Abstract
We conducted this double-blind, parallel-group, placebo-controlled, randomized, multiple-ascending-dose study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of GS-9851 (formerly PSI-7851) in treatment-naïve patients infected with hepatitis C virus (HCV) genotype 1. Thirty-two patients received active doses up to 400 mg of GS-9851 once daily for 3 days. GS-9851 and the metabolite GS-566500 (formerly PSI-352707) were rapidly cleared from the plasma, with half-life (t1/2) values of approximately 1 h for GS-9851 and 3 h for GS-566500. Accumulation (21%) was observed only for GS-331007 (formerly PSI-6206) after multiple dosing. GS-331007 was the primary drug-related moiety in the plasma and urine. Increases in the GS-9851, GS-566500, and GS-331007 maximum concentrations in plasma (Cmax) and area under the concentration-time curve (AUC) were less than dose proportional, particularly at the highest doses. The decline in plasma HCV RNA levels was dose dependent, and a mean maximal change from the baseline of −1.95 log10 IU/ml was obtained for 400 mg GS-9851, compared with −0.090 log10 IU/ml for the placebo. Most patients had a decrease in HCV RNA of ≥1.0 log10 IU/ml after 3 days' dosing with 400 mg GS-9851. No virologic resistance was observed. GS-9851 was generally well tolerated, with no notable differences in adverse event frequency across doses. The pharmacokinetic profile observed in this study was similar to that seen in a single-ascending-dose study in healthy subjects.
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease, cirrhosis, and liver cancer (1–4). A weekly injection of pegylated alpha interferon combined with daily oral administration of ribavirin and a protease inhibitor is now considered the standard of care for the treatment of genotype 1 HCV infection (5–7). This treatment regimen leads to sustained virologic response (SVR) rates in up to 75% of treatment-naïve patients (5, 7). However, despite improved SVR rates with this treatment, efficacy is somewhat variable and can be associated with serious side effects, such as rash and anemia (5, 7). Therefore, in spite of recent advances, there is an ongoing need for more consistently effective treatment options that offer improved safety and tolerability profiles and that can be used across all HCV genotypes.
The HCV RNA-dependent RNA polymerase, NS5B, a component of the replication complex that transcribes the HCV genome (8–10), is critical to HCV replication and infectivity (11, 12). There is no equivalent human homologue protein for NS5B, which makes it a suitable target for HCV-directed therapies (11). Inhibitors of NS5B fall into two major classes: nonnucleotide inhibitors (NNIs) and nucleoside/nucleotide analog inhibitors. Variations in the NS5B coding region introduce the potential for viral escape from inhibition by NNIs, and the therapeutic response to NNI treatment varies significantly (13–15). In contrast, nucleoside/nucleotide analogs appear to be equally effective across HCV genotypes. In vitro evidence of resistance to this class was obtained from two variants (S96T and S282T) (13, 15), which conferred resistance to R1479 (4′-azidocytidine) and 2′-C-methyl-modified nucleotide inhibitors, respectively (16, 17).
GS-9851 (formerly PSI-7851), a 50:50 mixture of the two isomers PSI-7976 and GS-7977 (formerly PSI-7977), is a nucleotide prodrug of the nucleoside analog GS-331007 (formerly PSI-6206) (18). GS-9851 potently inhibits HCV NS5B polymerase and has demonstrated pangenotypic activity in vitro (19). Preclinical studies of GS-9851 demonstrated a favorable profile in terms of antiviral potency, distribution, and metabolism (18). GS-9851 is first hydrolyzed to the intermediate GS-566500 (formerly PSI-352707), which is then metabolized to either the inactive metabolite, GS-331007, or the monophosphate GS-606965 (formerly PSI-7411) (20, 21). Inside the hepatocyte, GS-606965 is further phosphorylated by a series of enzymatic steps to an active triphosphate metabolite, GS-461203 (formerly PSI-7409), that selectively inhibits recombinant NS5B (22). A first-time-in-human study described in our accompanying article demonstrated that GS-9851 (25 to 800 mg) is generally safe and well tolerated and has a pharmacokinetic profile consistent with once-daily dosing (20).
The primary objectives of the current study were to investigate whether administration of GS-9851 up to 400 mg once daily for 3 days was safe and tolerated in patients with chronic HCV infection and to characterize the pharmacokinetics of GS-9851 and its systemic metabolites. The secondary objective was to assess plasma HCV RNA concentrations and monitor for viral sequence changes associated with GS-9851 exposure.
(This work was previously presented in part at the 60th Annual Meeting of the American Association for the Study of Liver Diseases, November 2009, Boston, MA, and at the International HIV & Hepatitis Virus Drug Resistance Workshop and Curative Strategies, 8 to 12 June 2010, Dubrovnik, Croatia.)
MATERIALS AND METHODS
Study population.
Forty males and females of ages between 18 and 65 years with body mass indices ranging from 18 to 36 kg/m2 were enrolled in the study. Females were to be surgically sterile, postmenopausal for at least 12 months at screening, or taking protocol-specified contraceptive measures. Only treatment-naïve, noncirrhotic patients with chronic HCV genotype 1 infection and HCV RNA levels of ≥50,000 IU/ml were eligible for inclusion in the study. Patients were in good health, with no significant comorbidities. Patients were excluded if they were positive for anti-hepatitis A virus immunoglobulin M (IgM) antibodies, hepatitis B surface antigen, anti-hepatitis B core protein IgM antibodies, or anti-human immunodeficiency virus antibodies. No medication associated with QT interval prolongation was permitted within 30 days prior to dosing or during the study, and any other concurrent medication required approval by the investigator and the sponsor. Patients who had received any systemic antineoplastic or immunomodulatory treatment within 6 months prior to the first dose of study drug or who might have needed such treatments at any time during the study were ineligible.
The study was conducted from May 2009 to September 2009. All patients provided informed consent prior to screening. The protocol was approved by the investigational review board (Western International Review Board, Olympia, WA), and the study (protocol number P7851-1102) was conducted according to good clinical practice and the Declaration of Helsinki.
Study design.
The study followed a double-blind, parallel-group, randomized, placebo-controlled, multiple-ascending-dose design with oral administration of once-daily doses of either GS-9851 or placebo for 3 days. Patients were allocated to one of four groups, each of which consisted of 10 patients who were randomized to receive either active GS-9851 or placebo (active, 8; placebo, 2). The starting dose of GS-9851 was 50 mg, followed by 100 mg, 200 mg, and 400 mg. Following the completion of each dose level, pharmacokinetic, pharmacodynamic (i.e., plasma HCV RNA), and safety data were examined by the sponsor and investigator to determine whether escalation to the next dose level was warranted.
Patients who had not undergone a liver biopsy within 3 years of dosing were asked to provide a biopsy sample to rule out cirrhosis or advanced fibrosis. Clinical laboratory tests were performed within 30 days of dosing to confirm patient eligibility for the study. The day before the first dose (day −1), patients were admitted to the study unit for baseline assessments that included vital signs, hematology, chemistry, and urinalysis. Patients received an oral dose of GS-9851 or placebo in the morning after a 10-h fast for 3 consecutive days. Study medication was administered with 240 ml of water. Patients were allowed to drink water freely throughout the study period except for a 3-h window that began 1 h prior to dosing until 2 h after each morning dose, during which the only water allowed was that taken with the study drug. Safety, pharmacokinetic, and pharmacodynamic assessments were performed from day 1 to the morning of day 4, after which the patients were discharged from the study unit provided they did not exhibit any ongoing safety issues. Patients returned to the study site on the mornings of days 5, 6, 7, 10, and 14 to undergo follow-up assessments.
Sample collection.
Blood samples for quantification of HCV RNA and viral genotyping were collected at screening and in the morning (predose on dosing days) on days 1 to 7, 10, and 14. Blood samples for pharmacokinetic analysis were drawn at predefined time points from day 1 until day 6. On day 1, blood samples were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 16 h postdose. On day 2, blood was collected predose only, and on day 3, it was collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 48, and 72 h following the last dose of study medication. In the 200-mg and 400-mg GS-9851 dose groups, urine was collected predose on day 1 and at the following intervals: 0 to 6, 6 to 12, and 12 to 24 h postdosing. On day 3, urine samples were collected at the following intervals following the last dose of study medication: 0 to 6, 6 to 12, 12 to 24, 24 to 48, and 48 to 72 h for analysis of the urinary concentrations of GS-9851 and its metabolites, GS-566500 and GS-331007.
Pharmacokinetic analysis.
Plasma concentrations of GS-9851, GS-566500, and GS-331007 were determined using a validated high-performance liquid chromatography method utilizing tandem mass spectroscopy (LC-MS/MS) (QPS, LLC, Newark, DE). The linear range of the plasma assay was 5 to 5,000 ng/ml for GS-9851 and 10 to 5,000 ng/ml for GS-566500 and GS-331007. Urine samples were analyzed using a validated LC-MS/MS method to determine concentrations of GS-9851, GS-566500, and GS-331007 (QPS, Newark, DE). The lower limit of quantitation (LLQ) of the assay was 10 ng/ml for each compound, with a linear quantifiable range of 10 to 10,000 ng/ml.
The plasma pharmacokinetic profiles of GS-9851, GS-566500, and GS-331007 were determined for each patient by noncompartmental analysis of individual plasma concentration-time data using the software program WinNonlin, Professional, version 5.2 (Pharsight Corporation, Mountain View, CA). The following pharmacokinetic parameters were determined: maximum concentration in plasma (Cmax), time of maximum concentration (tmax), half-life (t1/2), area under the plasma concentration-time curve to the last measurable concentration (AUC0–t), AUC from time zero to 24 h (AUC0–24), AUC from time zero and extrapolated to infinity (AUC0–∞), percentage of AUC extrapolated (AUC extrapolated), amount excreted in urine from time zero to 24 h (Ae0–24), renal clearance (CLrenal), and percentage of study drug excreted in urine (urine excreted). The percentages of urine excreted for GS-566500 and GS-331007 were adjusted for molecular weight differences between GS-9851 and the respective metabolite. Molecular-weight-adjusted GS-331007/GS-9851 AUC ratios were calculated.
Clinical virology assessments.
Hepatitis C virus genotyping was performed by Cenetron Central Laboratories, Ltd. (Austin, TX), using the Siemens Versant Inno-LiPA HCV genotyping assay, version 2.0 (Tarrytown, NY). This assay was validated independently using NS5B sequencing as a standard, and the concordance obtained between the two methods at the genotype and subtype levels was greater than 99%. Quantification of plasma HCV RNA was performed by Cenetron Central Laboratories, Ltd., using Cobas TaqMan HCV (Roche, Indianapolis, IN). The established lower limit of this assay is 15 HCV IU/ml, which is defined by a 95% hit rate according to World Health Organization standards. Hepatitis C virus genotypic monitoring was performed using population sequencing of the HCV NS5B-encoding region of the polymerase at baseline and end of treatment for all patients by DDL Diagnostic Laboratory (Voorburg, The Netherlands). Any amino acid substitutions found in samples after GS-9851 exposure were compared with those in the respective baseline sequence for each patient.
Safety and tolerability assessments.
Safety and tolerability were assessed by the reporting of adverse events, including time of onset, duration, intensity, and potential causal relationship with the study drug, from first dose until day 14. Vital signs, including blood pressure and heart rate, were recorded at screening, on the day of admission, and on days 1, 2, 3, 4, and 14. On day 1, vital signs were recorded predose and at 1, 2, 3, 4, and 12 h postdose. On day 2 and day 3, data were collected predose and at 1, 2, 3, and 4 h postdose, and on day 4, data were collected prior to patient discharge. Twelve-lead electrocardiograms (ECGs) were performed at screening, on days 1, 2, and 3, at 1 h predose and at 1, 2, 3, and 4 h postdose, and on day 14. Samples for clinical laboratory testing, including hematology, chemistry, and urinalysis, were obtained at screening, day −1, and days 3, 6, and 14. Clinical laboratory changes were graded according to criteria specified in the protocol using the 2004 version of the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (http://www.mtnstopshiv.org/sites/default/files/attachments/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf). Urinalysis included a qualitative dipstick test and quantitative spot measurements of albumin, creatinine, protein, and microscopic analysis.
Statistical analysis.
No formal sample size calculations were performed. Pharmacokinetic parameters, except tmax, underwent log transformation prior to statistical analysis. The pharmacokinetic parameters AUC0--t, AUC0–24, AUC0–∞, and Cmax were also dose normalized. Standard summary statistics were determined for all pharmacokinetic parameters.
Dose proportionality was evaluated based on AUC0–24 and Cmax on day 1 and day 3. Dose proportionality was determined by utilizing a power model and analysis of variance (ANOVA) using log-transformed pharmacokinetic data for each dose normalized to a 100-mg reference dose. Accumulation was evaluated by comparing AUC0–24 on day 3 with AUC0–24 on day 1. A mixed-effects model with ln(AUC) as the dependent variable, a fixed effect for day, and a random patient effect was used to estimate the difference between AUC0–24 for day 3 and AUC0–24 for day 1 on the log scale. A 90% confidence interval (CI) was also estimated for each dose group. Steady state was determined through examination of time-concentration plots of plasma trough concentration data.
Hepatitis C virus RNA data were summarized using log10-transformed data. Summary statistics for HCV RNA values over time and change from the baseline over time were generated. For categorical analysis of virologic response, HCV data were summarized with respect to the number and percentage of patients experiencing an HCV RNA decrease of ≥1.0 log10. Percentages were based on the number of patients within the data set.
RESULTS
Patient disposition.
Forty patients were enrolled, and all completed the study. Thirty-two patients were randomized to receive GS-9851 and eight to receive placebo. The majority of patients were male (90%) and Caucasian (70%). The mean (range) age of the patients was 43 (24 to 61) years.
Safety and tolerability.
GS-9851 was generally safe and well tolerated. There was no apparent dose or exposure-related increase in the frequency or severity of adverse events over the 3 days of GS-9851 administration at doses ranging from 50 mg to 400 mg. Ten (31%) patients who received GS-9851 experienced at least one adverse event. The most frequently reported adverse event was headache (four patients), followed by abdominal pain, anemia, increased blood creatine phosphokinase (CK), nausea, and urinary tract infection (two patients each). Four (13%) patients who received GS-9851 experienced adverse events that were judged to be drug related by the investigator: abdominal pain, anemia, and headache (one patient each) after receiving 50 mg GS-9851 and nausea (one patient) after 400 mg GS-9851.
Two patients experienced increased blood CK following GS-9851 dosing. One experienced the increase after receiving 50 mg (mild intensity; CK = 2,181 IU/liter) and one after receiving 100 mg GS-9851 (severe intensity; CK = 7,304 IU/liter, first reported, and 12,559 IU/liter after repeating the test); both elevations resolved without intervention and were considered to be unrelated to study drug by the investigator. Clinical laboratory evaluations showed no detectable trends for changes from baseline for clinical chemistry and hematology values over time following study drug administration. Generally, alanine aminotransferase (ALT) declined over time in the 200-mg and 400-mg GS-9851 dosing groups. There were no observed changes in hemoglobin. Five patients who received GS-9851 and one who received placebo had eight laboratory values that were judged to be grade 3 or grade 4 according to the DAIDS table as follows: reduced sodium (50 mg GS-9851, n = 1; placebo, n = 1), high CK (100 mg GS-9851, n = 1), high ALT (100 mg GS-9851, n = 1), high aspartate aminotransferase (100 mg GS-9851, n = 2), and reduced absolute neutrophil levels (200 mg GS-9851, n = 1; 400 mg GS-9851, n = 1). There was no discernible pattern of safety laboratory abnormalities with the increase of the GS-9851 daily dose from 50 mg to 400 mg. One patient experienced two adverse events of hypertension; the first event resolved, and the second event was ongoing at follow-up. No other abnormalities in vital signs or 12-lead ECG parameters were considered clinically significant.
Pharmacokinetic results.
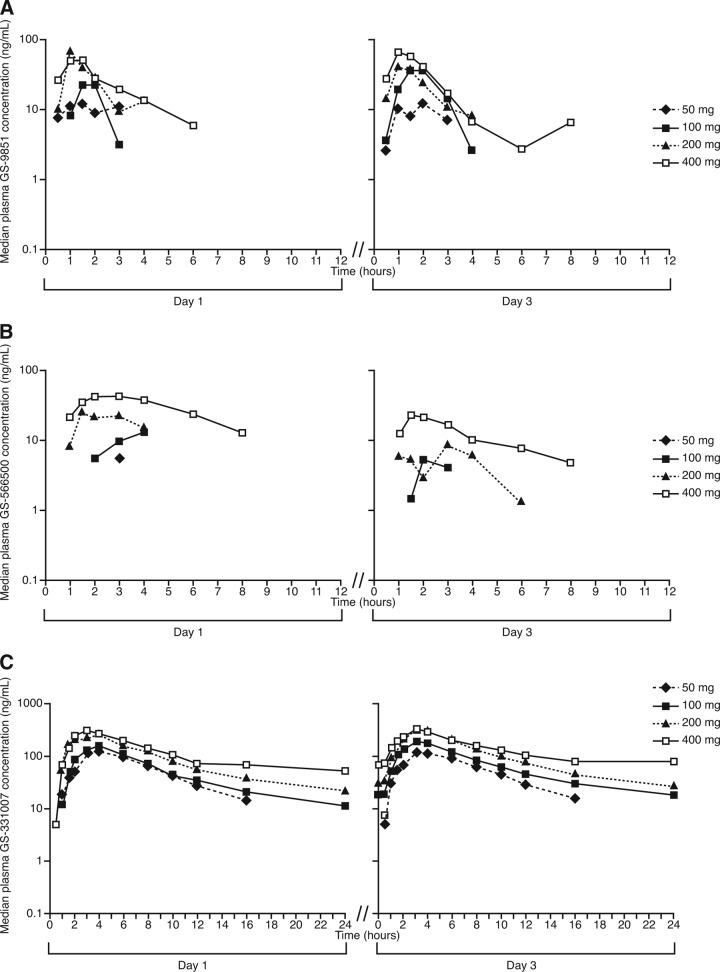
GS-9851 was readily absorbed, and plasma concentrations of GS-9851, GS-566500, and GS-331007 generally declined in a similar manner for all dose groups. Concentrations of GS-9851 and GS-566500 were not quantifiable throughout a dosing interval at any dose of GS-9851; in contrast, GS-331007 concentrations were quantifiable throughout a dosing interval at doses of ≥100 mg GS-9851 (Fig. 1).
Fig 1.
Median plasma concentration-time profiles after multiple daily doses of GS-9851 for GS-9851 (A), GS-566500 (B), or GS-331007 (C) (semilogarithmic scale).
For GS-9851, Cmax increased with dose on day 1, whereas on day 3, doses above 100 mg GS-9851 did not led to increases in Cmax (Table 1). On day 1 and day 3, values of AUC0–24 for GS-9851 were similar within a given dose level and also increased with the dose. Median GS-9851 tmax values ranged from 1.0 to 2.0 h on days 1 and 3 across the dose range studied. Concentrations of GS-9851 following Cmax quickly fell below the limit of detection of the assay, precluding the calculation of the terminal elimination slope and day 1 AUC0–∞ for most subjects. For those patients with sufficient quantifiable concentrations in the terminal phase, values of median t1/2 were 0.8 to 2.1 h on day 1 and approximately 1 h on day 3. The percentage of extrapolated AUC on day 1 was 7.4% for 100 mg GS-9851 and 21.9% for 200 mg GS-9851; however, these values were obtained from only a few subjects. The percentage of GS-9851 recovered in the urine on days 1 and 3 was less than 1% of an administered dose of GS-9851 (Table 2). Renal clearance values for GS-9851 were relatively stable across dosing days and doses (Table 2).
Table 1.
Derived plasma pharmacokinetic parameters for GS-9851, GS-566500, and GS-331007
| Parameter | Mean (SD) value for day and dose [na]b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 3 |
|||||||
| 50 mg | 100 mg | 200 mg | 400 mg | 50 mg | 100 mg | 200 mg | 400 mg | |
| GS-9851 | ||||||||
| AUC0–t (h · ng/ml) | 37.4 (19.9) [8] | 84.4 (87.6) [8] | 149.5 (139.8) [8] | 234.0 (127.6) [8] | 30.8 (24.2) [7] | 102.6 (96.0) [8] | 148.9 (185.3) [8] | 185.2 (135.7) [8] |
| AUC0–24 (h · ng/ml) | 45.7 (24.7) [8] | 90.2 (91.6) [8] | 162.1 (144.3) [8] | 236.4 (130.5) [8] | 35.1 (25.2) [7] | 113.2 (101.0) [8] | 159.7 (184.8) [8] | 191.5 (136.3) [8] |
| Cmax (ng/ml) | 23.1 (8.9) [8] | 61.2 (72.5) [8] | 75.4 (38.5) [8] | 165.6 (130.4) [8] | 21.5 (15.4) [7] | 81.0 (117.2) [8] | 77.1 (74.9) [8] | 81.7 (65.3) [8] |
| t1/2 (h) | 0.8 (0.4, 1.2) [2] | 2.1 (1.1, 4.2) [3] | 1.0 (1.0, 1.1) [2] | 1.0 (0.9, 1.2) [2] | 1.3 (0.6, 2.1) [2] | 1.0 (0.6, 1.3) [2] | ||
| tmax (h) | 1.8 (1.0, 3.0) [8] | 1.0 (0.8, 1.8) [8] | 1.0 (1.0, 1.6) [8] | 1.3 (1.0, 2.4) [8] | 2.0 (1.0, 2. 0) [7] | 1.5 (1.0, 1.5) [8] | 1.0 (0.8, 1.3) [8] | 1.0 (0.8, 1.5) [8] |
| AUC extrapolated (%) | 7.4 (1.93) [2) | 21.9 (15.0) [3) | ||||||
| GS-566500 | ||||||||
| AUC0–t (h · ng/ml) | 16.6 (17.7) [7] | 82.1 (70.2) [5] | 125.8 (135.7) [7] | 286.9 (260.7) [8)] | 15.8 (10.2) [4] | 81.3 (110.7) [7] | 93.3 (107.3) [8] | 201.6 (157.5) [8] |
| AUC0–24 (h · ng/ml) | 24.4 (23.4) [7] | 97.3 (70.5) [5] | 138.5 (136.8) [7] | 301.4 (268.8) [8] | 21.3 (11.8) [4] | 91.5 (113.2) [7] | 105.7 (110.2) [8] | 215.0 (162.9) [8] |
| Cmax (ng/ml) | 13.4 (4.3) [7] | 36.7 (24.1) [5] | 38.2 (24.1) [7] | 72.2 (61.5) [8] | 13.3 (0.8) [4] | 32.8 (32.6) [7] | 29.1 (17.3) [8] | 46.2 (31.9) [8] |
| t1/2 (h) | 2.4 (2.4, 2.4) [2] | 2.0 (1.9, 3.8) [4] | 3.1 (2.7, 3.5) [7] | 2.3 [1] | 3.3 (2.3, 4.3) [2] | 3.7 (2.9, 5.0) [3] | ||
| tmax (h) | 3.0 (1.5, 3.1) [7] | 2.0 (1.0, 3.0) [5] | 1.5 (1.5, 4.0) [7] | 1.9 (1.5, 3.0) [8] | 1.8 (1.5, 2.5) [4] | 2.0 (2.0, 3.0) [7] | 2.0 (1.3, 3.0) [8] | 2.0 (1.3, 3.3) [8] |
| AUC extrapolated (%) | 29.6 (15.5) [2] | 27.0 (13.8) [4] | 19.4 (9.8) [7] | |||||
| GS-331007 | ||||||||
| AUC0–t (h · ng/ml) | 899.9 (357.5) [8] | 1,256.4 (617.4) [8] | 2,554.1 (919.2) [8] | 2,854.4 (1,069.3) [8] | 918.9 (393.8) [7] | 2,100.8 (562.1) [8] | 3,196.4 (1,364.7) [8] | 5,311.0 (3,182.8) [8] |
| AUC0–24 (h · ng/ml) | 942.7 (364.1) [8) | 1,277.4 (600.6) [8) | 2,555.5 (919.4) [8) | 2,856.4 (1,070.6) [8) | 951.5 (390.3) [7) | 1,719.9 (372.13) [8) | 2,947.7 (1,097.4) [8) | 3,554.4 (1,380.1) [8) |
| AUC0–∞ (h · ng/ml) | 1,115.3 (300.6) [7] | 1,488.9 (564.4) [7] | 2,943.5 (1,053.1) [6] | 4,179.1 (1,615.0) [5] | ||||
| Cmax (ng/ml) | 129.8 (47.2) [8) | 174.2 (80.1) [8] | 318.5 (100.4) [8] | 335.1 (98.3) [8) | 117.7 (41.9) [7] | 218.9 (52.2) [8] | 336.6 (68.5) [8] | 340.4 (109.5) [8] |
| t1/2 (h) | 4.1 (2.9, 7.9) [7] | 5.8 (5.4, 7.7) [7] | 6.8 (6.6, 9.0) [6] | 11.7 (11.1, 12.0) [5] | 6.0 (3.2, 7.0) [5] | 10.3 (8.1, 14.9) [4] | 7.2 (5.5, 9.6) [7] | 17.9 (15.4, 21.4) [3] |
| tmax (h) | 3.5 (3.0, 4.0) [8] | 3.5 (3.0, 4.0) [8] | 3.5 (3.0, 4.0) [8] | 3.0 (2.5, 4.0) [8] | 4.0 (3.0, 4.0) [7] | 3.0 (2.5, 3.5) [8] | 3.5 (3.0, 5.0) [8] | 3.0 (2.5, 3.5) [8] |
| GS-331007/GS-9851 AUC0–t ratioc | 71.0 (66.2) | 93.0 (108.2) | 55.7 (32.9) | 37.6 (28.4) | 113.6 (87.5) | 91.5 (81.3) | 64.2 (23.5) | 84.7 (68.2) |
n, no. of subjects.
Data for t1/2 and tmax are presented as median (first quartile, third quartile).
Ratio calculated after conversion of GS-331007 AUC0–t into nanogram equivalents of GS-9851 · h/ml.
Table 2.
Urine pharmacokinetic parameters for GS-9851, GS-566500, and GS-331007
| Drug and parameter | Mean (SD) value for day and dosea |
|||
|---|---|---|---|---|
| Day 1 |
Day 3 |
|||
| 200 mg | 400 mg | 200 mg | 400 mg | |
| GS-9851 | ||||
| Urine recovered (%)c | 0.81 (0.66) | 0.54 (0.41) | 0.72 (0.46) | 0.35 (0.31) |
| Ae0–24 (mg) | 1.61 (1.32) | 2.14 (1.66) | 1.44 (0.92) | 1.40 (1.23) |
| CLrenal (liters/min) | 0.192 (0.078) | 0.167 (0.090) | 0.185 (0.059) | 0.142 (0.096) |
| GS-566500 | ||||
| Urine recovered (%)c | 0.91 (0.95) | 0.87 (0.63) | 0.80 (0.37) | 0.56 (0.26) |
| Ae0–24 (mg) | 1.42 (1.47) | 2.70 (1.94) | 1.23 (0.57) | 1.70 (0.79) |
| CLrenal (liters/min) | 0.315 (0.241)b | 0.223 (0.204) | 0.449 (0.397) | 0.272 (0.312) |
| GS-331007 | ||||
| Urine recovered (%)c | 26.22 (6.93) | 14.01 (3.47) | 38.45 (9.58) | 25.81 (8.33) |
| Ae0–24 (mg) | 25.77 (6.81) | 27.55 (6.83) | 32.86 (8.38) | 35.76 (8.01) |
| CLrenal (liters/min) | 0.178 (0.048) | 0.172 (0.041) | 0.194 (0.046) | 0.183 (0.053) |
Values are for 8 subjects except where otherwise noted.
n = 7.
Percentage of study drug excreted in urine. Values for GS-566500 and GS-331007 are presented as GS-9851 equivalents given the difference in molecular weights between GS-9851 and its metabolites.
Maximum plasma concentrations for the metabolite GS-566500 generally increased with increasing dose levels of GS-9851; however, values increased only marginally with doses of ≥100 mg. Median tmax values for GS-566500 ranged from 1.5 to 3.0 h across doses. GS-566500 concentrations quickly fell below the limit of detection of the assay post-Cmax; median t1/2 values of 2.0 to 3.7 h across doses and days were calculated for subjects with available data. The percentage of extrapolated AUC on day 1 was 29.6% for 100 mg GS-9851, 27.0% for 200 mg GS-9851, and 19.4% for 400 mg GS-9851; however, only a fraction of the subjects provided available data. On day 1 and day 3, values of AUC0–24 for GS-566500 increased with the dose. The percentage of GS-566500 recovered in the urine on days 1 and 3 was less than 1% of an administered dose of GS-9851 (Table 2).
Following single and multiple oral dose administration of GS-9851, the median GS-331007 tmax ranged from 3.0 to 4.0 h on day 1 and day 3 across the 50-mg to 400-mg dose range. After reaching Cmax, GS-331007 concentrations declined, with median t1/2 values ranging from 6.0 to 17.9 h on day 3 across the 50-mg to 400-mg dose range. Median GS-331007 t1/2 values generally increased with the increase in the GS-9851 dose; however, it should be noted that for several dose levels, median t1/2 values were obtained from a fraction of treated subjects. For GS-331007, Cmax marginally increased with doses over the 200-mg to 400-mg GS-9851 dose range on day 1 and day 3 (Table 1). On day 1, values of AUC0–∞ increased with dose escalation; values of AUC0–24 increased with the dose on day 1 and day 3. If the increase in median t1/2 with increasing GS-9851 dose was meaningful, a higher degree of increase in the AUC would be expected than that observed.
The majority of drug exposure after a dose of GS-9851 was from GS-331007, the inactive nucleoside metabolite. The geometric least-squares mean value of AUC0–t was at least 48-fold higher (after adjustment for molecular weight differences) for GS-331007 than for GS-9851 on day 3. Geometric mean GS-331007/GS-9851 metabolic ratios were variable, but no dose-related trends were noted on days 1 and 3. Most of the drug recovered in the urine was recovered as GS-331007. On days 1 and 3, the percentage of GS-331007 excreted in the urine after administration of GS-9851 ranged from 14.01% to 38.45% (Table 2), with the lowest values on both days at 400 mg GS-9851. Renal clearance values for GS-331007 were stable across doses and days, ranging from 0.183 liters/min to 0.194 liters/min on day 3, values that are slightly higher than the glomerular filtration rate.
Trough concentrations of GS-9851 and GS-566500 in plasma were below the limit of detection of the assay for all dosing levels. Mean plasma trough concentration-time profiles for GS-331007 showed that three daily doses of GS-9851 up to 200 mg may be sufficient to establish steady-state conditions of GS-331007. Steady-state conditions appeared to be established in five of eight patients who received 400 mg GS-9851 (data not shown).
Dose proportionality results.
A power model analysis demonstrated a lack of dose proportionality in terms of Cmax and AUC for GS-9851 and its metabolites across the dose range studied (50 mg to 400 mg GS-9851). Dose proportionality was also assessed using a pairwise ANOVA (Table 3). For GS-9851 and GS-566500, results of the ANOVA suggested that increases in Cmax and AUC on day 1 and day 3 occurred in a less than dose-proportional manner, with the greatest deviations from proportionality at the highest doses. However, high variability was observed in the estimation of the pharmacokinetic parameters. For the metabolite GS-331007, ANOVA results indicated that all GS-331007 exposure parameters on day 1 increased linearly from 100 mg to 200 mg GS-9851. On day 3, increases in GS-331007 Cmax and AUC values were linear from 50 mg to 100 mg GS-9851. Less than proportional increases in Cmax and AUC values for GS-331007 were especially evident at the 400-mg dose of GS-9851.
Table 3.
Analysis of variance of dose proportionality results for GS-9851, GS-566500, and GS-331007
| Day | Parameter | Comparison (mg) | Analysis of variancea |
|||||
|---|---|---|---|---|---|---|---|---|
| GS-9851 |
GS-566500 |
GS-331007 |
||||||
| GLSM ratio | 90% CI | GLSM ratio | 90% CI | GLSM ratio | 90% CI | |||
| 1 | AUC0–24 (ng · h/ml) | 50/100 | 1.64 | 0.72, 3.77 | 0.46 | 0.16, 1.27 | 1.52 | 1.03, 2.25 |
| 200/100 | 1.23 | 0.53, 2.81 | 0.56 | 0.20, 1.58 | 1.06 | 0.72, 1.57 | ||
| 400/100 | 1.02 | 0.44, 2.33 | 0.70 | 0.26, 1.90 | 0.58 | 0.39, 0.86 | ||
| Cmax (ng/ml) | 50/100 | 1.30 | 0.64, 2.62 | 0.84 | 0.46, 1.56 | 1.56 | 1.08, 2.25 | |
| 200/100 | 0.96 | 0.48, 1.93 | 0.53 | 0.29, 0.98 | 0.99 | 0.68, 1.43 | ||
| 400/100 | 0.95 | 0.47, 1.91 | 0.45 | 0.25, 0.81 | 0.52 | 0.36, 0.75 | ||
| 3 | AUC0–24 (ng · h/ml) | 50/100 | 0.72 | 0.32, 1.63 | 0.73 | 0.20, 2.67 | 1.01 | 0.71, 1.43 |
| 200/100 | 0.78 | 0.35, 1.72 | 0.58 | 0.20, 1.69 | 0.83 | 0.59, 1.16 | ||
| 400/100 | 0.48 | 0.22, 1.06 | 0.71 | 0.24, 2.05 | 0.49 | 0.35, 0.69 | ||
| Cmax (ng/ml) | 50/100 | 0.82 | 0.37, 1.81 | 1.14 | 0.53, 2.43 | 1.03 | 0.78, 1.37 | |
| 200/100 | 0.71 | 0.33, 1.51 | 0.53 | 0.28, 1.00 | 0.77 | 0.59, 1.02 | ||
| 400/100 | 0.37 | 0.17, 0.79 | 0.38 | 0.20, 0.71 | 0.38 | 0.29, 0.50 | ||
GLSM, geometric least-squares mean.
Accumulation ratio results.
The overall accumulation ratios determined for GS-9851 and GS-566500 ranged from 0.75 to 0.94, indicating no accumulation after multiple dosing (Table 4). There was no accumulation of GS-331007 for the 50-mg GS-9851 dose; from 100 mg to 400 mg GS-9851, the accumulation ratios ranged from 16% to 48%. The overall accumulation ratio for GS-331007 across the dose range studied was 21%, indicating a modest accumulation of this metabolite.
Table 4.
Plasma accumulation values (day 3 AUC0–24 vs day 1 AUC0–24) for GS-9851, GS-566500, and GS-331007
| Analyte | GS-9851 treatment (mg) | Accumulation, GLSMa |
90% CI | ||
|---|---|---|---|---|---|
| Test | Reference | Ratio | |||
| GS-9851 | Overall | 74.99 | 79.44 | 0.94 | 0.63, 1.41 |
| 50 | 26.45 | 38.60 | 0.69 | 0.33, 1.41 | |
| 100 | 73.46 | 46.96 | 1.56 | 0. 53, 4.63 | |
| 200 | 114.65 | 115.14 | 1.00 | 0.48, 2.08 | |
| 400 | 141.93 | 190.80 | 0.74 | 0.34, 1.62 | |
| GS-566500 | Overall | 51.40 | 68.16 | 0.75 | 0.45, 1.28 |
| 50 | 17.98 | 16.86 | 1.07 | 0.40, 2.83 | |
| 100 | 49.08 | 74.12 | 0.66 | 0.21, 2.13 | |
| 200 | 57.13 | 83.44 | 0.68 | 0.22, 2.17 | |
| 400 | 138.44 | 206.94 | 0.67 | 0.25, 1.82 | |
| GS-331007 | Overall | 1,909.48 | 1,584.03 | 1.21 | 1.01, 1.45 |
| 50 | 851.38 | 863.61 | 0.99 | 0.61, 1.60 | |
| 100 | 1,685.25 | 1,136.42 | 1.48 | 1.03, 2.13 | |
| 200 | 2,797.72 | 2,418.59 | 1.16 | 0.85, 1.57 | |
| 400 | 3,311.80 | 2,652.34 | 1.25 | 0.86, 1.81 | |
Geometric least-squares mean.
Clinical virology results.
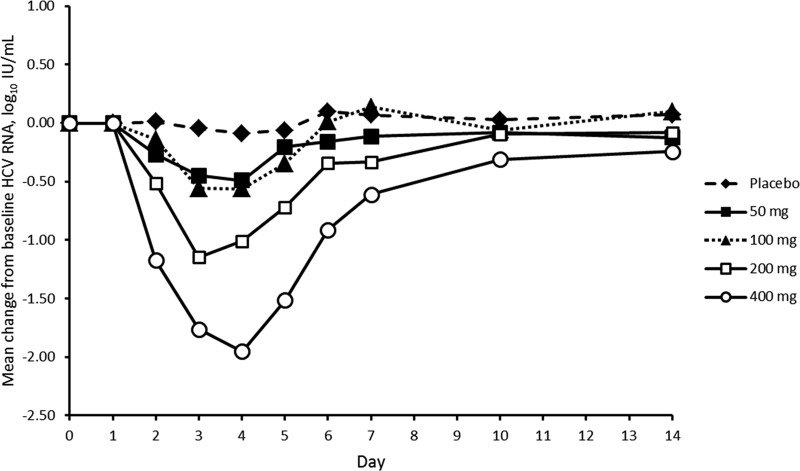
Patients who received GS-9851 experienced a marked decrease in mean HCV RNA from the baseline, whereas values for patients who received a placebo remained unchanged (Fig. 2). The largest HCV change from the baseline occurred 24 h after the last dose (day 4) for all active dosing regimens. Administration of 50 mg or 100 mg GS-9851 resulted in a similar change from baseline values of HCV RNA (day 4, 50 mg GS-9851, 0.492 log10 IU/ml; 100 mg GS-9851, 0.561 log10 IU/ml). A dose-dependent decrease was clearly observed for the 200-mg and 400-mg dose levels of GS-9851. Most (7/8; 87.5%) patients who received the 400-mg GS-9851 dose experienced a decrease in HCV RNA of ≥1.0 log10 2 days after receiving their last dose of GS-9851 (day 5). The mean change from baseline in HCV RNA appeared greater for patients infected with genotype 1a who received 400 mg GS-9851 than for patients infected with genotype 1b; there was a mean maximum change of −2.176 for genotype 1a (n = 4) and −1.728 for genotype 1b (n = 4). However, the magnitude of the difference in the response of the patients of each genotype is not remarkable, and similar mean HCV RNA declines between genotypes were observed at the 200-mg dose of GS-9851 (n = 6 and 2, genotypes 1a and 1b). Therefore, it is possible that the difference observed after receiving 400 mg GS-9851 may be a consequence of the small number of patients analyzed for each genotype.
Fig 2.
Mean HCV RNA change from baseline following multiple doses of GS-9851.
No preexisting or treatment-emergent S282T mutations were detected in tested HCV samples; there was no evidence of viral resistance after 3 days of monotherapy based upon population sequencing of the NS5B region or clonal analysis of plasma samples collected from all subjects at day 1 pretreatment (baseline) and at day 4 (24 h after last dosing) from those subjects that received GS-9851. Population sequencing of HCV samples revealed that several amino acid changes from baseline occurred in the patients who received GS-9851. Of note, most of these changes occurred in a single patient each and may thus represent sequence polymorphisms. However, there were three amino acid changes that were detected in more than one patient each: Q/R544Q (n = 2; one patient with genotype 1a and one patient with genotype 1b), D559D/N (n = 3; all patients with genotype 1a), and L588L/P (n = 2; one patient with genotype 1a and one patient with genotype 1b). The Q544 and R544 polymorphisms were detected at baseline among a number of GS-9851-treated subjects and did not appear to correlate with any specific virologic response. Position 588 is within the hydrophobic transmembrane C terminus of NS5B and is therefore unlikely to be directly involved in the RdRp activity of NS5B, since truncated NS5B, in which the transmembrane C-terminal 21 amino acids are deleted, retained RdRp activity. The D559D/N change, observed in three HCV genotype 1a subjects, is at a less polymorphic position. The two subjects with this change were in the 50-mg and 400-mg treatment groups. Analysis of the virologic response indicated that these two subjects had HCV RNA reductions comparable to those of other patients in their respective treatment groups. For these reasons, these amino acid changes are unlikely to be associated with reduced sensitivity to treatment with GS-9851.
DISCUSSION
As intended, GS-9851 is rapidly eliminated from systemic circulation, with apparent liver uptake and intracellular conversion to GS-461203, the active uridine triphosphate, given the on-treatment HCV RNA reductions observed in this patient population. Plasma exposures of GS-9851 and metabolites were consistent with the results of the single-ascending-dose (SAD) study in healthy subjects (20), with the highest systemic exposure observed for GS-331007 (the nucleoside metabolite), followed by GS-9851 and GS-566500.
In general, there was no dose-proportional exposure for any analyte, with less than proportional increases in exposure particularly at the highest doses of GS-9851 (200 and 400 mg) compared to results with a 100-mg reference dose. GS-9851 and GS-331007 were less than dose proportional to similar degrees, as indicated by relatively stable metabolite-to-parent exposure ratios across the dose range. This may suggest that the mechanism of this lack of dose proportionality is related to drug absorption and not alteration of drug clearance at higher doses.
Accumulation of GS-331007, but not GS-9851 or GS-566500, was observed in plasma following 3 days of GS-9851 administration. The modest degree of GS-331007 accumulation observed with GS-9851 was predictable based upon a t1/2 of approximately 6 to 18 h. GS-331007 median t1/2 values were variable between doses and did increase with dose; however, this is likely to be a function of increased bioanalytical detectability. In addition, a meaningful increase in t1/2 with an increasing GS-9851 dose is also not consistent with the degree of AUC increase observed with increasing dose. Steady-state conditions of GS-331007 were established after three doses in subjects receiving GS-9851 at 50 mg, GS-9851 at 100 mg, and GS-9851 at 200 mg and for most subjects receiving the 400-mg dose, which is also consistent with the t1/2 of GS-331007. Approximately 27% to 40% of a GS-9851 dose was recovered cumulatively as GS-9851, GS-566500, and GS-331007 on day 3, with most of the urinary recovery being attributed to GS-331007. Despite a relatively stable renal clearance rate for GS-9851 and GS-331007, the percent recovery of both parent and metabolite was higher at the 20-mg dose than at the 400-mg dose. This finding, coupled with the observation of less than dose-proportional exposure of GS-9851 and GS-331007 at higher GS-9851 doses, may suggest an alteration in GS-9851 intestinal solubility or absorption with increasing GS-9851 dose.
Clinical virology assessments revealed decreases from the baseline in mean plasma HCV RNA levels after administration of GS-9851 at all dose levels but not after administration of a placebo. The 400-mg GS-9851 dose produced the earliest and most potent antiviral effects in the greatest percentage of patients. Development of viral genotypic changes from the baseline were also assessed as it is known that RNA polymerase NS5B has a weak proofreading capacity and introduces errors during transcription of the HCV genome (23). These coding errors give rise to changes in the amino acid sequences of viral proteins that can lead to the emergence of treatment-resistant HCV variants. In the current study, no preexisting or treatment-emergent S282T mutations were detected, and there was no evidence of viral resistance after 3 days of therapy. This is of particular interest because this mutation has been previously associated with resistance to 2′-C-methyl-modified nucleotide inhibitors, such as GS-9851.
GS-9851 was generally well tolerated. The majority of events from both the placebo and GS-9851 cohorts were mild in severity, with the exception of an episode of elevated blood CK that was considered severe but judged to be unrelated to GS-9851. This episode resolved without intervention. No dose-limiting toxicity was identified, and there was no increase in the frequency or severity of adverse events with increasing doses of GS-9851 over the 3-day treatment period. In addition, no adverse events relating to liver function were reported, and no clinically significant trends in treatment-emergent vital signs and 12-lead ECG assessments were observed. The tolerability profile in this study was similar to that observed in a single-ascending-dose clinical study, in which healthy patients received single doses of GS-9851 ranging from 25 mg to 800 mg (20).
To date, few nucleoside analog inhibitors have been investigated for patients infected with HCV. The first nucleoside analog studied in such a patient population was NM823. In contrast with the results reported here for GS-9851, NM823 showed little antiviral efficacy and a poor safety profile. Therefore, the clinical development of NM823 was discontinued (24). Another nucleoside analog evaluated in patients with HCV was R7128. R7128 is a prodrug of PSI-6130, an oral cystidine nucleoside analog polymerase inhibitor (25). A study in patients with HCV showed that the parent compound, PSI-6130, was rapidly absorbed (tmax, 2 to 3 h), with a t1/2 of approximately 5 h (25). The t1/2 of the metabolite GS-331007 was close to 20 h. R7128 was generally well tolerated and resulted in significant, dose-dependent suppression of HCV replication following 14 days of monotherapy (25). After administration of R7128 at 1,500 mg once daily for 14 days, the mean HCV RNA decrease was of 1.48 log10 IU/ml. In our study, a once-daily 400-mg dose of GS-9851 led to a decrease of 1.761 log10 IU/ml after only 3 days' treatment. Nonetheless, the study with R7128 provided positive proof of concept that a direct-acting antiviral could deliver sufficient antiviral potency via monotherapy (25).
In conclusion, GS-9851 was well tolerated and readily absorbed in this population of patients chronically infected with HCV. Administration of GS-9851 led to significant reductions in plasma HCV RNA levels without the evolution of known resistance mutations. The findings of the current study indicate that further studies of longer duration are warranted to investigate the potential role of GS-9851 in the treatment of chronic HCV infection.
ACKNOWLEDGMENTS
We thank the patients and staff who participated in the study.
E. Lawitz reports receiving research/grant support from Abbott Laboratories, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, GlobeImmune, Idenix Pharmaceuticals, Idera Pharmaceuticals, Inhibitex Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Medarex, Medtronic, Merck & Co., Novartis, Pharmasset, Roche, Schering-Plough, Santaris Pharmaceuticals, Scynexis Pharmaceuticals, Vertex Pharmaceuticals, ViroChem Pharma, and ZymoGenetics, lecture fees from Gilead, Merck, and Vertex, and consulting fees from Abbott Laboratories, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Biotica, GlobeImmune, Inhibitex Pharmaceuticals, Merck & Co., Pharmasset, Santaris Pharmaceuticals, Tibotec, and Theravance; M. Rodriguez-Torres reports receiving research/grant support from Abbott Laboratories, Akros Pharmaceutical, Anadys Pharmaceutical, Beckman Coulter, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Gilead Pharmaceuticals, GlaxoSmithKline, Hoffman-La Roche, Human Genome Sciences, Idenix Pharmaceutical, Idera Pharmaceutical, Inhibitex, Johnson & Johnson, Merck Sharp & Dohme Corp., Mochida Pharmaceutical, Novartis, Pfizer, Pharmasset, Santaris Pharma. A/S, Scynexis, Inc., Siemens Healthcare Diagnostics, Vertex Pharmaceutical Inc., and Zymogenetics and consulting fees from Akros Pharmaceutical, Bristol-Myers Squibb, Genentech, Hoffman-La Roche, Inhibitex, Janssen R & D Ireland, Merck Sharp & Dohme Corp., Pharmasset, Santaris Pharma A/S, and Vertex Pharmaceutical Inc. J. Denning, E. Albanis, M. Cornpropst, M. M. Berrey, and W. Symonds were employees of Pharmasset at the time the study was conducted. J. Denning and W. Symonds are currently employees of Gilead Sciences. This study was funded by Pharmasset, Inc. Severina Moreira and Justin Cook of Niche Science and Technology Ltd. (Richmond-Upon-Thames, United Kingdom) provided writing and editorial support during the preparation of the manuscript and were paid for these services by Pharmasset, Inc.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Birerdinc A, Younossi ZM. 2010. Emerging therapies for hepatitis C virus. Expert Opin. Emerg. Drugs 15:535–544 [DOI] [PubMed] [Google Scholar]
- 2. Lauer GM, Walker BD. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41–52 [DOI] [PubMed] [Google Scholar]
- 3. Rong L, Perelson AS. 2010. Treatment of hepatitis C virus infection with interferon and small molecule direct antivirals: viral kinetics and modeling. Crit. Rev. Immunol. 30:131–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schinazi RF, Bassit L, Gavegnano C. 2010. HCV drug discovery aimed at viral eradication. J. Viral Hepat. 17:77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferenci P. 2011. Safety and efficacy of treatment for chronic hepatitis C with a focus on pegylated interferons: the backbone of therapy today and in the future. Expert Opin. Drug Saf. 10:529–544 [DOI] [PubMed] [Google Scholar]
- 6. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB, American Association for Study of Liver Diseases 2011. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 54:1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vermehren J, Sarrazin C. 2011. New HCV therapies on the horizon. Clin. Microbiol. Infect. 17:122–134 [DOI] [PubMed] [Google Scholar]
- 8. Ishii K, Tanaka Y, Yap CC, Aizaki H, Matsuura Y, Miyamura T. 1999. Expression of hepatitis C virus NS5B protein: characterization of its RNA polymerase activity and RNA binding. Hepatology 29:1227–1235 [DOI] [PubMed] [Google Scholar]
- 9. Lohmann V, Körner F, Herian U, Bartenschlager R. 1997. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J. Virol. 71:8416–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamashita T, Kaneko S, Shirota Y, Qin W, Nomura T, Kobayashi K, Murakami S. 1998. RNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal region. J. Biol. Chem. 273:15479–15486 [DOI] [PubMed] [Google Scholar]
- 11. Kolykhalov AA, Mihalik K, Feinstone SM, Rice CM. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeuzem S, Berg T, Moeller B, Hinrichsen H, Mauss S, Wedemeyer H, Sarrazin C, Hueppe D, Zehnter E, Manns MP. 2009. Expert opinion on the treatment of patients with chronic hepatitis C. J. Viral Hepat. 16:75–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herlihy KJ, Graham JP, Kumpf R, Patick AK, Duggal R, Shi ST. 2008. Development of intergenotypic chimeric replicons to determine the broad-spectrum antiviral activities of hepatitis C virus polymerase inhibitors. Antimicrob. Agents Chemother. 52:3523–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim A, Timm J. 2008. Resistance mechanisms in HCV: from evolution to intervention. Expert Rev. Anti Infect. Ther. 6:463–478 [DOI] [PubMed] [Google Scholar]
- 15. Ludmerer SW, Graham DJ, Boots E, Murray EM, Simcoe A, Markel EJ, Grobler JA, Flores OA, Olsen DB, Hazuda DJ, LaFemina RL. 2005. Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay. Antimicrob. Agents Chemother. 49:2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ali S, Leveque V, Le Pogam S, Ma H, Philipp F, Inocencio N, Smith M, Alker A, Kang H, Najera I, Klumpp K, Symons J, Cammack N, Jiang WR. 2008. Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack cross-resistance with R1479. Antimicrob. Agents Chemother. 52:4356–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Migliaccio G, Tomassini JE, Carroll SS, Tomei L, Altamura S, Bhat B, Bartholomew L, Bosserman MR, Ceccacci A, Colwell LF, Cortese R, De Francesco R, Eldrup AB, Getty KL, Hou XS, LaFemina RL, Ludmerer SW, MacCoss M, McMasters DR, Stahlhut MW, Olsen DB, Hazuda DJ, Flores OA. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164–49170 [DOI] [PubMed] [Google Scholar]
- 18. Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Wang P, Zhang HR, Bansal S, Espiritu C, Keilman M, Lam AM, Steuer HM, Niu C, Otto MJ, Furman PA. 2010. Discovery of a β-D-20-deoxy-20-r-fluoro-20-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 53:7202–7218 [DOI] [PubMed] [Google Scholar]
- 19. Lam AM, Murakami E, Espiritu C, Steuer HM, Niu C, Keilman M, Bao H, Zennou V, Bourne N, Julander JG, Morrey JD, Smee DF, Frick DN, Heck JA, Wang P, Nagarathnam D, Ross BS, Sofia MJ, Otto MJ, Furman PA. 2010. PSI-7851, a pronucleotide of β-D-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob. Agents Chemother. 54:3187–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denning J, Cornpropst M, Flach SD, Berrey MM, Symonds WT. 2013. Pharmacokinetics, safety, and tolerability of GS-9851, a nucleotide analog polymerase inhibitor for hepatitis C virus, following single ascending doses in healthy subjects. Antimicrob. Agents Chemother. 57:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murakami E, Tolstykh T, Bao H, Niu C, Steuer HM, Bao D, Chang W, Espiritu C, Bansal S, Lam AM, Otto MJ, Sofia MJ, Furman PA. 2010. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J. Biol. Chem. 285:34337–34347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murakami E, Niu C, Bao H, Micolochick Steuer HM, Whitaker T, Nachman T, Sofia MA, Wang P, Otto MJ, Furman PA. 2008. The mechanism of action of β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine involves a second metabolic pathway leading to β-D-2′-deoxy-2′-fluoro-2′-C-methyluridine 5′-triphosphate, a potent inhibitor of the HCV RNA-dependent RNA polymerase. Antimicrob. Agents Chemother. 52:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boonstra A, van der Laan LJ, Vanwolleghem T, Janssen HL. 2009. Experimental models for hepatitis C viral infection. Hepatology 50:1646–1655 [DOI] [PubMed] [Google Scholar]
- 24. Welsch C, Jesudian A, Zeuzem S, Jacobson I. 2012. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 61:i36–i46 [DOI] [PubMed] [Google Scholar]
- 25. Reddy R, Rodriguez-Torres M, Gane E, Robson R, Lalezari J, Everson GT, DeJesus E, McHutchison JG, Vargas HE, Beard A, Rodriguez CA, Hill GZ, Symonds WT, Berrey MM. 2007. Antiviral activity, pharmacokinetics, safety and tolerability of R7128, a novel nucleoside HCV RNA polymerase inhibitor, following multiple, ascending, oral doses in patients with HCV genotype 1 infection who have failed prior interferon therapy. Hepatology. 46(Suppl 1):862A [Google Scholar]