Abstract
Polymyxins are old antimicrobials, discontinued for many years because of nephrotoxicity and neurotoxicity reports and reintroduced recently due to the increasing frequency of multiresistant Gram-negative bacterial infections. There are very few data related to toxicity and efficacy from transplanted patients, the major subjects of this study. All solid-organ-transplanted patients from our institution during January 2001 to December 2007 who used polymyxins were retrospectively assessed for nephrotoxicity and treatment efficacy. Microbiological and clinical cure rates were 100% and 77.2%, respectively. Only transplant patients subjected to at least 72 h of intravenous polymyxin were entered in the study. Overall, 92 transplant patients were included, and the nephrotoxicity rate was 32.6%. Multivariate analysis showed a statistically significant association between duration of polymyxin treatment (P = 0.037; odds ratio [OR], 1.06; 95% confidence interval [CI], 1.00 to 1.12) and significant renal dysfunction. Polymyxin use is associated with very high rates of significant decrease in renal function; therefore, polymyxin must be used only when no other option is available and for as briefly as possible in the solid organ transplant setting.
INTRODUCTION
Polymyxins are old antimicrobials, discovered in the 1940s (1), and their clinical use started in the late 1960s (2), acting against Gram-negative bacteria such as Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli (1). Nephrotoxicity and neurotoxicity reports (3–6) and the development of new broad-spectrum and less toxic anti-Gram-negative-organism agents such as cephalosporins and carbapenems led to a brief discontinuation of polymyxin use. However, since the 1990s, an increasing number of reports regarding the emergence of multidrug-resistant Gram-negative bacteria, mainly A. baumannii and P. aeruginosa, have been published (7). As a result, polymyxins have reemerged as an alternative against such multiresistant organisms.
Although recent studies have disclosed lower nephrotoxicity rates than those in earlier reports, toxicity is still a major concern and a reason for not employing this class of drugs (8, 9). Renal transplant patients are more likely to develop renal toxicity by different mechanisms (10), and renal dysfunction is adversely related to organ and recipient survival (11). On the other hand, due to the severity of the patients' conditions and the frequent use of antimicrobials, Gram-negative bacterial multiresistance is a major issue in hematopoietic stem cell transplantation and in solid organ transplantation (12–14), showing the ever-increasing need for polymyxin employment in this setting. Very few data regarding polymyxin toxicity, especially nephrotoxicity, are available for transplant patients. Our study aimed at analyzing nephrotoxicity rates and the clinical and microbiological efficacy of polymyxin in solid organ transplant patients.
MATERIALS AND METHODS
Objectives.
The objectives of this study were to evaluate nephrotoxicity rates and independent risk factors for nephrotoxicity in solid organ transplant patients receiving polymyxin, as well as to describe the clinical and laboratorial efficacy of this antimicrobial in this particular population.
Patients and methods.
All medical records from solid organ transplant patients were reviewed. Patients 18 years or older, who were treated with polymyxin from January 2001 to December 2007 for at least 72 h and with no need for dialysis for the first 48 h of treatment, were included.
This study was conducted in 2 university-affiliated-hospitals in São Paulo, Brazil, Hospital São Paulo and Hospital do Rim e Hipertensão. The former is a 700-bed general hospital, and the latter is a 100-bed hospital specializing in renal transplantation.
The study was approved by the hospital ethics commission.
Demographic data.
We reviewed demographic data for donors, recipients, transplantation procedure, polymyxin use, nephrotoxic drug use 15 days before and/or during polymyxin use, renal function, and infection.
Main definitions.
Infections were defined according to the clinical judgment of assistant physicians as depicted in medical records. Basal serum urea and creatinine were defined as urea and creatinine values before polymyxin use. Multiresistant bacteria were defined as pathogens that were resistant to carbapenems. Microbiologic cure was defined as clearance in subsequent cultures of a pathogen initially isolated before polymyxin use. Cure or clinical improvement was defined as total or partial resolution of signs and symptoms of infection at the end of polymyxin treatment. We considered death for any cause after 30 days of polymyxin B use and total hospital mortality rate after polymyxin B use. Potentially concomitant nephrotoxic drugs were vancomycin, amphotericin B, ganciclovir, and calcineurin inhibitors. Polymyxin infusion time is the time necessary for complete infusion in a single infusion.
Polymyxin dose and adjustment for renal correction (1).
The polymyxin dose employed was 1.5 g to 2.5 g (1 g = 10,000 IU) per kg of body weight per day, and adjustment for renal function was as follows for polymyxin B: creatinine clearance (CLCR) of >80 ml/min, 1.5 to 2.5 mg/kg/day; CLCR of 30 to 80 ml/min, loading dose of 2.5 mg/kg/day on the first day and then 1.0 to 1.5 mg/kg/day; and CLCR of <30 ml/min, loading dose of 2.5 mg/kg/day on the first day and then 1.0 to 1.5 mg/kg/day every 2 to 3 days. For anuric patients, the loading dose was 2.5 mg/kg/day on the first day and then 1.0 to 1.5 mg/kg/day every 5 to 7 days.
Renal dysfunction criteria (15).
The creatinine clearance rate was calculated using the equation of Cockcroft and Gault (16). In patients with normal renal function (serum creatinine level, <1.2 mg/dl), renal failure was defined as a serum creatinine value of >2 mg/dl, as a reduction in the calculated creatinine clearance of 50% relative to the value at antibiotic therapy initiation, or as a decline in renal function that resulted in the need for renal replacement therapy (i.e., intermittent hemodialysis or continuous venovenous hemofiltration). In patients with preexisting renal dysfunction, renal failure was defined as an increase of 50% over the baseline creatinine level, as a reduction in the calculated creatinine clearance of 50% relative to the value at antibiotic therapy initiation, or as a decline in renal function that resulted in the need for renal replacement therapy.
Microbiologic method.
Antibiotic susceptibilities were determined by disc diffusion and microdilution broth methods according to CLSI (Clinical and Laboratory Standards Institute) standards.
Statistical analysis.
For the evaluation of risk factors for polymyxin nephrotoxicity, we compared groups with and without renal dysfunction at any moment during polymyxin use according to renal dysfunction criteria.
Univariate risk factor analysis for categorical variables was performed using Pearson's chi-square analysis (χ2), or Fisher's exact test (FET) when the supposition for χ2 application was not satisfied. We performed the Student t test for normal-distribution continuous variables, and we performed the Mann-Whitney test for non-normal-distribution variables.
For multivariate analysis, the multiple nonconditional logistic regression model with stepwise variable selection to identify independent risk factors was employed. All significant probabilities presented were bilateral type, and values lower than 0.05 were considered statistically significant. Statistical analysis of data was performed with SPSS software version 16.0 (Statistical Package for the Social Sciences software; Chicago, IL).
RESULTS
We retrospectively reviewed medical records from 94 solid organ transplant patients who used polymyxin (polymyxins B and E) according to study inclusion criteria. Two patients were excluded because they needed dialysis within 48 h of polymyxin use. Therefore, 92 patients were included in the final analysis. The majority of the patients were renal transplants (83.7%), and 70.7% of them received organs from deceased donors. The mean age was 47 ± 14.4 years (range, 20 to 72 years), and 57 patients were males (62%), with a medium hospital stay length of 75.1 days (range, 3 to 889 days). Other demographic data are presented in Table 1. We considered only the first polymyxin course of treatment (90 patients with polymyxin B and 2 patients with polymyxin E). Polymyxin B was used for a mean of 16.6 days (range, 3 to 46 days). Mean and median polymyxin B doses employed were 922,282 and 1,000,000 IU/day, respectively. For statistical analysis related to drug dose, time of infusion, and length of treatment, we considered only polymyxin B courses.
Table 1.
Demographic data for 92 solid organ transplant patients treated with polymyxin
| Variable | Mean or no. of patients | Range or % |
|---|---|---|
| Mean recipient age (yr) | 47 | 20–72 |
| Mean donor age (yr) | 42 | 4–71 |
| Mean total hospital stay (days) | 75.1 | 3–889 |
| Gender (male/female) | 57/35 | 62/38 |
| Location where polymyxin prescribed | ||
| Medical-surgical ward | 77 | 83.7 |
| ICU | 15 | 16.3 |
| Transplantation center | ||
| Hospital do Rim e Hipertensão | 71 | 77.2 |
| Hospital São Paulo | 21 | 22.8 |
| Organ(s) transplanted | ||
| Kidney | 77 | 83.7 |
| Kidney-pancreas | 6 | 6.5 |
| Liver | 8 | 8.7 |
| Heart | 1 | 1.1 |
| Immunosuppressant at time of polymyxin use | 79 | 85.9 |
| Prednisone | 69 | 75 |
| Hydrocortisone | 2 | 2.2 |
| Dexamethasone | 1 | 1.1 |
| Tacrolimus | 27 | 29.3 |
| Mycophenolate mofetil | 32 | 34.8 |
| Azathioprine | 10 | 10.9 |
| Cyclosporine | 27 | 29.3 |
| Rapamycin | 3 | 3.3 |
Of all infections, 83.7% were microbiologically confirmed. The major type of infection was urinary tract infection (UTI) (41.3%), followed by surgical site infection (SSI) (17.4%), pneumonia (16.3%), primary bloodstream infection (5.4%), intra-abdominal infection and soft tissue infection (2.2% each), and bloodstream infection transmitted from the donor (1.1%); 8.7% of patients were treated empirically with no source of infection detected. P. aeruginosa was the main etiologic agent, at 76.1% of isolates; 5.4% of isolates were A. baumannii, 1.1% each were K. pneumoniae and Enterobacter sp., and for 16.3% of patients, the etiologic agent was not recovered. Microbiological cure was observed in 25 patients (100%) (24 urine cultures from recipients and one blood culture from a donor, all of them kidney transplant patients), clinical cure was observed in 71/92 patients (77.2%), and hospital mortality occurred in 21/92 patients (22.8%) (Table 2).
Table 2.
Outcome and nephrotoxicity data for 92 solid organ recipients who received polymyxin therapy
| Variable | No. of patients or mean | % or range |
|---|---|---|
| Hospital mortality | 21 | 22.8 |
| 30-day mortality | 6 | 6.5 |
| Microbiological cure | 25a | 100 |
| Clinical cure | 71 | 77.2 |
| Graft loss in renal transplant | 5 | 5.4 |
| Acute pyelonephritis | 1 | 1.1 |
| Acute rejection | 1 | 1.1 |
| Chronic dysfunction | 3 | 3.3 |
| Mean serum creatinine level before polymyxin (mg/dl) | 2.22 | 0.7–9.6 |
| Mean days to nephrotoxicity | 11 | 3–24 |
| Nephrotoxicity | 30 | 32.6 |
| Hemodialysis during therapy | 15 | 16.3 |
Data were available for 25 patients with a culture result before polymyxin use. Twenty-four results were urine cultures, and for 1 patient, we examined a positive donor blood culture that did not become positive in the recipient.
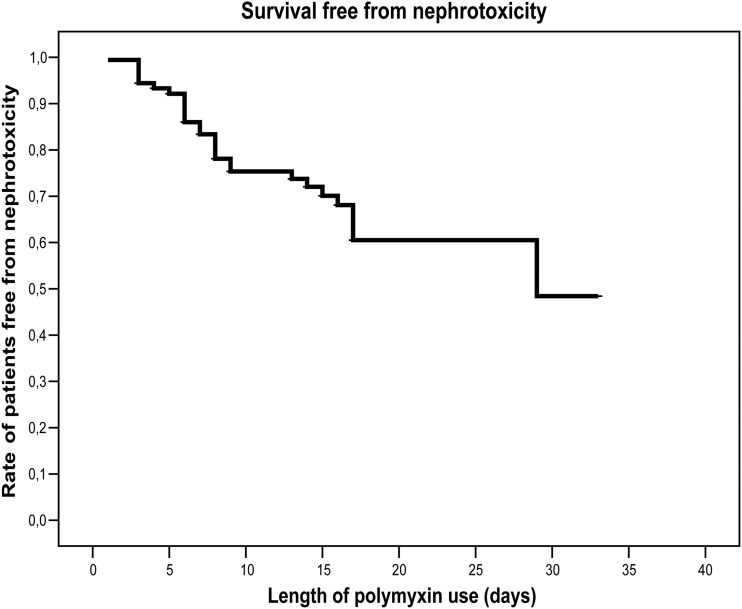
Thirty patients (32.6%) developed nephrotoxicity; the mean time for renal dysfunction development was 11 days (range, 3 to 24 days). Nephrotoxicity rates were 25%, 30%, and 51% on days 9, 16, and 29, respectively, after polymyxin was started (Fig. 1). Fifteen patients (16.3%) required dialysis during polymyxin treatment. Graft loss after polymyxin use was recorded for five patients (5.4%) (Table 2).
Fig 1.
Survival free from nephrotoxicity in 92 solid organ transplant patients who received polymyxin therapy.
Using our criteria as the standard, by multivariate analysis, the mean duration of polymyxin treatment (P = 0.037; odds ratio [OR], 1.06; 95% confidence interval [CI], 1.00 to 1.12) was independently associated with renal failure (Table 3).
Table 3.
Univariate and multivariate analysis of nephrotoxicity risk factors of 92 solid organ transplant patients
| Variable | Univariate analysis |
Multivariate analysis, P; OR (95% CI) | ||
|---|---|---|---|---|
| Nephrotoxicity | No nephrotoxicity | P; OR (95% CIa) | ||
| Deceased donor, no. (%) | 22 (73.3) | 43 (69.4) | 0.69; 1.22 (0.46–3.21) | |
| Donor mean age (yr) | 42.5 | 42 | 0.87; 1.00 (0.97–1.04) | |
| Recipient mean age (yr) | 47.5 | 50 | 0.73; 1.01 (0.97–1.04) | |
| Previous induction, no. (%) | 19 (63.3) | 28 (45.2) | 0.10; 2.10 (0.86–5.14) | |
| Previous rejection, no. (%) | 12 (40) | 16 (25.8) | 0.17; 1.92 (0.76–4.84) | |
| Organ transplanted, no. (%) | ||||
| Kidney | 23 (76.7) | 54 (87.1) | 0.21; 0.49 (0.16–1.50) | |
| Kidney/pancreas | 2 (3.2) | 4 (13.3) | 0.08; 4.62 (0.80–26.8) | |
| Liver | 3 (10) | 5 (8.1) | 0.76; 1.27 (0.28–5.69) | |
| Nephrotoxic drug use 15 days before and/or during polymyxin administration, no. (%) | 29 (96.7) | 55 (88.7) | 0.23; 3.69 (0.43–31.5) | |
| Cyclosporine | 9 (30) | 17 (27.4) | 0.80; 1.13 (0.43–2.96) | |
| Tacrolimus | 10 (33.3) | 18 (29) | 0.87; 1.08 (0.42–2.80) | |
| Vancomycin | 24 (80) | 29 (46.8) | 0.004; 4.55 (1.63–12.7) | 0.07; 2.8 (0.9–8.68) |
| Amphotericin B | 3 (10) | 5 (8.1) | 0.76; 1.27 (0.28–5.69) | |
| Ganciclovir | 5 (16.7) | 11 (17.7) | 0.90; 0.93 (0.29–2.96) | |
| Mean daily polymyxin dose (IU)b | 931,667 | 917,742 | 0.88; 1.0 (1.0–1.0) | |
| Polymyxin use, mean time (days)b | 19.5 | 15.2 | 0.025; 1.06 (1.01–1.1) | 0.037; 1.06 (1.00–1.12) |
| Polymyxin infusion time < 12 h, no. (%)b | 10 (34.5) | 10 (16.4) | 0.058; 2.68 (0.97–7.46) | |
| Mean serum creatinine level before polymyxin (mg/dl) | 1.87 | 2.39 | 0.125; 0.74 (0.50–1.09) | |
| Type of infection, no. (%) | ||||
| UTI | 6 (20) | 32 (51.6) | 0.005; 0.23 (0.08–0.65) | 0.053; 0.32 (0.10–1.01) |
| SSI | 8 (26.7) | 8 (12.9) | 0.11; 2.46 (0.82–7.36) | |
| Pneumonia | 6 (20) | 9 (14.5) | 0.51; 1.47 (0.47–4.60) | |
| ICU, no. (%) | 15 (50) | 20 (32.3) | 0.10; 2.10 (0.86–5.12) | |
| SOFAc score | 7.4 | 9.15 | 0.29; 0.92 (0.80–1.07) | |
| Presence of shock, no. (%) | 6 (9.7) | 5 (16.7) | 0.34; 1.87 (0.52–6.69) | |
CI, confidence interval.
For these analyses, data were available for 90 patients receiving polymyxin B, excluding two patients receiving polymyxin E.
SOFA, sequential organ failure assessment.
DISCUSSION
Recent studies suggest that polymyxin nephrotoxicity does not occur at a level as high as was initially described and that the use of polymyxin is safe with variable efficacy (15, 17–22). Although Gram-negative bacterial resistance is common in the setting of organ transplantation, little information on polymyxin use is available for this group of patients (23, 24). Moreover, renal dysfunction is a frequent complication and may affect both patient and graft survival (11).
Efficacy rates of polymyxin are variable and depend on the study population. Michalopoulos et al. (25) demonstrated in a series of 43 intensive care unit (ICU) hospitalized patients a clinical cure rate of 69.8% and a microbiological cure rate of 67.4%. Garnacho-Montero et al. (15), studying a cohort of 35 patients using colistin or imipenem for treatment of A. baumannii ventilator-associated pneumonia (VAP), reported a clinical cure rate of 57% in both groups. In our study, we showed higher clinical (77.2%) and microbiological (100%) cure rates. This may be explained by the higher frequency of patients with UTI diagnosis than with VAP diagnosis, which was more frequent in the ICU studies. Moreover, most of our patients were not critically ill, rendering the comparison with a population of exclusively ICU patients not adequate. Nevertheless, polymyxin has been shown to be very effective for UTI treatment, probably reflecting a high concentration in urine (26–28).
Recent data regarding polymyxin pharmacokinetics/pharmacodynamics in experimental and clinical studies suggest that the dose traditionally used is probably insufficient, because of low concentrations in plasma in critical patients (29–32). Some authors suggest not adjusting for renal failure in any situation for critical patients when using polymyxin B because of some evidence that renal clearance of the drug is very low (32, 33). This phenomenon may be leading to the emergence of strains resistant to polymyxins (33).
Nephrotoxicity rates range from 8 to 45% (34–40), mainly in critical care unit patients in recent studies. No data exclusively for transplanted patients are available in such studies. Using the same criteria, we found a high rate of nephrotoxicity (32.6%), although the rate was lower than those in earlier studies (renal impairment ranging from 20.2 to 36%) (3, 8, 9, 41, 42). A high proportion of patients needed substitutive therapy during polymyxin use. This rate of renal dysfunction is similar to rates described for drugs being replaced by less toxic agents, such as amphotericin B. Bates et al. (43), using the same toxicity score employed in our study and reporting data on the treatment of 707 critically patients, reported a renal dysfunction rate of 30% for deoxycholate-amphotericin B.
The high nephrotoxicity rate observed in our study overestimates the toxic effect of polymyxin, since kidney transplant patients are prone to renal dysfunction. In fact, our patients had initial mean creatinine levels in plasma of 2.22 mg/dl and 85.4% had a serum creatinine level greater than or equal to 1 mg/dl at the beginning of the study.
Multivariate analysis showed that the length of polymyxin use is statistically associated with a worsening of renal function (P < 0.037; OR, 1.06; 95% CI, 1.00 to 1.12). The medical literature contains controversial data on this subject. Falagas et al. (17) in a prospective study of 21 patients who used at least 7 days of colistin treatment showed a statistically significant correlation between cumulative antibiotic dose and nephrotoxicity. The same author in another publication (44) retrospectively evaluated 19 courses of colistin for 4 weeks or more and showed that the average rise in serum creatinine compared to baseline value after polymyxin E use was only 0.25 mg/dl. Our study reinforces the hypothesis that length of treatment and cumulative polymyxin use are related to renal dysfunction in solid organ transplantation.
The diagnosis of UTI had a marginally protective effect in multivariate analysis. We regard it as a spurious association, since patients with this diagnosis were less severely ill, based on a lower rate of ICU admission than that of patients with other infections (7.9% versus 59.3%, P < 0.001).
The retrospective nature of the study imposes some limitations regarding methodological aspects, mainly for diagnostic and microbiological characterization, and also for renal function monitoring. Since we did not have daily creatinine measurements for all patients, the time to reach an endpoint for nephrotoxicity may be underestimated. Serum urea and creatinine are not ideal markers for renal function assessment, although they are reliable for comparison with literature data. Finally, the lack of a control group and the analysis of a population subjected to multiple interventions impacting renal function may overestimate the real nephrotoxic effect of polymyxin.
Although we have disclosed a prohibitive rate of nephrotoxicity related to polymyxin use in kidney transplant patients, we understand that for multidrug-resistant bacteria such as P. aeruginosa and A. baumannii, polymyxin is sometimes the only viable therapeutic alternative. We suggest, for the purpose of reducing nephrotoxicity rates, using polymyxin judiciously only for true infections (restricting, for instance, the treatment of asymptomatic bacteriuria and tracheal colonization) and for as brief a time as possible.
Footnotes
Published ahead of print 7 January 2013
REFERENCES
- 1. Evans ME, Feola DJ, Rapp RP. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960–967 [DOI] [PubMed] [Google Scholar]
- 2. Lieberson AD, Winter LW, Behnke RH, Martin RR. 1969. Extensive pseudomonal pneumonia ultimately responding to polymyxin therapy. Am. Rev. Respir. Dis. 100:558–564 [DOI] [PubMed] [Google Scholar]
- 3. Fekety FR, Jr, Norman PS, Cluff LE. 1962. The treatment of gram-negative bacillary infections with colistin. The toxicity and efficacy of large doses in forty-eight patients. Ann. Intern. Med. 57:214–229 [DOI] [PubMed] [Google Scholar]
- 4. Gold GN, Richardson AP., Jr 1966. An unusual case of neuromuscular blockade seen with therapeutic blood levels of colistin methanesulfonate (Coly-Mycin). Am. J. Med. 41:316–321 [DOI] [PubMed] [Google Scholar]
- 5. Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perkins RL. 1964. Apnea with intramuscular colistin therapy. JAMA 190:421–424 [DOI] [PubMed] [Google Scholar]
- 7. Navon-Venezia S, Ben-Ami R, Carmeli Y. 2005. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis. 18:306–313 [DOI] [PubMed] [Google Scholar]
- 8. Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857–868 [DOI] [PubMed] [Google Scholar]
- 9. Tallgren LG, Liewendahl K, Kuhlbaeck B. 1965. The therapeutic success and nephrotoxicity of colistin in acute and chronic nephropathies with impaired renal function. Acta Med. Scand. 177:717–728 [DOI] [PubMed] [Google Scholar]
- 10. Nankivell BJ, Chapman JR. 2006. Chronic allograft nephropathy: current concepts and future directions. Transplantation 81:643–654 [DOI] [PubMed] [Google Scholar]
- 11. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. 2003. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 349:931–940 [DOI] [PubMed] [Google Scholar]
- 12. Dobbin C, Maley M, Harkness J, Benn R, Malouf M, Glanville A, Bye P. 2004. The impact of pan-resistant bacterial pathogens on survival after lung transplantation in cystic fibrosis: results from a single large referral centre. J. Hosp. Infect. 56:277–282 [DOI] [PubMed] [Google Scholar]
- 13. Linares L, Cervera C, Cofan F, Ricart MJ, Esforzado N, Torregrosa V, Oppenheimer F, Campistol JM, Marco F, Moreno A. 2007. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transplant. Proc. 39:2222–2224 [DOI] [PubMed] [Google Scholar]
- 14. Moreno A, Cervera C, Gavalda J, Rovira M, de la Camara R, Jarque I, Montejo M, de la Torre-Cisneros J, Miguel Cisneros J, Fortun J, Lopez-Medrano F, Gurgui M, Munoz P, Ramos A, Carratala J. 2007. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am. J. Transplant. 7:2579–2586 [DOI] [PubMed] [Google Scholar]
- 15. Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar AE, Garcia-Garmendia JL, Bernabeu-Wittel IM, Gallego-Lara SL, Madrazo-Osuna J. 2003. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin. Infect. Dis. 36:1111–1118 [DOI] [PubMed] [Google Scholar]
- 16. Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 17. Falagas ME, Fragoulis KN, Kasiakou SK, Sermaidis GJ, Michalopoulos A. 2005. Nephrotoxicity of intravenous colistin: a prospective evaluation. Int. J. Antimicrob. Agents 26:504–507 [DOI] [PubMed] [Google Scholar]
- 18. Kallel H, Bahloul M, Hergafi L, Akrout M, Ketata W, Chelly H, Hamida CB, Rekik N, Hammami A, Bouaziz M. 2006. Colistin as a salvage therapy for nosocomial infections caused by multidrug-resistant bacteria in the ICU. Int. J. Antimicrob. Agents 28:366–369 [DOI] [PubMed] [Google Scholar]
- 19. Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L. 2003. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit. Care 7:R78–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ouderkirk JP, Nord JA, Turett GS, Kislak JW. 2003. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob. Agents Chemother. 47:2659–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rios FG, Luna CM, Maskin B, Saenz Valiente A, Lloria M, Gando S, Sosa C, Baquero S, Llerena C, Petrati C, Apezteguia C. 2007. Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. Eur. Respir. J. 30:307–313 [DOI] [PubMed] [Google Scholar]
- 22. Sobieszczyk ME, Furuya EY, Hay CM, Pancholi P, Della-Latta P, Hammer SM, Kubin CJ. 2004. Combination therapy with polymyxin B for the treatment of multidrug-resistant Gram-negative respiratory tract infections. J. Antimicrob. Chemother. 54:566–569 [DOI] [PubMed] [Google Scholar]
- 23. Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008–1011 [DOI] [PubMed] [Google Scholar]
- 24. Linden PK, Kusne S, Coley K, Fontes P, Kramer DJ, Paterson D. 2003. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 37:e154–e160 [DOI] [PubMed] [Google Scholar]
- 25. Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. 2005. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin. Microbiol. Infect. 11:115–121 [DOI] [PubMed] [Google Scholar]
- 26. Bergan T, Fuglesang J. 1982. Polymyxin antibiotics: chemical and pharmacokinetic properties. Antibiot. Chemother. 31:119–144 [PubMed] [Google Scholar]
- 27. Kunin CM, Bugg A. 1971. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J. Infect. Dis. 124:394–400 [DOI] [PubMed] [Google Scholar]
- 28. Kuntzman R, Jacobson M, Tsai I, Burns JJ, Burchall J, Koch A. 1973. Certain aspects of drug binding to nonplasma proteins as illustrated by studies with cyclizine, chlorcyclizine, and polymyxin B. Ann. N. Y. Acad. Sci. 226:131–147 [DOI] [PubMed] [Google Scholar]
- 29. Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. 2012. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin. Infect. Dis. 54:1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. 47:1298–1304 [DOI] [PubMed] [Google Scholar]
- 33. Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 11:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng CY, Sheng WH, Wang JT, Chen YC, Chang SC. 2010. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 35:297–300 [DOI] [PubMed] [Google Scholar]
- 35. Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int. J. Antimicrob. Agents 35:194–199 [DOI] [PubMed] [Google Scholar]
- 36. Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 48:1724–1728 [DOI] [PubMed] [Google Scholar]
- 37. Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME. 2005. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob. Agents Chemother. 49:3136–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim J, Lee KH, Yoo S, Pai H. 2009. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int. J. Antimicrob. Agents 34:434–438 [DOI] [PubMed] [Google Scholar]
- 39. Mendes CA, Cordeiro JA, Burdmann EA. 2009. Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. Ann. Pharmacother. 43:1948–1955 [DOI] [PubMed] [Google Scholar]
- 40. Santamaria C, Mykietiuk A, Temporiti E, Stryjewski ME, Herrera F, Bonvehi P. 2009. Nephrotoxicity associated with the use of intravenous colistin. Scand. J. Infect. Dis. 41:767–769 [DOI] [PubMed] [Google Scholar]
- 41. Baines RD, Jr, Rifkind D. 1964. Intravenous administration of sodium colistimethate. JAMA 190:278–281 [DOI] [PubMed] [Google Scholar]
- 42. Olesen S, Madsen PO. 1967. Intravenous administration of sodium colistimethate in urinary tract infections. Curr. Ther. Res. Clin. Exp. 9:283–287 [PubMed] [Google Scholar]
- 43. Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Dasbach EJ, Platt R. 2001. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 32:686–693 [DOI] [PubMed] [Google Scholar]
- 44. Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A. 2005. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect. Dis. 5:1 doi:10.1186/1471-2334-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]