Abstract
Fosfomycin is a potential option for vancomycin-resistant enterococcus (VRE) infections despite limited in vitro and clinical data. In this study, 32 VRE isolates from renal transplant patients with urinary stent infections were susceptible to fosfomycin, daptomycin, and linezolid and resistant to amoxicillin, minocycline, and nitrofurantoin based on their MIC50s and MIC90s. Fosfomycin was bacteriostatic at 0.5 to 16× the MIC (32 to 2,048 μg/ml); synergy occurred when fosfomycin was combined with daptomycin (2.8 to 3.9 log10 CFU/ml kill; P < 0.001) or amoxicillin (2.6 to 3.4; P < 0.05). These combinations may be potent options to treat VRE urinary infections pending investigation of clinical efficacy.
TEXT
Solid-organ transplant recipients are at increased risk for colonization with vancomycin-resistant enterococcus (VRE) and vulnerable to active infections with this organism (1). At our institution, a 7-French, 16-cm double-J stent is inserted through the ureter into the renal pelvis and then into the bladder at the time of kidney transplantation. Biofilm development often precipitates colonization of the stents and results in sequestered, stationary-phase bacteria that further resist the effects of antibiotics.
Fosfomycin, a phosphonic acid derivative initially isolated in 1969 from cultures of Streptomyces species, is an oral therapy for uncomplicated urinary tract infections (UTIs) (2). It is often bactericidal against multidrug-resistant Gram-positive and Gram-negative pathogens, although it has not been well studied against VRE. The antibacterial activity of fosfomycin is achieved by inhibiting the enzyme N-acetylglucosamine (UDP-GlcNAc) enolpyruvyl transferase (MurA), which synthesizes UDP-N-acetylenolpyruvylglucosamine, an essential component in the biosynthesis of peptidoglycan (2). It is highly concentrated in the urine, with peak values of 1,053 to 4,415 μg/ml within 4 h after a single oral 3-g dose (2). Fosfomycin has gained recent interest as a potential therapeutic option for treating infections caused by VRE despite limited efficacy data (3).
The aim of this study was to investigate fosfomycin activity in vitro alone and in combination with other antibiotic treatment options against VRE urine isolates collected from renal transplant patients with urinary stent infections. We present synergistic combinations with fosfomycin that may be useful treatment options for these situations.
(Portions of this work were presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2011 [4].)
Thirty-two VRE (Enterococcus faecium) isolates were collected from renal transplant patients with urinary stent infections from 2007 to 2010 at a tertiary medical center. Enterococcus faecalis ATCC 29212 was used as a control strain. The antibiotics evaluated were amoxicillin, fosfomycin, nitrofurantoin, and minocycline, purchased from Sigma-Aldrich (St. Louis, MO), as well as linezolid (Pfizer, NY) and daptomycin (Cubist, Lexington, MA), which were commercially purchased.
Antibiotic activities were determined in calcium-adjusted Mueller-Hinton broth (MHB) appropriate for the antibiotics tested (5). For assays involving fosfomycin, MHB was also supplemented with 25 μg/ml glucose-6 phosphate. All biofilm assays were evaluated in tryptic soy broth plus 1% dextrose and supplemented with the appropriate requirements for selected antibiotics (6).
MICs and minimum bactericidal concentrations (MBCs) against planktonic cultures were determined by broth microdilution (5). The MIC and minimum biofilm eradication concentration (MBEC) were determined using a transferable solid-phase pin lid (Nunc) as described by Ceri et al. (7).
Antibiotic activity against four of the clinical isolates as well as ATCC 29212 was analyzed in a 24-h time-kill curve in duplicate with an inoculum of 5 × 105 CFU/ml. The standard kill curve method for synergy (8) utilized variable concentrations of the primary antibiotic (fosfomycin, 0.5 to 16× the MIC) in combination with a static subinhibitory concentration (0.5× the MIC) of the secondary antibiotic. Synergy, additive effect, antagonism, and indifference were defined as ≥2 log kill, <2 but >1 log kill, >1 log growth, and ±1 log kill, respectively (8). Bactericidal activity was defined as 99.9% kill from the initial inoculum.
Clinical VRE isolates were susceptible to fosfomycin, daptomycin, and linezolid and resistant to amoxicillin, minocycline, and nitrofurantoin based on MIC50 and MIC90 results (Table 1). Linezolid and daptomycin were the most active antibiotics, with MIC90s of 2 and 4 μg/ml, respectively. Only linezolid maintained the same MIC profile when tested against biofilm cultures, while fosfomycin and daptomycin had at least a 2-fold increase in the MIC90 in biofilm (Table 1).
Table 1.
Susceptibility results of Enterococcus faecium isolatesa
| Antibiotic | Susceptibility (μg/ml)b |
|||||
|---|---|---|---|---|---|---|
| Planktonic cultures |
Biofilm cultures |
|||||
| MIC50 | MIC90 | MBC range | MIC50 | MIC90 | MBEC range | |
| Amoxicillin | 64 | 128 | 16–1,024 | 64 | 128 | 64–1,024 |
| Daptomycin | 2 | 4 | 1–32 | 2 | 8 | 1–32 |
| Fosfomycin | 64 | 64 | 128 | 64 | 128 | 256–512 |
| Linezolid | 2 | 2 | 16–32 | 2 | 2 | 16–32 |
| Minocycline | 16 | 32 | 4–32 | 8 | 32 | 4–256 |
| Nitrofurantoin | 64 | 128 | 32–1,024 | 64 | 128 | 512–1,024 |
Susceptibility results in planktonic and biofilm cultures of 32 vancomycin-resistant Enterococcus faecium isolates collected from renal transplant patients with urinary stent infections.
MBC, minimum bactericidal concentration; MBEC, minimum biofilm eradication concentration.
Fosfomycin was bacteriostatic against VRE in the kill curve at concentrations of 0.5 to 16× the MIC. Concentrations of 0.5 to 4× the MIC resulted in 0.2 to 1.6 log10 CFU/ml kill at 8 h followed by regrowth. Fosfomycin concentrations of 16× the MIC were the most active, with 0.9 to 2.9 log10 CFU/ml maximum 24-h kill. Fosfomycin was bactericidal and considerably more active against vancomycin-susceptible E. faecalis ATCC 29212 (maximum kill, 3.4 to 3.5 log10 CFU/ml; P < 0.001).
The addition of daptomycin, amoxicillin, or linezolid to fosfomycin in the kill curve increased bacterial killing (Fig. 1, each antibiotic at 0.5× the MIC), while combination with nitrofurantoin or minocycline had no further antimicrobial effects, with ±1 log difference in kill compared to any agent alone (data not shown). The most potent and highly synergistic combination was fosfomycin plus daptomycin, which often achieved bactericidal activity against clinical strains (maximum kill, 2.8 to 3.9 log10 CFU/ml; P < 0.001 versus either agent alone). Fosfomycin plus amoxicillin was also synergistic (2.6 to 3.4 log10 CFU/ml kill; P < 0.05) but was bactericidal in only half of the clinical strains tested. Fosfomycin in combination with linezolid in the kill curve was bacteriostatic and produced a synergistic or additive effect. No antagonism was detected.
Fig 1.
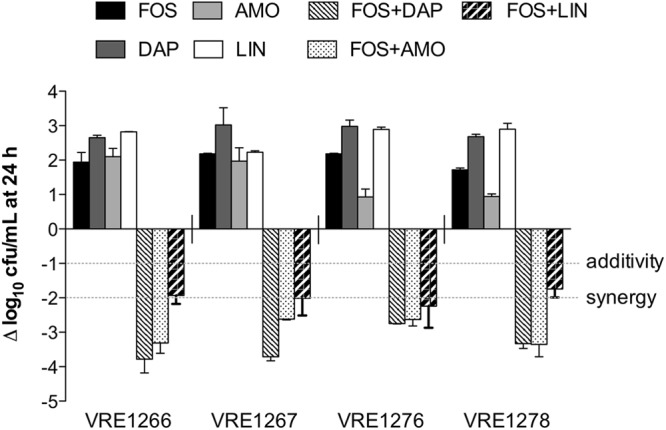
Change in log10 CFU/ml after 24-h antibiotic exposures in the kill curve. Data represent antibiotics evaluated alone or in combination at 0.5× the MIC. Fosfomycin (FOS) combined with either daptomycin (DAP) or amoxicillin (AMO) was synergistic and often bactericidal (≥3 log kill), while FOS combined with linezolid (LIN) was either synergistic or additive yet bacteriostatic.
The use of fosfomycin in the treatment of VRE has recently gained renewed interest although few in vitro or clinical data support its use. Of the VRE isolates in our study, 91% were susceptible (MIC, ≤64 μg/ml) to fosfomycin (5), consistent with the limited prior reports of fosfomycin susceptibility in VRE (3, 9). Fosfomycin exhibited a concentration-dependent effect in the standard kill curve. The antibacterial activity of fosfomycin increased from low to high concentrations but still remained bacteriostatic up to 16× the MIC. Although fosfomycin concentrations are high at this exposure (1,024 to 2,048 μg/ml), they represent concentrations comparable to those achieved in the urine of patients after a single 3-g oral dose (9). Interestingly, low exposures of fosfomycin (0.5× the MIC) in combination with daptomycin, amoxicillin, or linezolid produced greater bacterial killing than high concentrations of fosfomycin alone (1 to 16× the MIC) and bacterial killing similar to that induced by high fosfomycin exposures in combination with 0.5× the MICs of secondary antibiotics.
Synergy studies with fosfomycin and daptomycin are limited, but one case report describes successful treatment with this combination for S. aureus endocarditis caused by a daptomycin-nonsusceptible strain (10). In our study, fosfomycin plus daptomycin was the most active combination against VRE and resulted in bacterial kill at or close to the detection limit. Based on the ability of fosfomycin to inhibit essential enzymes in the biosynthesis of peptidoglycan (11), we hypothesize that this synergistic effect is largely due to fosfomycin increasing the sensitivity of the bacterial cell envelope to daptomycin.
Aminopenicillins are key agents used in the treatment of Enterococcus sp. infections, although the activities of ampicillin and amoxicillin alone against VRE are poor (9). Fosfomycin modifies the production of penicillin binding proteins (PBPs) in Staphylococcus aureus and Streptococcus pneumoniae (12). This suggests that the combination of fosfomycin and amoxicillin has potential to overcome beta-lactam resistance caused by PBPs (12). In our study, synergy was observed for fosfomycin in combination with amoxicillin despite amoxicillin resistance in VRE. This combination may be a potent and sought-after oral option to treat VRE urinary infections, since both agents achieve high urine concentrations.
Linezolid has in vitro activity against VRE, with susceptibility rates over 99% (13). The isolates in our study displayed similar susceptibility rates (97%), and importantly linezolid susceptibility in biofilm was maintained (MIC, ≤2 μg/ml). Fosfomycin combined with linezolid was either synergistic or additive in the kill curve. One previous study with fosfomycin and linezolid suggests potential for this combination therapy when used against Staphylococcus epidermis and Staphylococcus aureus (11). This combination could be an effective oral option for the treatment of VRE UTIs, including those with biofilm development on urinary stents.
The treatment of UTIs caused by VRE in renal transplant patients is often difficult to manage due to host immunosuppression, hardware placement, and lack of optimally studied antibiotic options (14). A recent case series of fosfomycin outcomes in complex urinary tract infections due to multidrug-resistant organisms, including VRE, reported that urinary stents were associated with microbiologic failure in fosfomycin monotherapy (3). In order to effectively treat such infections, early hardware removal and complex antimicrobial therapy are often required (9). Based on our findings, combination therapy with fosfomycin and either daptomycin or amoxicillin is highly synergistic and results in enhanced bactericidal activity compared to fosfomycin alone against VRE. Additional modeling of these two combinations using pharmacokinetic concentrations achieved in urine will help to define their potential utilities. These combinations should be further explored for clinical efficacy.
ACKNOWLEDGMENT
This work was supported by the University of Wisconsin Hospital and Clinics.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Sood S, Malhotra M, Das BK, Kapil A. 2008. Enterococcal infections & antimicrobial resistance. Indian J. Med. Res. 128:111–121 [PubMed] [Google Scholar]
- 2. Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents. 34:506–515 [DOI] [PubMed] [Google Scholar]
- 3. Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob. Agents Chemother. 56:5744–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Descourouez JL, Jorgenson M, Rose W. 2011. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., abstr E-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Rose WE, Poppens PT. 2009. Impact of biofilm on the in vitro activity of vancomycin alone and in combination with tigecycline and rifampicin against Staphylococcus aureus. J. Antimicrob. Chemother. 63:485–488 [DOI] [PubMed] [Google Scholar]
- 7. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 61:365–370 [DOI] [PubMed] [Google Scholar]
- 9. Heintz BH, Halilovic J, Christensen CL. 2010. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy 30:1136–1149 [DOI] [PubMed] [Google Scholar]
- 10. Chen LY, Huang CH, Kuo SC, Hsiao CY, Lin ML, Wang FD, Fung CP. 2011. High-dose daptomycin and fosfomycin treatment of a patient with endocarditis caused by daptomycin-nonsusceptible Staphylococcus aureus: case report. BMC Infect. Dis. 11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grif K, Dierich MP, Pfaller K, Miglioli PA, Allerberger F. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J. Antimicrob. Chemother. 48:209–217 [DOI] [PubMed] [Google Scholar]
- 12. Utsui Y, Ohya S, Magaribuchi T, Tajima M, Yokota T. 1986. Antibacterial activity of cefmetazole alone and in combination with fosfomycin against methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 30:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhanel GG, Laing NM, Nichol KA, Palatnick LP, Noreddin A, Hisanaga T, Johnson JL, Hoban DJ, NAVRESS Group 2003. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J. Antimicrob. Chemother. 52:382–388 [DOI] [PubMed] [Google Scholar]
- 14. de Souza RM, Olsburgh J. 2008. Urinary tract infection in the renal transplant patient. Nat. Clin. Pract. Nephrol. 4:252–264 [DOI] [PubMed] [Google Scholar]


