Abstract
Chronic infection with hepatitis B virus (HBV) is associated with impairment of T and NK cell immunity. This study was aimed at investigating the impact of treatment with telbivudine (LDT) on T and NK cell immunity in patients with chronic hepatitis B (CHB). A total of 54 CHB patients and 30 healthy controls (HC) were recruited. Individual patients were treated orally with 600 mg LDT daily for 13 months. The serum HBV DNA loads, the levels of the HBV-related biomarkers alanine aminotransferase (ALT) and aspartate transaminase (AST), and the numbers of different subsets of peripheral T and NK cells in subjects were measured before and longitudinally after LDT treatment. Following treatment with LDT, the serum HBV DNA loads and the percentages of HBsAg- or HBeAg-seropositive cases were gradually reduced, accompanied by decreased levels of serum ALT and AST. In comparison with the HC, fewer CD3− CD56+ and CD244+ NK cells and CD3+ CD8+ T cells, lower frequencies of cytokine+ CD4+ T cells, and more CD3+ CD4+, CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD8+ PD-1+ T cells were detected in CHB patients. Treatment with LDT increased the numbers of NK and CD8+ cells and the frequencies of cytokine+ CD4+ T cells but reduced the numbers of CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD8+ PD-1+ T cells in CHB patients. The frequencies of cytokine+ CD4+ T cells were negatively associated with the levels of serum HBV DNA, ALT, and AST. Thus, treatment with LDT inhibits HBV replication, modulates T and NK cell immunity, and improves liver function in Chinese patients with CHB.
INTRODUCTION
Hepatitis B is a potentially life-threatening liver disease caused by infection with hepatitis B virus (HBV). Approximately 2 billion people have been infected with HBV, and more than 360 million patients worldwide have chronic hepatitis B (CHB) (1). Although the HBV-specific vaccine is highly effective and safe and has been used for more than 20 years, HBV is still the most common cause of chronic liver disease in the world (2). Furthermore, many patients with CHB are at high risk of developing liver cirrhosis and hepatocellular carcinoma (HCC) (3). Hence, understanding the pathogenesis of CHB is of great importance in the management of patients with CHB.
T cell responses are crucial for viral clearance in HBV-infected individuals (4). Both CD4+ and CD8+ T cells are responsible for the control of acute HBV infection (5). Previous studies have shown that circulating HBV-specific CD4+ and CD8+ T cells are rarely detected in patients with CHB (6, 7), suggesting an impairment of HBV-specific T cell responses. In addition, recent studies have suggested that polyclonal and multispecific T-helper (Th) cell responses, including Th1 and Th2 responses, determine the outcome of HBV infection (8, 9). Th1 cells produce interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), whereas Th2 cells secrete IL-4, IL-6, IL-10, and IL-13 (10). Moreover, the profiles of these circulating cytokines are associated with the levels of viral replication and liver function (11). Regulatory T cells (Tregs) are important regulators of immune responses, and a higher frequency of Tregs is commonly detected in patients with CHB (12–14). Furthermore, higher percentages of Tregs are associated with increased levels of serum HBV DNA, suggesting that Tregs inhibit HBV-specific T cell immunity in patients with CHB (15, 16). In addition, Tregs can modulate HBV-specific CD8+ T cell responses (16). NK cells participate in the control of HBV infection (17). NK cells also produce proinflammatory cytokines such as IFN-γ, which regulates antiviral immunity (17). The CD244 antigen is an activating receptor on NK cells, and engagement of CD244 enhances NK cell activity and IFN-γ production (18). Numerous studies have shown that a lower frequency of CD244+ NK cells is present in CHB patients (19). Therefore, different subsets of lymphocytes control HBV replication and disease progression in patients with CHB. However, there are few longitudinal studies of lymphocyte profiles and function during the pathogenic process of CHB, particularly in Chinese patients.
Our previous studies have shown that treatment with antiviral drugs such as adefovir dipivoxil (ADV) or entecavir (ETV) can inhibit HBV replication, reduce the levels of serum HBeAb, and improve liver function (20, 21). Long-term treatment with ADV or ETV enhances HBV-specific T cell immunity and reduces the frequency of Tregs in patients with CHB (20, 21). However, a previous study has indicated that long-term treatment with ADV or ETV has a very low HBeAg+ seroconversion rate in CHB patients (22). Hence, application of new antiviral drugs may be valuable for the management of patients with CHB. Telbivudine (LDT) is a synthetic thymidine nucleoside analogue with activity against HBV (23). The available data from clinical trials indicate that LDT is a potent inhibitor of HBV replication and a better inducer of HBeAg+ seroconversion than other antiviral reagents in CHB patients (24, 25). A recent study showed that treatment with LDT rapidly reduces the HBV DNA load, which is associated with an increased frequency of Th2 cells and decreased frequencies of Th17 cells and Tregs in a few patients with CHB (26). However, little is known about the impact of treatment with LDT on the numbers of CD8+ T cells and NK cells and their functions in patients with CHB.
In this study, we examined the effect of treatment with LDT on the numbers of CD4+ and CD8+ T cells and NK cells and their functions, as well as the potential association of different populations of lymphocytes with liver function, in 54 patients with CHB. We found that treatment with LDT inhibited HBV replication and improved liver function, accompanied by modulation of NK and T cells, in patients with CHB.
MATERIALS AND METHODS
Patients.
A total of 54 patients with CHB were recruited at the First Hospital of Jilin University from September 2008 to October 2010. Individual subjects with CHB were diagnosed if they were seropositive for HBsAg, HBeAg, and HBV DNA for at least 13 months (27). All hepatitis B patients had evidence of infection with genotype C HBV. Another 30 gender-, age-, and ethnicity-matched healthy subjects were also recruited as healthy controls (HC). Individuals with seropositive hepatitis C virus, hepatitis G virus, hepatitis D virus, or HIV-1 infection or autoimmune liver disease were excluded. All patients denied being drug users or having been exposed to hepatotoxins, so far as they knew (27). Written informed consent was obtained from individual participants. The experimental protocol was established according to the guidelines of the 1975 Declaration of Helsinki and was approved by the Human Ethics Committee of Jilin University, China. The demographic and clinical characteristics of the patients are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Parametera | Value for groupb |
|
|---|---|---|
| CHB patients | HC | |
| No. of individuals | 54 | 30 |
| Age (yr) | 41.8 ± 6.7 | 42.9 ± 8.1 |
| No. (%) of: | ||
| Males | 44 (81.5) | 24 (80) |
| Females | 10 (18.5) | 6 (20) |
| HBV DNA level (log10 copies/ml) | 8.1 ± 1.2 | NA |
| ALT level (U/liter) | 167.2 ± 1.2* | 22.6 ± 4.3 |
| AST level (U/liter) | 132.9 ± 9.9* | 22.3 ± 5.3 |
| HBsAg level (log10 IU/ml) | 1.6 ± 2.5 | NA |
Normal values are as follows: ALT, ≤40 IU/liter; AST, ≤40 IU/liter; and HBV DNA, ≤3 log10 copies/ml.
Data are means ± standard deviations (SD), except as specified. HC, healthy controls; CHB, chronic hepatitis B; NA, not applicable.
, P < 0.05 versus the HC.
Individual patients were treated orally with 600 mg LDT (Novartis Pharmaceutics) once per day for 13 months, along with other short-term common medicines to reduce clinical symptoms, including metoclopramide (Fahrenheit Pharmaceutical, Shanghai, China) three times per day for 10 days for two patients and ibuprofen (Zhengzhou Chengwang Chemical Pharmaceutical, Zhengzhou, China) for 7 days for four patients. The patients visited the outpatient service every 3 months for a physical examination and laboratory tests. Their clinical symptoms were recorded, and the patients were monitored for 1 year.
Virological and immunological assessments.
Peripheral blood venous samples were obtained from individual patients before treatment and 3, 6, 9, and 13 months after the initial treatment. The levels of plasma HBsAg, anti-HBs, anti-HBc, HBeAg, and anti-HBe in individual participants were measured using commercially available kits according to the manufacturer's instructions (Abbott Laboratories). The levels of serum alanine aminotransferase (ALT) and aspartate transaminase (AST) were detected using an automatic biochemistry analyzer (Roche Diagnostics) (21). The levels of serum HBV DNA were determined by quantitative real-time PCR (RT-PCR) using a specific kit (Amplicor; Roche) according to the manufacturer's instructions. The limit of detection for HBV DNA was 300 copies/ml (21). Patients' clinical symptoms were recorded, and the patients were monitored for 1 year.
Flow cytometry analysis.
The frequencies of peripheral blood NK cells and T cells in individual patients were analyzed by flow cytometry using specific antibodies, as previously described (28). Briefly, individual blood samples (100 μl) were incubated in duplicate with a mixture of fluorescein isothiocyanate (FITC)-conjugated anti-CD3, allophycocyanin (APC)-conjugated anti-CD56, and phycoerythrin (PE)-conjugated anti-NK2B4 (BD Bioscience, San Diego, CA) for 30 min at room temperature. FITC-IgG1, PE-IgG1, and APC-IgG1 were used as negative controls. Additional cells were stained with peridinin chlorophyll protein (PerCP)–anti-CD4, FITC–anti-CD25, and PE–anti-CD127 or PerCP–anti-CD3, PE–anti-CD8, and FITC–PD-1. The erythrocytes were lysed with BD FACS lysing solution (BD Bioscience) according to the manufacturer's instructions. Some PerCP–anti-CD4- and FITC–anti-CD25-stained cells were fixed, permeabilized, and stained intracellularly with PE–anti-Foxp3. After being washed, the cells were characterized using a FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software (v7.6.2) (TreeStar, Ashland, OR). At least 20,000 events for each sample were acquired for analysis. To ensure the quality of each batch of data during the longitudinal study, we used the same lots of antibodies from identical manufacturers according to the same protocol, in a blinded manner (28).
Flow cytometry analysis of intracellular cytokine staining (ICS).
For analysis of intracellular cytokine production, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, United Kingdom). Human PBMCs (106 cells/well) were stimulated with 50 ng/ml of phorbol-12-myristate-13-acetate (PMA; Sigma Chemical, St. Louis, MO) and 2 μg/ml of ionomycin in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; HyClone) for 6 h, with the addition of monensin (GolgiStop; BD Sciences) for the last 2 h of incubation. PBMCs cultured in medium alone were used as negative controls. The cells were stained in duplicate with PerCP–anti-CD3 and FITC–anti-CD4 at room temperature for 30 min. After being washed, the cells were fixed and permeabilized using a fixing/permeabilizing reagent (Caltag), followed by staining with PE–anti-IL-2, PE–anti-TNF-α, PE–anti-IL-4, PE–anti-IL-6, PE–anti-IL-10, PE–anti-IFN-γ, or isotype-matched control antibodies. After being washed, the cells were characterized by flow cytometry analysis, and at least 50,000 events per sample were analyzed.
Statistical analysis.
Data are expressed as medians and ranges, unless specified otherwise. All clinical and flow cytometry data were analyzed by the Kruskal-Wallis test and the chi-square test, using SPSS 16.0 software. The relationship between two variables was evaluated using the Spearman rank correlation test. Individual data were stratified according to the levels of cytokines, HBV DNA load (>300 copies/ml or <300 copies/ml), and seropositive HBeAg status, and the differences between them were analyzed using Kruskal-Wallis, chi-square, and Fisher's exact tests, where applicable. A two-sided P value of <0.05 was considered statistically significant.
RESULTS
Treatment with LDT modulates HBV-related biochemical markers in CHB patients.
To determine the impact of treatment with LDT on immune profiles and liver function, a total of 54 CHB patients and 30 HC were recruited. There was no statistically significant difference in the distributions of age and gender between the CHB patients and the HC (Table 1). As expected, patients but not HC had various levels of HBV loads and high levels of serum HBsAg. Furthermore, the concentrations of serum ALT and AST in CHB patients were significantly higher than those in the HC.
Following treatment with LDT, the HBV DNA loads in the CHB patients gradually decreased and were significantly lower than those before treatment (see Fig. S1 in the supplemental material). There were 4 cases with undetectable serum HBV DNA at 3 months post-initial treatment, and the number of cases with undetectable HBV DNA increased with time (Table 2). Similarly, following treatment with LDT, the number of cases with seronegative HBeAg and HBsAg gradually increased, and the levels of serum HBsAg were significantly reduced with time (Table 2; see Fig. S1). Furthermore, the levels of serum ALT and AST rapidly decreased and reached the normal range at 13 months post-initial treatment. Together, our data indicate that treatment with LDT effectively inhibited HBV replication and improved liver function in patients with CHB.
Table 2.
Effects of telbivudine on HBV DNA loads and HBeAg status
| Parameter | Valuea |
||||
|---|---|---|---|---|---|
| Baseline | 3 mo | 6 mo | 9 mo | 13 mo | |
| Mean HBV DNA load ± SD (log10 copies/ml) | 8.1 ± 1.2 | 4.6 ± 1.7* | 3.9 ± 1.2* | 4.0 ± 1.5* | 3.9 ± 1.4* |
| No. of patients | |||||
| HBV DNA load | |||||
| >300 copies/ml | 54 | 50 | 36 | 30 | 13 |
| <300 copies/ml | 0 | 4* | 18* | 24* | 31* |
| HBeAg status | |||||
| Positive | 54 | 49 | 46 | 42 | 39 |
| Negative | 0 | 5* | 8* | 12* | 15* |
| HBeAg status | |||||
| Positive | 54 | 51 | 45 | 44 | 39 |
| Negative | 0 | 3* | 9* | 10* | 15* |
*, P < 0.05 versus the basal value.
Treatment with LDT restores the number of activated NK cells in patients with CHB.
We next characterized the numbers of total PBMCs in patients and HC and found that the numbers of PBMCs in CHB patients (median, 9.2 × 106/ml; range, 3.6 × 106/ml to 10.2 × 106/ml) before LDT treatment were significantly greater than those in the HC (median, 5.5 × 106/ml; range, 3.2 × 106/ml to 9.6 × 106/ml) (P = 0.012). Following treatment with LDT for 13 months, the median number of PBMCs in the patients decreased from 9.22 × 106/ml to 5.8 × 106/ml, which was similar to the median for the HC. Therefore, treatment with LDT reduced the number of PBMCs in patients with CHB.
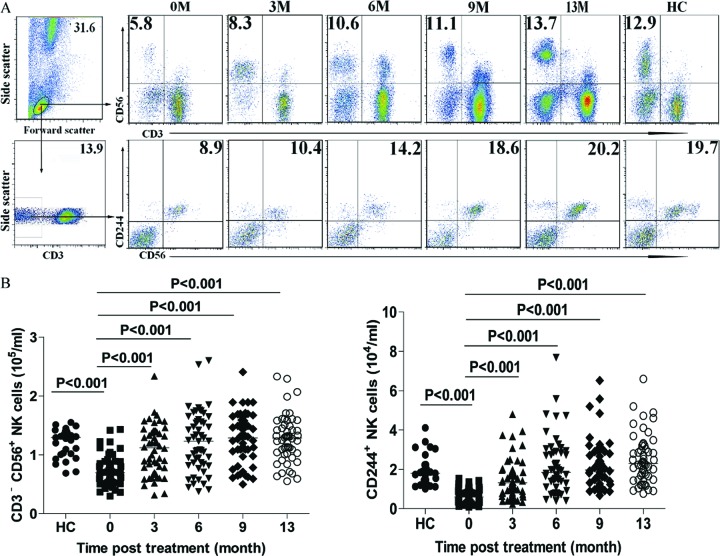
Characterization of peripheral blood NK cells revealed that the number of CD3− CD56+ NK cells in CHB patients before treatment was significantly lower than that in the HC (medians of 1.29 × 105 versus 0.70 × 105/ml) (P < 0.001) (Fig. 1). Following treatment with LDT for 3 months, the number of NK cells increased significantly (medians of 1.12 × 105, 1.23 × 105, 1.29 × 105, and 1.29 × 105/ml for 3, 6, 9, and 13 months posttreatment, respectively) compared with that before treatment (P < 0.001) and was gradually elevated at the later time points, to a level similar to that in the HC. A similar pattern for the number of CD244+ NK cells in total NK cells (medians of 0.59 × 104, 1.32 × 104, 1.85 × 104, 1.98 × 104, and 2.13 × 104/ml in CHB patients at 0, 3, 6, 9, and 12 months posttreatment versus 1.80 × 104/ml in HC) was observed in this population. Hence, treatment with LDT increased the number of activated NK cells in patients with CHB.
Fig 1.
Fluorescence-activated cell sorter (FACS) analysis of NK cells. The numbers of CD56+ NK and CD56+ CD244+ NK cells in CHB patients and HC subjects were determined longitudinally by flow cytometry analysis. PBMCs were obtained from individual subjects at the indicated time points and stained in duplicate with anti-CD56, anti-CD3, and anti-CD244. The cells were gated on living mononuclear cells and then analyzed for the number of CD3− CD56+ NK cells. Additional cells were gated on CD3− cells and analyzed for the number of CD3− CD56+ CD244+ cells. Data shown are representative charts or the mean numbers of NK and CD244+ NK cells per ml peripheral blood for individual patients at each time point from two separate experiments. (A) Representative flow cytometry charts. (B) Numbers of CD56+ NK and CD56+ CD244+ NK cells. Data were analyzed by the Kruskal-Wallis test. The horizontal lines indicate the median values for the groups.
Treatment with LDT significantly modulates the number of peripheral T cells in CHB patients.
Further characterization of peripheral T cells indicated that the numbers of CD3+ and CD3+ CD4+ T cells in CHB patients before treatment were significantly higher than those in the HC (medians of 7.44 × 105 versus 6.29 × 105/ml [P < 0.001] and 5.01 × 105 versus 3.81 × 105/ml [P < 0.001], respectively) (see Fig. S2 in the supplemental material). In contrast, the number of CD3+ CD8+ T cells in CHB patients before treatment was significantly lower than that in the HC (medians of 2.33 × 105 versus 2.89 × 105/ml; P = 0.004). As a result, the ratios of CD3+ CD4+ T cells to CD3+ CD8+ T cells in patients were higher than those in the HC. Following treatment with LDT for 3 months, the number of CD3+ cells increased significantly (medians of 7.94 × 105, 7.86 × 105, 7.85 × 105, and 7.77 × 105/ml for 3, 6, 9, and 13 months posttreatment, respectively) and was maintained at a similar level throughout the observation period in CHB patients. Furthermore, the number of CD3+ CD4+ T cells in CHB patients was slightly reduced at 9 months post-initial treatment and was maintained at a low level at 13 months post-initial treatment (medians of 5.16 × 105, 4.45 × 105, 4.11 × 105, and 4.19 × 105/ml for 3, 6, 9, and 13 months posttreatment, respectively). In addition, the number of CD3+ CD8+ T cells in CHB patients at 6 months post-initial treatment was significantly greater than that before treatment and further increased with time (medians of 2.76 × 105, 3.15 × 105, 3.62 × 105, and 3.59 × 105/ml for 3, 6, 9, and 13 months posttreatment, respectively). Accordingly, following LDT treatment, the ratios of CD3+ CD4+ to CD3+ CD8+ T cells in patients with CHB were gradually reduced. Therefore, treatment with LDT modulated the number of different subsets of T cells and reduced the ratio of CD4+ to CD8+ T cells in patients with CHB.
Treatment with LDT reduces the numbers of regulatory CD4+ and CD8+ T cells in CHB patients.
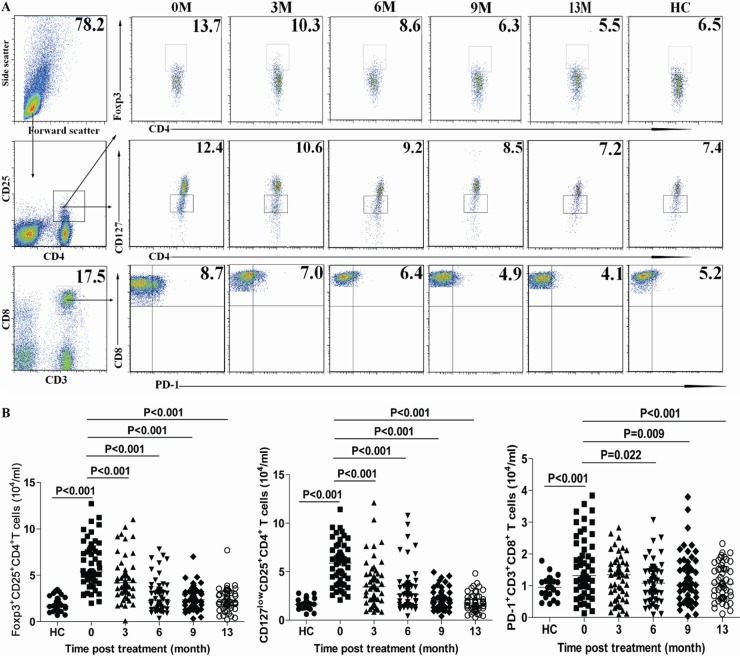
We further determined the numbers of circulating CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ T cells. We found that the numbers of CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ T cells in CHB patients before LDT treatment were significantly greater than those in the HC (medians of 5.38 × 104 versus 1.66 × 104/ml [P < 0.001], 5.87 × 104 versus 1.65 × 104/ml [P < 0.001], and 1.32 × 104 versus 0.95 × 104/ml [P < 0.001], respectively) (Fig. 2). Following treatment with LDT, the numbers of CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ T cells were gradually reduced compared with those before treatment. Therefore, treatment with LDT modulated regulatory CD4+ and CD8+ T cells.
Fig 2.
FACS analysis of peripheral blood CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ cells. The numbers of peripheral blood CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ T cells in individual CHB patients and HC subjects were determined longitudinally by flow cytometry analysis at the indicated time points. Data shown are representative FACS charts or mean numbers of each type of cell per ml of peripheral blood for individual subjects from two separate experiments. (A) Representative FACS charts. (B) Numbers of CD4+ CD25+ Foxp3+, CD4+ CD25+ CD127low, and CD3+ CD8+ PD-1+ T cells. Data were analyzed by the Kruskal-Wallis test. The horizontal lines indicate the median values for the groups.
Treatment with LDT modulates the frequency of effector T cell responses in patients with CHB.
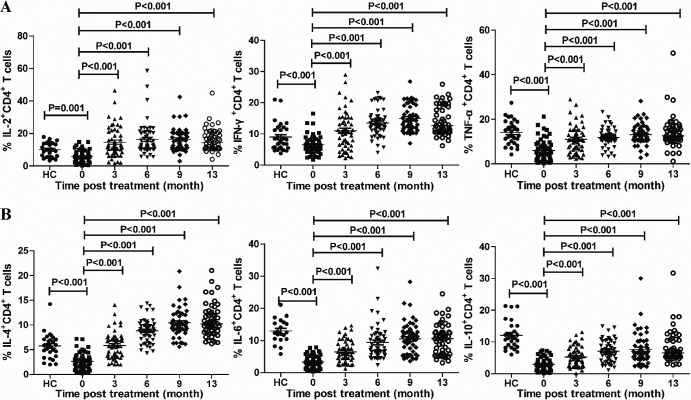
Effector T cells can secrete cytokines in response to stimulators. To determine the impact of treatment with LDT on effector T cell responses, PBMCs were isolated from individual CHB patients and healthy subjects and then stimulated with PMA and ionomycin. The frequencies of cytokine-secreting CD4+ T cells were characterized by intracellular staining and flow cytometry analysis. The frequencies of IL-2+, IFN-γ+, TNF-α+, IL-4+, IL-6+, and IL-10+ CD4+ T cells in the patients with CHB were significantly lower than those in the HC (medians of 12.16% versus 5.55% [P < 0.001], 9.14% versus 6.18% [P < 0.001], 15.75% versus 5.01% [P < 0.001], 8.49% versus 2.44% [P < 0.001], 12.45% versus 3.19% [P < 0.001], and 11.70% versus 2.76% [P < 0.001], respectively). Following treatment with LDT for 3 months, the frequencies of cytokine-secreting CD4+ T cells increased significantly and were further elevated at later time points for CHB patients (Fig. 3). Thus, treatment with LDT increased the frequencies of cytokine-secreting CD4+ effector T cells in patients with CHB.
Fig 3.
FACS analysis of different functional CD4+ T cells. PBMCs were isolated from individual CHB patients and HC subjects at the indicated time points and stimulated with PMA-ionomycin in vitro. Subsequently, the cells were stained with anti-CD4, fixed, permeabilized, and stained with antibodies against the indicated cytokines. The frequencies of cytokine+ CD4+ T cells were determined by flow cytometry. Data are mean values for each measure for individual subjects from two separate experiments. Data were analyzed by the Kruskal-Wallis test. The horizontal lines indicate the median values for the groups.
Treatment with LDT reduces serum HBV loads but enhances T cell immunity in CHB patients.
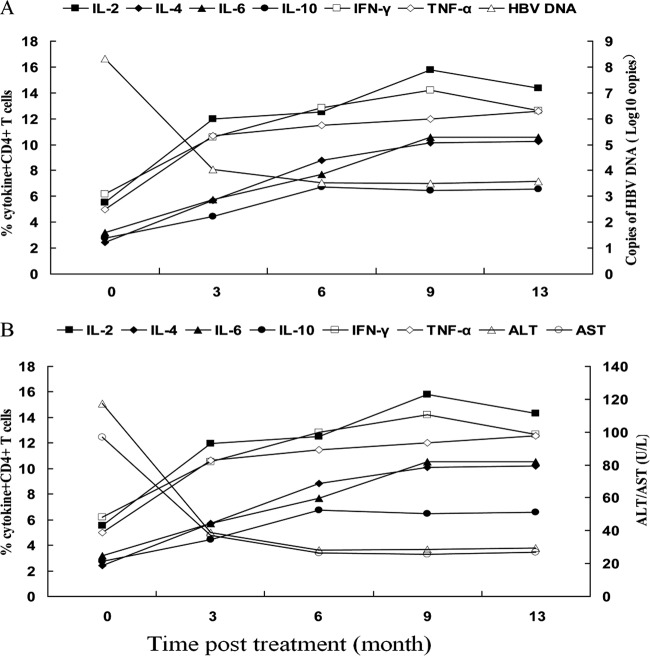
Further analysis indicated that there was a negative association between the frequencies of cytokine-secreting CD4+ T cells and the levels of HBV DNA loads in patients with CHB. While high serum HBV DNA loads were detected in patients with CHB before LDT treatment, the frequency of each type of cytokine-secreting CD4+ T cell was at its lowest level (Fig. 4A). Treatment with LDT decreased the serum HBV DNA loads but increased the frequency of each type of cytokine-secreting CD4+ T cell. As a result, the serum HBV DNA loads in CHB patients were negatively associated with the frequencies of cytokine-secreting CD4+ T cells. Similarly, the frequencies of cytokine-secreting CD4+ T cells were negatively associated with the levels of serum ALT and AST in CHB patients (Fig. 4B). Collectively, these data indicate that treatment with LDT inhibited HBV replication, enhanced T cell immunity, and improved liver function in these patients.
Fig 4.
Associations between percentages of cytokine+ CD4+ T cells and concentrations of serum HBV DNA, HBsAg, ALT, and AST in CHB patients. The relationships between the percentages of cytokine+ CD4+ T cells and the levels of serum HBV DNA, ALT, or AST in CHB patients were analyzed by logistic regression correlation analysis. Data shown are median values for each measure for CHB patients at the indicated time points (n = 54 per time point). (A) Correlation between percentages of cytokine-secreting CD4+ T cells and the levels of serum HBV DNA in CHB patients. (B) Association between frequencies of cytokine-secreting CD4+ T cells and the levels of serum AST and ALT in CHB patients.
DISCUSSION
LDT is an orally bioavailable l-nucleoside with potent and specific anti-HBV activity and has been proved to have better therapeutic responses and less virological resistance in CHB patients (25, 29, 30). Several studies have also shown that LDT inhibits HBV replication by modulating T cell immunity (20, 21). In this study, we longitudinally investigated the therapeutic effect and immunomodulatory function of LDT in CHB patients. We found that treatment with LDT significantly reduced the levels of serum HBV DNA, ALT, and AST, demonstrating that treatment with LDT inhibited HBV DNA replication and improved liver function in CHB patients, consistent with a previous report (31). In addition, treatment with LDT did not cause obvious side effects in the CHB patients tested. Therefore, LDT may be safe and effective for the treatment of patients with CHB.
Longitudinal characterization of NK and CD244+ NK cells revealed that while the numbers of NK and CD244+ NK cells in CHB patients were significantly lower than those in the HC, treatment with LDT for 3 months significantly increased the numbers of both NK and CD244+ NK cells, with further increases at later time points, in CHB patients. Our data support the notion that CHB patients develop impaired NK responses (32, 33) and indicate that treatment with LDT corrected immune impairment of NK responses in CHB patients. It is possible that LDT inhibited HBV replication and reduced HBV loads, which mitigated the inhibitory effect of HBV on NK cell activity, increasing NK cell responses in CHB patients. Therefore, the NK cell response to viral antigens is important for viral clearance during HBV infection (34, 35). In addition, activated NK cells, particularly CD244+ NK cells, secrete high levels of IFN-γ, which is crucial for the clearance of HBV (18, 19). The significantly increased CD244+ NK responses induced by treatment with LDT may inhibit HBV replication via negative feedback, contributing to anti-HBV immunity in CHB patients.
Previous studies have shown that persistent infection of HBV can modulate T cell immunity (6, 7). We found that the numbers of CD3+ and CD4+ T cells in CHB patients were significantly higher than those in the HC, while the number of CD8+ T cells was significantly lower than that in HC. Given that CD8+ T cells are crucial for anti-HBV immunity, the smaller number of CD8+ T cells in CHB patients further indicated that persistent infection with HBV impaired T cell immunity (7). Following treatment with LDT, the numbers of CD3+ and CD4+ T cells gradually decreased, while the number of CD8+ T cells increased significantly. The increased number of CD8+ T cells may reflect anti-HBV immunity in CHB patients. Therefore, the number of different subsets of T cells in CHB patients may be a biomarker for evaluating HBV replication and anti-HBV T cell immunity in CHB patients.
Tregs are important for the maintenance of immune tolerance. A previous study has shown that the level of Foxp3 expression is inversely correlated with that of CD127 expression in human T cells (36). Similarly, we found larger numbers of CD4+ CD25+ Foxp3+ and CD4+ CD25+ CD127low T cells in CHB patients, supporting the notion that Tregs contribute to immunological hyporesponsiveness against HBV infection (15, 17, 37, 38). These data also suggest that CD127low may be a good marker for Tregs. PD-1 is expressed on activated T cells, and engagement of PD-1 by its ligands promotes a negative signal for T cell immunity. We found a significantly larger number of PD-1+ CD8+ T cells in CHB patients than in the HC, consistent with a previous report (39). Furthermore, treatment with LDT decreased the numbers of Tregs and PD-1+ CD8+ T cells in CHB patients, which may have contributed to the inhibition of HBV replication.
Previous studies have shown that antigen-specific CD4+ T cell immunity also plays important roles in anti-HBV immunity (40, 41). IFN-γ, IL-2, and TNF-α are crucial for Th1 responses and can inhibit HBV DNA replication (42–44). IL-4 can promote the production of anti-HBV neutralizing antibodies and help in the clearance of circulating virus (20, 21). In contrast, IL-6 and IL-10 are involved in the pathogenesis of CHB (20, 21, 45). We characterized different functional CD4+ T cells and found that the frequencies of IL-2+, IFN-γ+, TNF-α+, IL-4+, IL-6+, and IL-10+ CD4+ T cells in CHB patients were significantly lower than those in the HC. These data suggest that although CHB patients had a greater number of CD4+ T cells, they had an impairment in CD4+ T cell function. We found that treatment with LDT increased the frequencies of cytokine-producing CD4+ T cells in CHB patients. Our data are consistent with a previous report that increased CD4+ T cell immunity is associated with the restoration of anti-HBV T cell immunity (46). The increased effector CD4+ T cell responses induced by treatment with LDT may also contribute to its anti-HBV activity in CHB patients. It is notably that the frequency of IL-10+ T cells remained lower than that in the HC after completing the treatment. Given that IL-10-secreting T cells usually negatively regulate anti-HBV immunity, our data suggest that treatment with LDT may preferably enhance anti-HBV T cell immunity but have a lesser effect on IL-10+ T cell responses in CHB patients. More importantly, we observed that the percentages of cytokine-producing effector CD4+ T cells were inversely correlated with the levels of serum HBV DNA, ALT, and AST. Given that these cytokines are crucial for the inhibition of HBV replication or in regulating T cell immunity (42–44, 47), the significantly increased cytokine responses may be responsible for the control of HBV replication and liver inflammation in CHB patients.
In summary, treatment with LDT not only dramatically reduced the levels of serum HBV DNA, ALT, and AST but also corrected the impairments of immune function in CHB patients. It is possible that LDT inhibits HBV replication, which reduces the inhibitory effect of HBV on T cell immunity. Alternatively, the reduced HBV replication in the liver may also mitigate HBV-related inflammation, which in turn reduces T cell infiltration in the liver. We are interested in further investigating the mechanisms underlying the action of LDT in regulating T cell immunity in CHB patients. Our findings highlight the possibility that the enhanced immune response seen with LDT treatment may play an important role in HBV clearance. We recognize that our study has some limitations, such as a relatively small sample size and the lack of studies on antigen-specific T cell responses and molecular mechanisms of the therapeutic effect. Therefore, further studies with a larger population are warranted.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant 30972610), the Jilin Province Science and Technology Agency (grants 200705128 and 20110716), the Chinese Medical Science and Technology Projects of Jilin Province (grant 08sys-086), the Health Department Research Projects in Jilin Province (grant 2009Z054), and the Cutting-Edge Science and Interdisciplinary Innovation Projects of Jilin University.
We thank Medjaden Bioscience for assisting in the preparation of the manuscript.
The authors declare no financial and commercial conflicts.
Footnotes
Published ahead of print 28 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02181-12.
REFERENCES
- 1. Hoofnagle JH, Dusheiko GM, Seef LB, Jones EA, Waggoner JG, Baies ZB. 1981. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann. Intern. Med. 94:744–748 [DOI] [PubMed] [Google Scholar]
- 2. Iloeje UH, Yang HI, Su J, Jen CL, Chen CJ. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130:678–686 [DOI] [PubMed] [Google Scholar]
- 3. Chen CJ, Yang HI, Su J, Jen SL, Lu SN, Huang GT, Lloeie UH. 2006. Risk of hepato-cellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295:65–73 [DOI] [PubMed] [Google Scholar]
- 4. Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 [DOI] [PubMed] [Google Scholar]
- 5. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boni C, Penna A, Bertoletti A, Lamonaca V, Rapti G, Missale G, Pilli M, Urbani S. 2003. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 39:595–605 [DOI] [PubMed] [Google Scholar]
- 7. Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J. 2000. The role of virus-specific CD8 cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mosmann TR, Sad S. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology 17:138–146 [DOI] [PubMed] [Google Scholar]
- 9. O'Garra A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275–283 [DOI] [PubMed] [Google Scholar]
- 10. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)17, T(H)1 and T(H)2 cells. Nat. Immunol. 10:864–887 [DOI] [PubMed] [Google Scholar]
- 11. Huang CF, Lin SS, Ho YC, Chen FL, Yang CC. 2006. The immune response induced by hepatitis B virus principal antigens. Cell. Mol. Immunol. 3:97–106 [PubMed] [Google Scholar]
- 12. Sakaguchi S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562 [DOI] [PubMed] [Google Scholar]
- 13. Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, Tang Z, Fu YX, Wang FS. 2006. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J. Immunol. 177:739–747 [DOI] [PubMed] [Google Scholar]
- 14. Manigold T, Racanelli V. 2007. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect. Dis. 7:804–813 [DOI] [PubMed] [Google Scholar]
- 15. Stoop JN, van der Molen RG, Kuipers EJ, Kusters JG, Janssen HL. 2007. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology 361:141–148 [DOI] [PubMed] [Google Scholar]
- 16. Franzese O, Kennedy PT, Gehring AJ. 2005. Modulation of the CD8+ T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J. Virol. 79:3322–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, Dariz A, Guillermet C, Pemollet M, Zarski JP, Marche PN, Leroy V. 2009. Fine characterization of intra-hepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J. Hepatol. 30:1010–1016 [DOI] [PubMed] [Google Scholar]
- 18. Tangye SG, Cherwinski H, Lanier LL, Phillips JH. 2000. 2B4-mediated activation of human natural killer cells. Mol. Immunol. 37:493–501 [DOI] [PubMed] [Google Scholar]
- 19. Sandusky MM, Messmer B, Watzl C. 2006. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur. J. Immunol. 12:3268–3276 [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y, Li W, Yu L, Liu J, Xin G, Yan H, Sun P, Zhang H, Xu D, Niu J. 2011. Enhancing the antihepatitis B virus immune response by adefovir dipivoxil and entecavir therapies. Cell. Mol. Immunol. 8:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang YF, Ma ZH, Xin GJ, Yan HQ, Li WY, Xu HN, Hao CH, Niu JQ, Zhao PW. 2010. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm. 2010:143026 doi:10.1155/2010/143026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liaw YF, Lau GK, Kao JH, Gane E. 2010. Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection. Dig. Dis. Sci. 55:2727–2734 [DOI] [PubMed] [Google Scholar]
- 23. Standring DN, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hemandez B, Juodawlkis A. 2010. Antiviral beta-l-nucleosides specific for hepatitis B virus infection. Antivir. Chem. Chemother. 12:119–129 [PubMed] [Google Scholar]
- 24. Rasenack J, Poynard T, Lai CL, Gane E, Brown NA, Healthcote J. 2007. Efficacy of telbivudine vs lamivudine at 2 years in patients with HBeAg-positive chronic hepatitis B who are eligible for treatment based on guidelines. J. Hepatol. 46(Suppl 1):S195 [Google Scholar]
- 25. Jia JD, Hou JL, Yin YK. 2007. Two-year results of a phase III comparative trial of telbivudine vs lamivudine in Chinese patients. J. Hepatol. 46(Suppl 1):S189. [DOI] [PubMed] [Google Scholar]
- 26. Ji-Yuan Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. 2010. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One 5:e13869 doi:10.1371/journal.pone.0013869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yim HJ, Lok AS. 2006. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 43:S173–S181 [DOI] [PubMed] [Google Scholar]
- 28. Duramad P, McMahon CW, Hubbard A. 2004. Flow cytometric detection of intracellular Th1/Th2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 13:1452–1458 [PubMed] [Google Scholar]
- 29. Standring DN, Bridges EG, Placidi L. 2010. Antiviral beta-l-nucleosides specific for hepatitis B virus infection. Antivir. Chem. Chemother. 1:119–129 [PubMed] [Google Scholar]
- 30. Palumbo E. 2008. Telbivudine for chronic hepatitis B. A review. Anti Infect. Agents Med. Chem. 7:245–248 [Google Scholar]
- 31. Zhu XF, Lu LX, Wang Y, Xu KW, Li DJ, Zhu X, Liu C, Wang JR, Tang H, Wang LC. 2011. Effect and predictive elements for 52 weeks' telbivudine treatment on naive HBeAg positive chronic hepatitis B. Hepat. Mon. 11:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crotta S, Stilla A, Wack A, Andrea A, Nuti S, Doro U, Mosca M, Filliponi F, Brunetto RM, Bonino F, Abrignani S, Vailiante NM. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tseng CT, Klimpel GR. 2002. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lanier LL. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274 [DOI] [PubMed] [Google Scholar]
- 35. Orange JS, Fassett MS, Koopman LA, Boyson JE. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006–1012 [DOI] [PubMed] [Google Scholar]
- 36. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. 2006. CD127 expression inversely correlates with Foxp3 and suppressive function of human CD4+ Treg cells. J. Exp. Med. 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kusters JG, Janseen HL. 2005. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 41:771–778 [DOI] [PubMed] [Google Scholar]
- 38. Feng IC, Koay LB, Sheu MJ. 2007. HBcAg-specific CD4+CD25+ regulatory T cells modulate immune tolerance and acute exacerbation on the natural history of chronic hepatitis B virus infection. J. Biomed. Sci. 14:43–57 [DOI] [PubMed] [Google Scholar]
- 39. Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. 2008. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol. Immunol. 45:963–970 [DOI] [PubMed] [Google Scholar]
- 40. Fourel I, Cullen JM, Saputelli J, Aldrich CE, Schaffer P, Averett DR, Pugh J, Mason WS. 1994. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J. Virol. 68:8321–8330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825–829 [DOI] [PubMed] [Google Scholar]
- 42. Cooksley H, Chokshi S, Maayan Y, Wedemeyer H, Andreone P, Gilson R, Warnes T, Paganin S, Zoulim F, Frederick D, Neumann AU, Brosgart CL, Naoumov NV. 2008. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD41 T-cell reactivity. Antimicrob. Agents Chemother. 52:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McClary H, Koch R, Chisari FV, Guidotti LG. 2000. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74:2255–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. 1996. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N. Engl. J. Med. 334:1422–1427 [DOI] [PubMed] [Google Scholar]
- 45. Kakumu S, Fukatsu A, Shinagawa T. 1992. Localization of intrahepatic interleukin 6 in patients with acute and chronic liver disease. Chin. Pathol. 45:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F, Ferrari C. 1998. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J. Clin. Invest. 102:968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lok AS, McMahon BJ. 2001. Chronic hepatitis B. Hepatology 34:1225–1241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.