Abstract
Macrolide antibiotics are important for clinical treatment of infections caused by Campylobacter jejuni. Development of resistance to this class of antibiotics in Campylobacter is a complex process, and the dynamic molecular changes involved in this process remain poorly defined. Multiple lineages of macrolide-resistant mutants were selected by stepwise exposure of C. jejuni to escalating doses of erythromycin or tylosin. Mutations in target genes were determined by DNA sequencing, and the dynamic changes in the expression of antibiotic efflux transporters and the transcriptome of C. jejuni were examined by real-time reverse transcription-PCR, immunoblotting, and DNA microarray analysis. Multiple types of mutations in ribosomal proteins L4 and L22 occurred early during stepwise selection. On the contrary, the mutations in the 23S rRNA gene, mediating high resistance to macrolides, were observed only in the late-stage mutants. Upregulation of antibiotic efflux genes was observed in the intermediately resistant mutants, and the magnitude of upregulation declined with the occurrence of mutations in the 23S rRNA gene. DNA microarray analysis revealed the differential expression of 265 genes, most of which occurred in the intermediate mutant, including the upregulation of genes encoding ribosomal proteins and the downregulation of genes involved in energy metabolism and motility. These results indicate (i) that mutations in L4 and L22 along with temporal overexpression of antibiotic efflux genes precede and may facilitate the development of high-level macrolide resistance and (ii) that the development of macrolide resistance affects the pathways important for physiology and metabolism in C. jejuni, providing an explanation for the reduced fitness of macrolide-resistant Campylobacter.
INTRODUCTION
Campylobacter jejuni is a leading cause of diarrhea and one of the most common bacterial causes of food-borne illnesses worldwide (1). Because of the decreasing therapeutic effectiveness of fluoroquinolones, macrolide antibiotics (e.g., erythromycin [Ery]) have become more and more important for the treatment of Campylobacter infections in humans (2, 3). Additionally, some macrolide antibiotics, including tylosin (Tyl), Ery, and tilmicosin, are also used in food animal production for both therapeutic and subtherapeutic purposes (4). As a zoonotic pathogen transmitted through the food-borne route, Campylobacter is subject to selection pressure from macrolide use in both veterinary medicine and human medicine. Although the overall prevalence of macrolide-resistant Campylobacter is relatively low, high incidences of macrolide resistance in Campylobacter have been reported in some studies (5–9). In order to curb the emergence of macrolide resistance, it is necessary for us to understand how Campylobacter develops resistance to this class of antibiotics.
In Campylobacter, macrolide resistance is mediated by target mutations and antibiotic efflux (2). Mutations in the L4 and L22 proteins appear to mediate low (MIC, 8 to 16 μg/ml)-to-intermediate (MIC, 16 to 128 μg/ml) levels of resistance to Ery, while mutations in the 23S rRNA gene are associated with high-level (MIC, ≥256 μg/ml) Ery resistance (5, 10, 11). Selection of mutations in the 23S rRNA gene seems to require prolonged exposure to macrolide antibiotics, suggesting that other mutations or changes in Campylobacter may be required prior to the occurrence of the 23S rRNA mutations (2, 10). Active efflux via the CmeABC multidrug efflux pump is another mechanism that confers resistance to macrolides on Campylobacter (10, 12, 13). By inactivation of the CmeABC efflux pump in resistant Campylobacter strains, previous studies revealed that CmeABC functions synergistically with the mutations in ribosomal proteins L4 (G74D) and L22 (insertions at position 86 or 98) or the 23S rRNA gene (A2075G) to confer macrolide resistance (10, 11, 13–15). In Campylobacter, expression of cmeABC is controlled by CmeR, which binds to the promoter of cmeABC and regulates the expression of the efflux operon (16, 17). In addition to CmeABC, previous studies (12, 18) also suggested that other efflux mechanisms might be associated with Ery resistance, but the identities of the suspected efflux pumps are unknown.
Despite the improved understanding of the mechanisms of macrolide resistance in Campylobacter, the molecular processes leading to the generation of stable high-level resistance are largely unknown. Understanding of these molecular events will potentially facilitate the clinical use of antibiotics and targeted control of macrolide-resistant Campylobacter. Toward this end, we conducted a systemic analysis of the development of macrolide-resistant mutants in culture medium under selection pressure. Multiple lineages of macrolide-resistant C. jejuni mutants were selected in vitro by stepwise exposure of C. jejuni NCTC 11168 and 81-176 to increasing concentrations of Ery or Tyl. The occurrence of mutations in the 23S rRNA gene (three copies) and the genes encoding ribosomal proteins L4 and L22 was monitored during this multistep selection process. The dynamic changes in the expression of four antibiotic efflux genes (cmeB, Cj1687, Cj1257c, and Cj1375 [cmeG]) in representative lineages were determined by real-time reverse transcription (RT)-PCR and immunoblotting (for CmeABC only). DNA microarray analysis was also carried out with a representative lineage to analyze the global transcriptional changes that occur during the development of macrolide resistance in C. jejuni.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
C. jejuni strains NCTC 11168 and 81-176 were used as the parent stains for in vitro selection of Eryr and Tylr mutants. Both strains were originally isolated from humans, are commonly used for laboratory studies, and have been sequenced (accession no. NC_002163.1 and NC_008787.1). Both strains are susceptible to Ery and Tyl, and the MICs are 1 and 4 μg/ml, respectively. The Campylobacter strains were grown in Mueller-Hinton (MH) broth or agar at 42°C under microaerobic conditions (5% O2, 10% CO2, 85% N2).
Susceptibility test.
MICs of macrolide antibiotics were measured by the agar dilution method as recommended by the Clinical and Laboratory Standards Institute (19). C. jejuni ATCC 33560 was used as the quality control strain.
Stepwise selection of macrolide-resistant mutants in vitro.
Ery and Tyl were used to select macrolide-resistant mutants in vitro. They were chosen because Ery is the drug of choice for treating human Campylobacter infections, while Tyl and Ery are macrolide antibiotics commonly used in food animal production for therapeutic and subtherapeutic purposes (4). For the first round of selection, cultures of parent strains C. jejuni NCTC 11168 and 81-176 were spread on plates containing a series of increasing concentrations (0.5 × MIC, 1 × MIC, and 2 × MIC) of Ery or Tyl (11). Following 3 to 5 days of incubation under microaerobic conditions at 42°C, single colonies on the plate with the highest concentration of the drugs were randomly selected and used for subsequent stepwise selection. Stepwise selection was performed by transferring and passaging colonies selected in the first round to MH broth with gradually increased drug (Ery or Tyl) concentrations. Briefly, each colony picked in the first-round selection was separately enriched in antibiotic-free MH broth to the late logarithmic phase to a density of 1 × 108 CFU/ml, from which 100 μl (containing approximately 107 CFU) was transferred to 10 ml MH broth containing the same concentration of drugs in the first-round selective plates. After 2 to 3 days of incubation, the cells in each culture were collected as a unit and subjected to MIC determinations. For the next selection step, 100 μl (containing approximately 107 CFU) of the culture from the previous step was inoculated into 10 ml of MH broth containing a 2-fold higher concentration of the antibiotic used in the previous step. During selection with each antibiotic concentration, multiple passages might be needed to allow adaptation and optimal growth of the selected mutants. The selective concentration of the antibiotic was gradually increased for each lineage up to the concentration at which the cultures were not able to grow. A lineage was defined as a series of derivatives selected from a single colony that was obtained in the first-round selection.
PCR amplification and DNA sequence analysis.
The primers used to amplify the sequences of the 23S rRNA gene, rplD (L4), rplV (L22), and cmeR along with the promoter of cmeABC are listed in Table 1. All three copies of the 23S rRNA gene in each of the mutant strains were amplified and sequenced as described by Gibreel et al. (20). All PCR products were sequenced in the DNA facility at Iowa State University.
Table 1.
Primers used for PCR amplification and real-time RT-PCR
| Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| 23S rRNA gene amplification | ||
| FI | CCCTAAGTCAAGCCTTTCAATCC | 20 |
| FII | CGTTATAGATACGCTTAGCGGTTATG | 20 |
| FIII | CATCGAGCAAGAGTTTATGCAAGC | 20 |
| CJ-copy-R | CTACCCACCAGACATTGTCCCAC | 20 |
| Fragment of 23S rRNA gene | ||
| 23S-domV-F | GTAAACGGCGGCCGTAACTA | This study |
| 23S-domV-R | GACCGAACTGTCTCACGACG | This study |
| 23S-2611F | ATGTCGGCTCATCGCATCCTGG | 20 |
| 23S-2611R | CATCCATTACACACCCAGCCTATC | 20 |
| Ribosomal protein L4 | ||
| L4-F | GTAGTTAAAGGTGCAGTACCA | This study |
| L4-R | GCGAAGTTTGAATAACTACG | This study |
| Ribosomal protein L22 | ||
| L22-F | GAATTTGCTCCAACACGC | This study |
| L22-R | ACCATCTTGATTCCCAGTTTC | This study |
| CmeR and cmeABC promoter | ||
| cmeR-F | TAGAAAAGTATATTTGTATACCCT | 16 |
| cmeR-R | CGCCACTAACTTGAGGCTTTA | 16 |
| Real-time RT-PCRa | ||
| 16S-F | TACCTGGGCTTGATATCCTA | 21 |
| 16S-R | GGACTTAACCCAACATCTCA | 21 |
| CmeB-F | ACGATTCAACCTTTTCCCAGC | This study |
| CmeB-R | TTTGCTACTTGAGCAATCGCTTC | This study |
| Cj1687-F | TGGTGGTTTGGACTCTTTCTGGGA | This study |
| Cj1687-F | TCTCTGCGATTAAAGCCACCACGA | This study |
| Cj1375-F | GCTTTACCACGATTTTCTTCTGTGA | This study |
| Cj1375-R | TACACCATGCTTTTAGGAAGAATGC | This study |
| Cj1257c-F | ATCGCCGTGATAGCGCCTAAAGAA | This study |
| Cj1257c-R | GCCACCAAACAAAGGCCCAAGTAA | This study |
Sequences and gene names are based on C. jejuni NCTC 11168.
RNA extraction and real-time quantitative RT-PCR (qRT-PCR).
To prepare RNA, C. jejuni cultures were grown on MH plates for 12 h and harvested with MH broth containing RNAprotect Bacterial Reagent (1:2; Qiagen, Valencia, CA). RNA extraction, purification, and quantification were performed as previously described (21). The extracted RNA was further treated with an On-Column DNase Digestion kit (Qiagen), followed by an additional DNase treatment to remove residual DNA contamination. The prepared RNA samples were used as the template for real-time qRT-PCR and microarray analysis. The relative expression of four antibiotic efflux transporter genes (cmeB, Cj1687, Cj1257c, and Cj1375) in four representative lineages (68Ex-1, 68Ex-3, 76Ex-6, and 76Tx-7) was determined by qRT-PCR, which was performed with the Bio-Rad IQ thermocycler and the iScript One-Step RT-PCR kit with SYBR green as described previously (21). The primer sets used for specific genes are listed in Table 1. 16S rRNA was used as a control for normalization (22).
SDS-PAGE and immunoblotting.
Whole-cell proteins of various Campylobacter strains were separated by SDS-PAGE and immunoblotted using anti-CmeA, anti-CmeB, and anti-CmeC antibodies as described previously (23).
DNA microarray analysis and data analysis.
Three Eryr mutants (68E1-3, 68E8-3, and 68E64-3) in lineage 68Ex-3 were analyzed by microarray analysis in comparison with C. jejuni NCTC 11168. The microarray analysis slides of C. jejuni (version 1) were obtained from the J. Craig Venter Institute. For each microarray analysis slide, a pair of cDNA samples prepared from NCTC 11168 and a mutant culture, respectively, were cohybridized. Four hybridizations using four independent RNA samples were performed for each pair of C. jejuni cultures. cDNA labeling, preparation, and hybridization were performed following the protocols from the J. Craig Venter Institute. Data collection and normalization were performed as previously described (17). After statistical analysis, the set of p values was converted to q values to control the false-discovery rate. For this study, genes with a q value of <0.1 and a change of ≥1.5-fold were identified as being differentially expressed by the resistant and wild-type strains. Differentially expressed genes were first classified on the basis of the genomic annotation at the Sanger Center and then subjected to clustering analysis (K-means/medians clustering [KMC]) and hierarchical clustering (HCL) analysis using Multiexperiment Viewer (MeV v4.4).
Microarray data accession number.
The microarray data obtained in this study have been deposited in the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/database) and assigned accession number GSE17881.
RESULTS
Lineages of the selected macrolide-resistant mutants.
Three individual colonies (68E-1, 68E-2, and 68E-3) of parent strain C. jejuni NCTC 11168 were randomly selected on plates containing 1 μg/ml Ery in the first-round selection and subjected to stepwise selection to obtain three representative lineages of Eryr mutants (68Ex-1, 68Ex-2, and 68Ex-3). Similarly, one representative colony (76E-6) of parent strain C. jejuni 81-176 was selected on a plate with 2 μg/ml Ery in the first-round selection and one lineage of Eryr mutants (76Ex-6) was obtained after stepwise selection. The characteristic features of the four lineages are described in Table 2.
Table 2.
Characteristics of C. jejuni mutants selected by Ery
| Lineagea and strainb | MIC (μg/ml) |
Mutation in 23S rRNA genec | Change in protein L4 | Change(s) in protein L22 | Mutation in cmeR | |
|---|---|---|---|---|---|---|
| Ery | Tyl | |||||
| Parent, NCTC 11168 | 1 | 4 | —d | — | — | |
| 68Ex-1 | ||||||
| 68E1-1 | 4 | 8 | — | — | A88E | — |
| 68E4-1 | 32 | 256 | — | — | A88E | Del490A |
| 68E8-1 | 64 | 128 | — | — | A88E | Del490A |
| 68E32-1 | 256 | 128 | — | G67V | A88E | Del490A |
| 68E64-1 | >512 | 256 | C2627A (3) | G67V | A88E | Del490A |
| 68Ex-2 | ||||||
| 68E1-2 | 4 | 8 | — | 59-G-60 | — | |
| 68E4-2 | 32 | 128 | — | 59-G-60 | G86E | — |
| 68E8-2 | 64 | 128 | — | 59-G-60 | A84D, G86E | — |
| 68E32-2 | 128 | 128 | — | 59-G-60 | A84D, G86V | — |
| 68E64-2 | 512 | 256 | — | 59-G-60 | A84D, G86V | — |
| 68Ex-268Ex-3 | ||||||
| 68E1-3 | 4 | 16 | — | R72I | — | — |
| 68E4-3 | 64 | 64 | — | R72I | V100L | H174N |
| 68E8-3 | 128 | 256 | — | R72I | 91-S-92 | H174N |
| 68E32-3 | >512 | >512 | — | R72I | — | H174N |
| 68E64-3 | >512 | >512 | A2074C (3) | R72I | — | H174N |
| Parent, 81176 | 1 | 4 | — | — | — | — |
| 68Ex-268Ex-376Ex-6 | ||||||
| 76E2-6 | 32 | 64 | — | — | — | — |
| 76E8-6 | 256 | >512 | A2074C (2) | — | — | — |
| 76E64-6 | >512 | >512 | A2074C (3) | — | — | — |
The lineages are named according to the parent strain and the antibiotic used for selection. 68 and 76 indicate that the parent strains are 11168 and 81-176, respectively, E represents selection by Ery, x depicts the various concentrations used for selection, and the last number indicates the lineage number.
The strains in the lineages are named similarly to the lineages, except that x is replaced by the actual antibiotic concentration from which the mutant was selected.
Each number in parentheses is the number of mutated copies of the 23S rRNA gene.
—, no mutations detected.
By using Tyl as the selective agent, four individual colonies (68T-1, 68T-2, 68T-3, and 68T-4) of parent strain C. jejuni NCTC 11168 were selected on plates with 4 μg/ml Tyl and one colony (76T-7) of C. jejuni 81-176 was selected on plates with 8 μg/ml Tyl in the first round of selection. After stepwise selection, five representative lineages of Tylr mutants (68Tx-1, 68Tx-2, 68Tx-3, 68Tx-4, and 76Tx-7) were obtained; their characteristic features are listed in Table 3.
Table 3.
Characteristics of C. jejuni mutants selected by Tyl
| Lineagea and strainb | MIC (μg/ml) |
Mutation in 23S rRNA gene | Change in protein L4 | Change(s) in protein L22 | Mutation in cmeR | |
|---|---|---|---|---|---|---|
| Ery | Tyl | |||||
| Parent, NCTC 11168 | 1 | 4 | —c | — | — | |
| 68Tx-1 | ||||||
| 68T4-1 | 4 | 8 | — | — | A88E | |
| 68T16-1 | 32 | 256 | — | R72I | A88E | H174N |
| 68T32-1 | 64 | 256 | — | R72I | A88E | H174N |
| 68T128-1 | 128 | 256 | — | R72I | A88E | H174N |
| 68Tx-2 | ||||||
| 68T4-2 | 4 | 8 | — | — | — | |
| 68T16-2 | 128 | 256 | — | G57V | — | — |
| 68T32-2 | 256 | 512 | — | G57V | G86E | — |
| 68T128-2 | 128 | 256 | — | G57V | G86E | — |
| 68 Tx-3 | ||||||
| 68T4-3 | 2 | 8 | — | — | — | — |
| 68T16-3 | 32 | 256 | — | A71D | — | S117Y |
| 68T32-3 | 64 | 512 | — | A71D | A88E | S117Y |
| 68T64-3 | 16 | 256 | — | A71D | A88E | S117Y |
| 68Tx-4 | ||||||
| 68T4-4 | 2 | 8 | — | — | — | — |
| 68T8-4 | 16 | 64 | — | A71D | — | — |
| 68T16-4 | 32 | 128 | — | A71D | A88E | G204L |
| 68T32-4 | 64 | 256 | — | A71D | A88E | G204L |
| 68T64-4 | 128 | 512 | — | A71D | A88E, G86V | G204L |
| Parent, 81176 | 1 | 4 | — | — | — | — |
| 76Tx-7 | ||||||
| 76T8-7 | 8 | 32 | — | — | — | — |
| 76T16-7 | 32 | 128 | — | G57D | G86E | — |
| 76T32-7 | 64 | 256 | — | G57D | G86E | — |
Dynamic changes in point mutations in target genes and proteins.
As shown in Tables 2 and 3, multiple mutations were observed in the target genes (the 23S rRNA gene, the genes for ribosomal proteins L4 and L22, and cmeR). Overall, the mutations in the genes for L4 and L22 occurred earlier in the stepwise selection process, while the mutations in the 23S rRNA gene occurred later in the selection process and were associated with high-level resistance to Ery (MIC, ≥256 μg/ml).
Six different changes in ribosomal protein L4 were observed in the macrolide-resistant mutants selected (Tables 2 and 3). The Gly-to-Val (G57V) and Gly-to-Asp (G57D) modifications identified at position 57 in L4 were previously reported by Caldwell et al. (11). Four other mutations in ribosomal protein L4, including R72I, G67V, and A71D and the insertion of a glycine at position 59, represent new mutations identified in this study. In a given lineage, only one type of mutation was observed in L4.
Modifications at positions 84, 86, and 88 were frequently observed mutations in ribosomal protein L22 in the macrolide-resistant mutants (Tables 2 and 3). G86E and A88E were more frequent than other types of mutations in the mutants analyzed. In lineage 68Ex-2, the G86E mutation in L22 was switched to G86V and the modification at position 86 coexisted with A84D (Table 2). With respect to the 23S rRNA gene, the A2074C mutation was observed in the high-level Eryr mutants of two lineages (68Ex-3 and 76Ex-6). In 68E64-3, the A2074C change was present in all three copies of the 23S rRNA gene. In the 76Ex-6 lineage, the A2074C transversion occurred earlier in the mutant selection process. This point mutation was present in only two of the three copies of the 23S rRNA gene in 76E8-6 but in all three copies of the 23S rRNA gene in 76E64-6 (Table 2). Interestingly, the high-level Eryr mutant (68E64-1) in lineage 68Ex-1 displayed a different type of point mutation in the 23S rRNA gene with a C→A point mutation at position 2627 (equivalent to position 2611 in Escherichia coli). In one of the lineages selected with Ery (68Ex-2), stepwise selection failed to select mutants containing mutations in the 23S rRNA gene (Table 2), while none of the Tyl-selected mutants displayed point mutations in the 23S rRNA gene (Table 3).
Dynamic changes in the expression of antibiotic efflux genes.
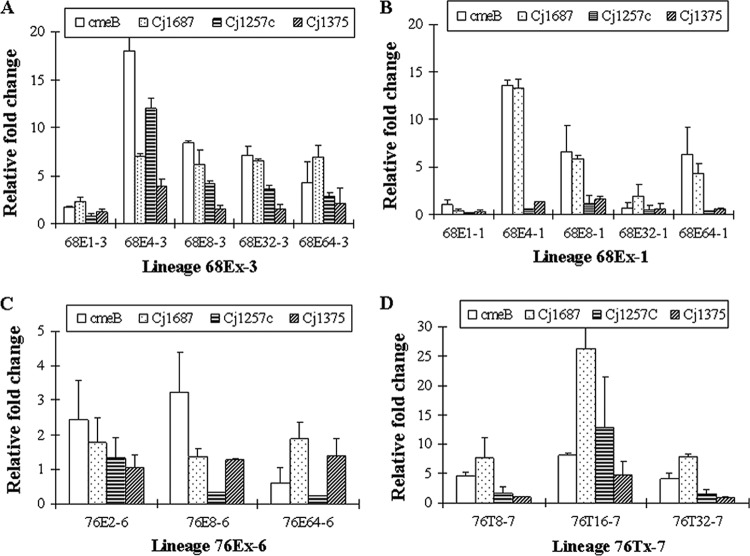
The qRT-PCR results (Fig. 1) showed that there was a general trend for increased expression of the four efflux genes (cmeB, Cj1687, Cj1257c, and Cj1375) in the lineages examined (68Ex-1, 68Ex-3, 76Ex-6, and 76Tx-7). In lineage 68Ex-1 (Fig. 1B), the most obvious change was a drastic increase in the expression of cmeB and Cj1687 in 68E4-1, and the magnitudes of overexpression of the two efflux genes subsequently declined in the later-stage mutants of the lineage. In the 68Ex-3 lineage (Fig. 1A), all four of the efflux genes tested were upregulated and the magnitudes of upregulation of cmeB, Cj1257c, and Cj1687 were greater than that of Cj1375. In lineage 76Ex-6 (Fig. 1C), overexpression of cmeB was observed in 76E2-6 and 76E8-6, while the expression level of cmeB in 76E64-6 returned to the level of the parent strain, C. jejuni 81-176. In lineage 76Tx-7 (Fig. 1D), cmeB and Cj1687 were significantly overexpressed and the change was especially obvious for Cj1687 in 76T16-7. Overall, there was a general trend for the overexpression of the efflux genes to be temporarily upregulated in the mutants with intermediate-level resistance and subsequently decline in the later-stage mutants.
Fig 1.
Differential expression of four efflux genes in selected mutant strains in four different lineages. The data were generated by real-time RT-PCR in comparison with the parent strain (NCTC 11168 or 81-176).
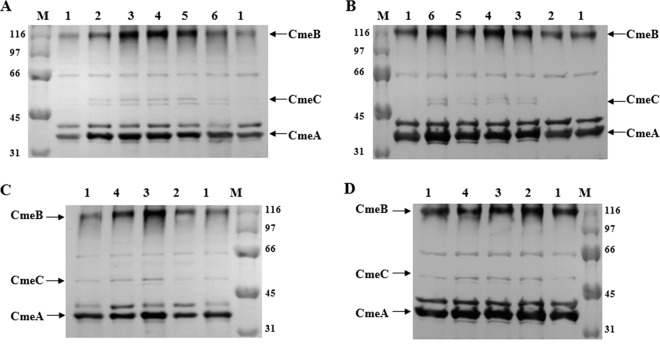
Immunoblotting analysis further showed the change in CmeABC expression in the lineages (Fig. 2). In each lineage, the production of CmeABC proteins increased during the mutant selection process. For example, the CmeB protein was significantly overproduced in 68E4-3, 68E8-3, and 68E32-3 (lanes 3, 4, and 5 in Fig. 2A) compared with the wild-type strain. Likewise, a significant increase in CmeB production was observed in 76E8-6 (lane 3 in Fig. 2C).
Fig 2.
Immunoblotting analysis of CmeABC expression in selected strains in four different lineages. In panel A, numbers 1, 2, 3, 4, 5, and 6 represent C. jejuni NCTC 11168, 68E1-3, 68E4-3, 68E8-3, 68E32-3, and 68E64-3, respectively. In panel B, numbers 1, 2, 3, 4, 5, and 6 correspond to C. jejuni NCTC 11168, 68E1-1, 68E4-1, 68E8-1, 68E32-1, and 68E64-1. In panel C, numbers 1, 2, 3, and 4 correspond to C. jejuni 81-176, 76E2-6, 76E8-6, and 76E64-6. In panel D, numbers 1, 2, 3, and 4 correspond to C. jejuni 81-176, 76T8-7, 76T16-7, and 76T32-7. Cell envelopes prepared from each strain were blotted with polyclonal antibodies to CmeA, CmeB, and CmeC. The same amount of total proteins was loaded in each lane. Prestained molecular mass markers (lane M; Bio-Rad) were used to estimate the sizes of the proteins (shown in kilodaltons). The positions of CmeA, CmeB, and CmeC are indicated.
Identification of mutations in cmeR.
Since cmeABC was overexpressed in the mutant selection process (Fig. 1 and 2), we further determined if any mutations occurred in cmeR and the promoter of cmeABC that might explain the overexpression of cmeABC. Three different types of point mutations (H174N, S117Y, and G204L) and a deletion (del490A) were detected in cmeR in some mutant strains of five different lineages, including 68Ex-1, 68Ex-3, 68Tx-1, 68Tx-3, and 68Tx-4 (Tables 2 and 3). The del490A nucleotide deletion would cause a frameshift and truncation of the CmeR open reading frame, which may explain the overexpression of cmeB in lineage 68Ex-1 (Fig. 1 and 2). The H174N substitution in CmeR also occurred concomitantly with the observed overexpression of cmeB in lineage 68Ex-3, suggesting that the H174N mutation may affect the function of CmeR. No mutations in the promoter of cmeABC were observed in any of the Eryr mutants.
Dynamic changes in the transcriptome during mutant selection.
To examine the global change at the transcriptional level in the mutant selection process, three Eryr mutants (68E1-3, 68E8-3, and 68E64-3) of lineage 68Ex-3 were subjected to microarray analysis in comparison with C. jejuni NCTC 11168. The transcriptional abundance of 265 genes was altered in at least one of the three strains. In 68E1-3, only nine genes were found differentially expressed in comparison with the parent strain. However, in 68E8-3, 85 genes were upregulated and 124 genes were downregulated, and in 68E64-3, 52 genes were upregulated and 93 genes were downregulated (see Fig. S1 and Table S1 in the supplemental material). Of the 265 genes, 86 were also changed in 68E8-3 and 68E64-3 (see Fig. S1 and Table S1). Only five genes showed differential expression in all three mutant strains, of which Cj0561c, Cj0365c (cmeC), and peb3 were upregulated while Cj1465 and Cj1357c were downregulated (see Table S1).
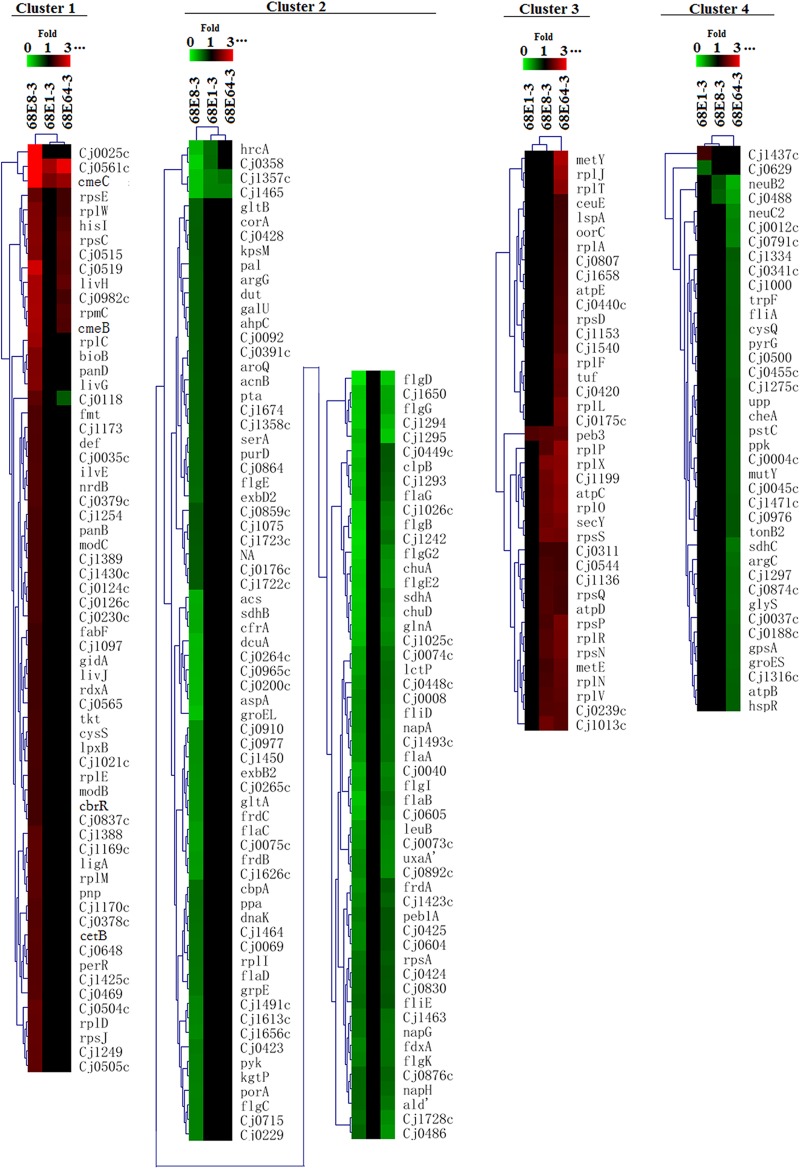
After KMC analysis, the 265 genes were grouped into four clusters, numbered 1, 2, 3, and 4 (Fig. 3). The clusters clearly visualized the trends of transcriptional changes during the development of macrolide resistance in C. jejuni. Cluster 1 includes 64 genes the expression of which was not changed in 68E1-3 but upregulated in 68E8-3; and then in 68E64-3, their overexpression was reduced, with many returning to a near-wild-type level. On the contrary, the 122 genes in cluster 2 were downregulated in 68E8-3 but the magnitudes of downregulation were reduced in 68E64-3. Cluster 3 includes 40 genes whose expression level continuously increased in the lineage as macrolide resistance increased. The 39 genes included in cluster 4 showed little change in expression in 68E1-3 and 68E8-3 but were downregulated in highly resistant strain 68E64-3. Additionally, from the order or distance of the three strains exhibited in the HCL-Tree (Fig. 3) in each cluster, it can be seen that the differentially expressed genes in 68E8-3 are characteristic changes in clusters 1 and 2, while the transcriptomic alterations in 68E64-3 better represent the characteristic changes in clusters 3 and 4.
Fig 3.
HCL analysis of C. jejuni genes differentially expressed in the three strains (68E1-3, 68E8-3, and 68E64-3) in lineage 68Ex-3. For each of the four clusters, hierarchical clustering was performed both on the genes and on the strains to visualize the relationships among the genes and strains, respectively. Four clusters are presented in four different panels. For each cluster, the color scale shows the n-fold change range of up- and down-regulated genes. The three mutant strains are indicated at the top of each panel.
The 64 genes in cluster 1 include those encoding broad regulatory functions (perR, cetB, and cbrR), efflux pumps (Cj1173, cmeB, cmeC, and Cj0035c), ribosomal proteins (rpmC, rpsCEJ, and rplCDEMW), aminoacyl tRNA synthetases (fmt and cysS), DNA replication/protein translation-associated proteins (gidA, ligA, def, and pnp), and small-molecule metabolism (tkt, hisL, ilvE, nrdB, bioB, panBD, and fabF). Additionally, the cluster includes genes encoding membrane proteins (Cj1170c, Cj0378c, and Cj0124c), surface polysaccharides (Cj1425c, Cj1430c, and lpxB), periplasmic proteins (Cj0561c, Cj1169c, Cj0515, and Cj1021c), ABC transporters (livHJG and Cj0469), a molybdenum transport system (modBC), and amino acid transporters, as well as other transporters (Cj0982c, Cj0025c, Cj1389, and Cj1097).
Cluster 2 includes genes encoding broad regulators (Cj0448 and Cj1491c), ribosomal proteins (rplI and rpsA), membrane proteins (Cj1493c, Cj1026c, pal, Cj0423, Cj0176c, porA, and Cj0830), surface polysaccharides (Cj1423c and kpsM), flagellar proteins (flgBCGG2EE2KI, fliDE, and flaABCDG), nutrient transport/binding proteins (peb1A, chuAD, corA, cfrA, lctP, dcuA, kgtP, Cj0486, Cj0654c, and exbB2D2), chaperons or heat shock proteins (clpB, cbpA, hrcA, dnaK, grpE, and groEL), and detoxification enzymes (ahpC and Cj0358). Additionally, the cluster includes genes involved in carbon compound degradation (galU and pta), energy metabolism (pyk, gltA, sdhAB, acnB, and frdABC), respiration (gpsA, Cj1357c, Cj1358c, Cj0074c, Cj0075c, Cj0265c, fdxA, and napAGH), central intermediary metabolism (ppa, gltB, aspA, and uxaA), amino acid biosynthesis (glnA, argG, serA, aroQ, and leuB), nucleoside biosynthesis (purD and dut), and fatty acid biosynthesis (Cj1537c).
Many of the 40 genes in cluster 3 are involved in protein synthesis, including those for ribosomal proteins (rplAJTLFPRNVXO and rpsDPNSQ) and elongation factor Tu (tuf). Additionally, the cluster includes genes for a transcriptional regulator (Cj0440c), membrane proteins (Cj1658, Cj0544, and Cj1013c), a surface polysaccharide (Cj1136), miscellaneous periplasmic proteins (Cj0420, Cj1540, and peb3), and cation transporter/binding proteins (ceuE and Cj0175c), as well as genes involved in energy metabolism (oorC, Cj1153, and atpCDE), amino acid biosynthesis (metEY), and peptide secretion (secY and lspA).
Cluster 4 includes genes encoding a transcriptional regulator (Cj1000), transport/binding proteins (Cj0012c, Cj0045c, pstC, and tonB2), chaperons or heat shock proteins (groES and hspR), and a chemotaxis histidine kinase (cheA). Additionally, the cluster includes genes involved in peptide secretion (Cj1471c), energy metabolism (sdhC, gpsA, Cj0037, Cj0874c, and atpB), central intermediary metabolism (ppk and cysQ), amino acid biosynthesis (argG and trpF), nucleoside biosynthesis (pryG and upp), aminoacyl tRNA synthesis (glyS), DNA replication (mutY), and the biosynthesis of membrane or surface structures (Cj0629, Cj0341c, Cj0455c, Cj1316c, neuB2C2, fliA, Cj1275c, and Cj0004c).
Several representative differentially expressed genes (Cj0561c, Cj1199, Cj1013c, flgI, and sdhA) identified by DNA microarray analysis were further confirmed by qRT-PCR. In most of the cases, the qRT-PCR data confirmed the up- or downregulation detected by microarray analysis (data not shown). The relative n-fold changes in Cj0561c and Cj1013c detected by qRT-PCR were much greater than those detected by microarray analysis (data not shown).
DISCUSSION
In this study, the dynamic changes in target gene mutations, expression of antibiotic efflux genes, and the global transcriptome were analyzed during the process of macrolide resistance development in Campylobacter. The findings provide a glimpse into the development of macrolide resistance in C. jejuni, which involves mutations in L4 and L22 preceding the occurrence of mutations in the 23S rRNA gene, temporal upregulation of multidrug efflux genes, and transcriptomic changes in metabolic pathways and cellular processes. These results indicate a complex adaptive process involved in the development of macrolide resistance in Campylobacter.
It is known that modifications in the large loop of the L4 protein (amino acids [aa] 55 to 77) (24, 25) and in the highly conserved large loop of the L22 protein (aa 78 to 98) (26, 27) were associated with macrolide resistance in various bacteria. In this study, several mutations in ribosomal proteins L4 (R72I, G67V, A71D, and the insertion of a glycine at position 60) and L22 (A84D, G86E, G86V, and A88E) were observed in macrolide-resistant mutants (Tables 2 and 3), indicating that mutations in the ribosomal proteins are actively involved in the adaptation to macrolide selection in Campylobacter. It appears that some modifications in ribosomal proteins L4 (R72I) and L22 (A88E and G86E) may facilitate the development of high-level resistance to macrolides in Campylobacter, because they were frequently observed in derivatives with different levels of macrolide resistance (5). However, some mutations in L4, such as G57D, G57V, and A71D, seem to impede the emergence of highly resistant mutants (mutations in the 23S rRNA gene), because subsequent selection of mutants carrying such mutations did not further increase the Ery MIC (Table 3). A similar phenomenon was observed in previous studies (11, 28). These observations suggest that mutations in L4 and L22 are commonly involved in the development of macrolide resistance, but certain mutations in the ribosomal proteins might be incompatible with the mutations in the 23S rRNA gene, preventing the emergence of highly resistant mutants.
Interestingly, there were some differences in the mutations selected with Tyl and Ery. The mutations G57D, G57V, and A71D in L4 were observed only in mutants selected with Tyl (Tables 2 and 3). On the other hand, the mutations in the 23S rRNA gene occurred only in mutants selected with Ery, and selection with Tyl was not able to obtain mutants highly resistant to Ery (MIC, ≥512). A previous study also found that Tyl failed to select Campylobacter mutants that harbor mutations in the 23S rRNA gene in culture medium (11). However, Eryr mutants that harbored mutations in the 23S rRNA gene developed in C. jejuni-infected chickens fed a growth-promoting dose of Tyl for a prolonged time (10). These observations suggest that the selection environment influences the development of macrolide-resistant mutants. Additionally, Ery is a 14-membered macrolide while Tyl is a 16-membered macrolide with slightly different ribosome binding sites (29). Thus, the structural variations between the two antibiotics might also contribute to the differences in the mutants selected.
C. jejuni has three copies of the rrn operon (20, 30, 31). Multiple studies have identified Eryr mutants (Ery MIC, >64 μg/ml) that carried the A2074C or A2075G mutation in two or three copies of the 23S rRNA gene (11, 20, 28, 32, 33). A previous study reported that the A2074G mutation in only one copy of the 23S rRNA gene resulted in a moderate increase in the Ery MIC (8 μg/ml) for a Campylobacter coli mutant that was selected with Ery (10). In this study, the A2074C transversion was observed in at least two copies of the 23S rRNA gene (Table 2). In lineage 76Ex-6, further selection of the mutant with the A2074C transversion in two copies of the 23S rRNA gene led to the occurrence of the same mutation in all three copies and a concurrent increase in the macrolide MICs (Table 2). Together, these observations suggest that the number of mutated copies of the 23S rRNA gene increasingly reduces the susceptibility of Campylobacter to macrolide antibiotics but a mutation in at least two copies of the 23S rRNA gene is required for high-level resistance to macrolides.
In Campylobacter, the frequency of emergence of single-step macrolide-resistant mutants is quite low (<10−9), and de novo development of macrolide-resistant mutants requires prolonged exposure to the antibiotic (10), suggesting that multiple mutations are required to raise the MICs of macrolide antibiotics. During the stepwise selection process in this study, 107 CFU of the organisms were inoculated into 10 ml of MH broth with an elevated antibiotic concentration. This inoculum was well below the number required for the presence of preexisting multiple mutations. Thus, multiple passages were required during certain selection steps to allow the selection of new mutations that support full growth at an elevated antibiotic concentration. Despite the difference between this methodology and the model of selection in an animal host (10), the evolutionary pathways appear to be similar, i.e., involving mutations in L4 and L22, followed by the occurrence of mutations in the 23S rRNA gene. It should be pointed out that this study measured mutations in only a few target genes. Mutations in other chromosomal genes likely also contribute to the development of macrolide resistance in Campylobacter and were not measured in this work.
The overexpression of CmeABC in the stepwise selection process was consistently shown with three techniques, including qRT-PCR, immunoblotting, and microarray analysis (Fig. 1, 2 and 3), suggesting that the differential expression of this predominant antibiotic efflux system facilitates the development of macrolide resistance. Besides cmeABC, two major facilitator superfamily (MFS) transporters (Cj1687 and Cj1257c) were particularly upregulated as detected by qRT-PCR (Fig. 1), while the microarray analysis result revealed the significant upregulation of a small multidrug resistance (SMR) superfamily member transporter (Cj1173) and an MFS transporter (Cj0035c) in 68E8-3 (Fig. 3, cluster 1). Although a previous study (34) found that insertional inactivation of Cj1687, Cj1257c, Cj1174, and Cj0035c did not alter the susceptibility of C. jejuni to Ery, the upregulation of these efflux transporters during stepwise selection suggests that they may play a role in the development of macrolide resistance. Cj1375 (cmeG) is another MFS transporter of C. jejuni and was shown to confer resistance to antibiotics and oxidative stress; however, overexpression of cmeG did not increase resistance to Ery (35). In this study, cmeG was upregulated in some lineages but the change was not as obvious as that in other efflux genes examined.
An interesting observation of this study is that the overexpression of the antibiotic efflux genes was most significant in the intermediately resistant mutants and the level of overexpression actually declined in the late-stage mutants (Fig. 1, 2, and 3). This finding suggests that the enhanced expression of the efflux genes is temporarily required to facilitate the development of highly resistant mutants, in which mutations in the 23S rRNA gene or other changes stabilize the resistance phenotype. The interplay of the antibiotic efflux pump CmeABC and ribosomal protein L4/L22 was also observed in previous studies in which it was found that the efflux system acted synergistically with some modifications in ribosomal proteins L4 and L22 to mediate intermediate-level macrolide resistance and facilitate the development of mutation in the 23S rRNA gene (14, 15). Together, these observations clearly indicate that the dynamic change in efflux gene expression interacts with target mutations to facilitate Campylobacter adaptation to the selection pressure from macrolide treatment.
In Campylobacter, expression of cmeABC is controlled by CmeR, which functions as a repressor of cmeABC (16). The overexpression of cmeABC during stepwise selection could be partly explained by mutations in CmeR, such as the nucleotide deletion at 490A and the H174N change in the protein (Tables 2 and 3). On the basis of the sequence and crystal structure of CmeR, these mutations are located in the C-terminal domain of CmeR (36–39). The deletion at 490A is expected to truncate the CmeR protein, which may explain the significant upregulation of cmeABC in the 68Ex-1 lineage (Table 2; Fig. 1B and 2B). The H174 residue of CmeR is in the ligand-binding pocket of CmeR and is important for the interaction with various ligands, including bile acid, glycerol, and drugs (38, 39). Thus, the H174N mutation likely affects the function of CmeR and leads to the upregulation of cmeABC in the 68Ex-3 lineage (Table 2; Fig. 1A and 2A). This notion is further supported by the fact that Cj0561c was also upregulated in lineage 68Ex-3 (Fig. 3). Cj0561c encodes a putative periplasmic protein and is subject to direct regulation by CmeR (17). Thus, overexpression of Cj0561c further suggested that the H174N mutation altered the function of CmeR. The direct impact of the CmeR mutations on the function of CmeR remains to be determined in future studies.
It should be pointed out that the overexpression of cmeABC cannot be totally explained by mutations in CmeR, as some lineages (such as 76Ex-6 and 76Tx-7) did not harbor any mutations in CmeR (or the promoter of cmeABC) but showed overexpression of cmeABC (Tables 2 and 3; Fig. 1 and 2). Additionally, although the same CmeR mutation (such as in lineages 68Ex-1 and 68Ex-3) existed in the late-stage mutants, the levels of cmeABC overexpression declined in these highly resistant mutants. These observations suggest that a CmeR-independent mechanism also modulates the expression of cmeABC. Indeed, a recent study indicated that CosR, an oxidative stress regulator, serve as the second repressor of cmeABC (40). Thus, any mutations that affect the function of CosR would potentially modulate the expression of cmeABC. It is also likely that the accumulation of other mutations offsets the need for cmeABC overexpression in late-stage mutants. These possibilities await further examination in future studies.
The microarray analysis produced several interesting findings. First, few genes were differentially expressed in the first-step mutant, while most of the differential gene expression was observed in the intermediate-level-resistant mutant (68E8-3) and the number of differentially expressed genes and the magnitudes of differential expression were significantly reduced in highly resistant strain 68E64-3, which contained the A2074C mutation in all three copies of the 23S rRNA gene. This finding strongly suggests that global changes at the transcriptomic level are temporarily involved in the development of macrolide-resistant mutants and that there is a tendency to restore the transcriptome to the wild-type state in the stable highly resistant mutant. Second, a large number of genes encoding ribosomal proteins (rplJPTXOLRFNVAWCDME, rpsPNSCDQEJ, and rpmC) were upregulated. Macrolides target the bacterial ribosome and inhibit protein synthesis, and the resistance-associated mutations in L4 and L22 alleviate the inhibitory effect of the antibiotics but might reduce the efficiency of protein synthesis (5). Thus, upregulation of the protein synthesis machinery is likely a compensatory response to the reduced rate of protein synthesis in the mutants. Third, a large number of genes involved in the heat shock response, motility, and energy metabolism were significantly downregulated in the macrolide-resistant mutants, suggesting that the development of macrolide resistance in C. jejuni profoundly impacts Campylobacter physiology and may result in a growth burden and fitness cost. Indeed, several recent studies found that macrolide-resistant Campylobacter shows a significant fitness cost in vitro and in vivo and is outcompeted by susceptible Campylobacter in the chicken host in the absence of antibiotic selection pressure (41–44).
In summary, this study has revealed dynamic changes in target gene mutations and differential gene expression during the stepwise selection of macrolide resistance in Campylobacter. Particularly, the results discovered a temporal overexpression of antibiotic efflux pumps in the selection process, which interacts with mutations in L4 and L22 and facilitates the development of stable and highly resistant mutants. Additionally, the study also revealed that there is an intermediate “chaos” state in terms of global gene expression in the development of macrolide resistance and further adaptation to antibiotic selection appears to favor the return of the transcriptome to the wild-type state in highly resistant mutants. These findings depict a complex process involving interplay between target mutations and differential gene expression and provide new insights into the molecular mechanisms underlying the development of macrolide resistance in C. jejuni.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the technical help provided by Byeonghwa Jeon, Taradon Luangtongkum, Yang Wang, Paul Plummer, and Rachel Sippy.
This work was supported by grant R01DK063008 from the National Institute of Diabetes and Digestive and Kidney Diseases. The contents are solely our responsibility and do not necessarily represent the official views of the funding agency.
Footnotes
Published ahead of print 28 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01927-12.
REFERENCES
- 1. Blaser MJ, Taylor DN, Feldman RA. 1983. Epidemiology of Campylobacter jejuni infections. Epidemiol. Rev. 5:157–176 [DOI] [PubMed] [Google Scholar]
- 2. Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 4:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Q, Lin J, Pereira S. 2003. Fluoroquinolone-resistant Campylobacter in animal reservoirs: dynamics of development, resistance mechanisms and ecological fitness. Anim. Health Res. Rev. 4:63–71 [DOI] [PubMed] [Google Scholar]
- 4. McEwen SA, Fedorka-Cray PJ. 2002. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 34(Suppl 3):S93–S106 [DOI] [PubMed] [Google Scholar]
- 5. Gibreel A, Taylor DE. 2006. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob. Chemother. 58:243–255 [DOI] [PubMed] [Google Scholar]
- 6. Lévesque S, Frost E, Michaud S. 2007. Comparison of antimicrobial resistance of Campylobacter jejuni isolated from humans, chickens, raw milk, and environmental water in Quebec. J. Food Prot. 70:729–735 [DOI] [PubMed] [Google Scholar]
- 7. van Hees BC, Veldman-Ariesen MJ, de Jongh BM, Tersmette M, van Pelt W. 2007. Regional and seasonal differences in incidence and antibiotic resistance of Campylobacter from a nationwide surveillance study in The Netherlands: an overview of 2000-2004. Clin. Microbiol. Infect. 13:305–310 [DOI] [PubMed] [Google Scholar]
- 8. Vlieghe ER, Jacobs JA, Van Esbroeck M, Koole O, Van Gompel A. 2008. Trends of norfloxacin and erythromycin resistance of Campylobacter jejuni/Campylobacter coli isolates recovered from international travelers, 1994 to 2006. J. Travel Med. 15:419–425 [DOI] [PubMed] [Google Scholar]
- 9. Pollett S, Rocha C, Zerpa R, Patino L, Valencia A, Camina M, Guevara J, Lopez M, Chuquiray N, Salazar-Lindo E, Calampa C, Casapia M, Meza R, Bernal M, Tilley D, Gregory M, Maves R, Hall E, Jones F, Arriola CS, Rosenbaum M, Perez J, Kasper M. 2012. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect. Dis. 12:193 doi:10.1186/1471-2334-12-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin J, Yan M, Sahin O, Pereira S, Chang YJ, Zhang Q. 2007. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob. Agents Chemother. 51:1678–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caldwell DB, Wang Y, Lin J. 2008. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 52:3947–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mamelli L, Prouzet-Mauleon V, Pages JM, Megraud F, Bolla JM. 2005. Molecular basis of macrolide resistance in Campylobacter: role of efflux pumps and target mutations. J. Antimicrob. Chemother. 56:491–497 [DOI] [PubMed] [Google Scholar]
- 13. Cagliero C, Mouline C, Payot S, Cloeckaert A. 2005. Involvement of the CmeABC efflux pump in the macrolide resistance of Campylobacter coli. J. Antimicrob. Chemother. 56:948–950 [DOI] [PubMed] [Google Scholar]
- 14. Cagliero C, Mouline C, Cloeckaert A, Payot S. 2006. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 50:3893–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibreel A, Wetsch NM, Taylor DE. 2007. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 51:3212–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin J, Akiba M, Sahin O, Zhang Q. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 49:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo B, Wang Y, Shi F, Barton YW, Plummer P, Reynolds DL, Nettleton D, Grinnage-Pulley T, Lin J, Zhang Q. 2008. CmeR functions as a pleiotropic regulator and is required for optimal colonization of Campylobacter jejuni in vivo. J. Bacteriol. 190:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pumbwe L, Randall LP, Woodward MJ, Piddock LJ. 2005. Evidence for multiple-antibiotic resistance in Campylobacter jejuni not mediated by CmeB or CmeF. Antimicrob. Agents Chemother. 49:1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jorgensen JH, Hindler JF. 2007. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin. Infect. Dis. 44:280–286 [DOI] [PubMed] [Google Scholar]
- 20. Gibreel A, Kos VN, Keelan M, Trieber CA, Lévesque S, Michaud S, Taylor DE. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han J, Sahin O, Barton YW, Zhang Q. 2008. Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog. 4:e1000083 doi:10.1371/journal.ppat.1000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin J, Cagliero C, Guo B, Barton YW, Maurel MC, Payot S, Zhang Q. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 187:7417–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin J, Michel LO, Zhang Q. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poehlsgaard J, Douthwaite S. 2005. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3:870–881 [DOI] [PubMed] [Google Scholar]
- 25. Roberts MC. 2004. Resistance to macrolide, lincosamide, streptogramin, ketolide, and oxazolidinone antibiotics. Mol. Biotechnol. 28:47–62 [DOI] [PubMed] [Google Scholar]
- 26. Davydova N, Streltsov V, Wilce M, Liljas A, Garber M. 2002. L22 ribosomal protein and effect of its mutation on ribosome resistance to erythromycin. J. Mol. Biol. 322:635–644 [DOI] [PubMed] [Google Scholar]
- 27. Malbruny B, Canu A, Bozdogan B, Fantin B, Zarrouk V, Dutka-Malen S, Feger C, Leclercq R. 2002. Resistance to quinupristin-dalfopristin due to mutation of L22 ribosomal protein in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2200–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lehtopolku M, Kotilainen P, Haanpera-Heikkinen M, Nakari UM, Hanninen ML, Huovinen P, Siitonen A, Eerola E, Jalava J, Hakanen AJ. 2011. Ribosomal mutations as the main cause of macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 55:5939–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kannan K, Mankin AS. 2011. Macrolide antibiotics in the ribosome exit tunnel: species-specific binding and action. Ann. N. Y. Acad. Sci. 1241:33–47 [DOI] [PubMed] [Google Scholar]
- 30. Taylor DE, Eaton M, Yan W, Chang N. 1992. Genome maps of Campylobacter jejuni and Campylobacter coli. J. Bacteriol. 174:2332–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newnham E, Chang N, Taylor DE. 1996. Expanded genomic map of Campylobacter jejuni UA580 and localization of 23S ribosomal rRNA [sic] genes by I-CeuI restriction endonuclease digestion. FEMS Microbiol. Lett. 142:223–229 [DOI] [PubMed] [Google Scholar]
- 32. Ladely SR, Meinersmann RJ, Englen MD, Fedorka-Cray PJ, Harrison MA. 2009. 23S rRNA gene mutations contributing to macrolide resistance in Campylobacter jejuni and Campylobacter coli. Foodborne Pathog. Dis. 6:91–98 [DOI] [PubMed] [Google Scholar]
- 33. Ladely SR, Harrison MA, Fedorka-Cray PJ, Berrang ME, Englen MD, Meinersmann RJ. 2007. Development of macrolide-resistant Campylobacter in broilers administered subtherapeutic or therapeutic concentrations of tylosin. J. Food Prot. 70:1945–1951 [DOI] [PubMed] [Google Scholar]
- 34. Ge B, McDermott PF, White DG, Meng J. 2005. Role of efflux pumps and topoisomerase mutations in fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 49:3347–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeon B, Wang Y, Hao H, Barton YW, Zhang Q. 2011. Contribution of CmeG to antibiotic and oxidative stress resistance in Campylobacter jejuni. J. Antimicrob. Chemother. 66:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Routh MD, Su CC, Zhang Q, Yu EW. 2009. Structures of AcrR and CmeR: insight into the mechanisms of transcriptional repression and multi-drug recognition in the TetR family of regulators. Biochim. Biophys. Acta 1794:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu R, Su CC, Shi F, Li M, McDermott G, Zhang Q, Yu EW. 2007. Crystal structure of the transcriptional regulator CmeR from Campylobacter jejuni. J. Mol. Biol. 372:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lei HT, Shen Z, Surana P, Routh MD, Su CC, Zhang Q, Yu EW. 2011. Crystal structures of CmeR-bile acid complexes from Campylobacter jejuni. Protein Sci. 20:712–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hwang S, Zhang Q, Ryu S, Jeon B. 2012. Transcriptional regulation of the CmeABC multidrug efflux pump and the KatA catalase by CosR in Campylobacter jejuni. J. Bacteriol. 194:6883–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hao H, Dai M, Wang Y, Peng D, Liu Z, Yuan Z. 2009. 23S rRNA mutation A2074C conferring high-level macrolide resistance and fitness cost in Campylobacter jejuni. Microb. Drug Resist. 15:239–244 [DOI] [PubMed] [Google Scholar]
- 42. Luangtongkum T, Shen Z, Seng VW, Sahin O, Jeon B, Liu P, Zhang Q. 2012. Impaired fitness and transmission of macrolide-resistant Campylobacter jejuni in its natural host. Antimicrob. Agents Chemother. 56:1300–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Almofti YA, Dai M, Sun Y, Haihong H, Yuan Z. 2011. Impact of erythromycin resistance on the virulence properties and fitness of Campylobacter jejuni. Microb. Pathog. 50:336–342 [DOI] [PubMed] [Google Scholar]
- 44. Han F, Pu S, Wang F, Meng J, Ge B. 2009. Fitness cost of macrolide resistance in Campylobacter jejuni. Int. J. Antimicrob. Agents 34:462–466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.