruby DE, Leeds JM. 2011. Comparison of the safety and pharmacokinetics of ST-246(R) after i.v. infusion or oral administration in mice, rabbits and monkeys. PLoS One 6:e23237 doi:10.1371/journal.pone.0023237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. FDA 2001. Guidance for industry: bioanalytical method validation. FDA, Washington, DC [Google Scholar]
- 23. Chinsangaram J, Honeychurch KM, Tyavanagimatt SR, Leeds JM, Bolken TC, Jones KF, Jordan R, Marbury T, Ruckle J, Mee-Lee D, Ross E, Lichtenstein I, Pickens M, Corrado M, Clarke JM, Frimm AM, Hruby DE. 2012. Safety and pharmacokinetics of the anti-orthopoxvirus compound ST-246 following a single daily oral dose for 14 days in human volunteers. Antimicrob. Agents Chemother. 56:4900–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holford NH. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329–332 [DOI] [PubMed] [Google Scholar]
- 25. Grambsch PM, Therneau TM. 1994. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526 [Google Scholar]
- 26. FDA 2005. Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. FDA, Washington, DC. [Google Scholar]
- 27. Boxenbaum H, DiLea C. 1995. First-time-in-human dose selection: allometric thoughts and perspectives. J. Clin. Pharmacol. 35:957–966 [DOI] [PubMed] [Google Scholar]
- 28. Aebersold P. 2012. FDA experience with medical countermeasures under the animal rule. Adv. Prev. Med. 2012:507571 doi:10.1155/2012/507571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135–144 [DOI] [PubMed] [Google Scholar]
- 30. Bergman KL. 2009. The animal rule and emerging infections: the role of clinical pharmacology in determining an effective dose. Clin. Pharmacol. Ther. 86:328–331 [DOI] [PubMed] [Google Scholar]