Abstract
Understanding the cellular pharmacology of antiretroviral agents in macrophages and subsequent correlation with antiviral potency provides a sentinel foundation for definition of the dynamics between antiretroviral agents and viral reservoirs across multiple cell types, with the goal of eradication of HIV-1 from these cells. Various clinically relevant nucleoside antiviral agents, and the integrase inhibitor raltegravir, were selected for this study. The intracellular concentrations of the active metabolites of the nucleoside analogs were found to be 5- to 140-fold lower in macrophages than in lymphocytes, and their antiviral potency was significantly lower in macrophages constitutively activated with macrophage colony-stimulating factor (M-CSF) during acute infection than in resting macrophages (EC50, 0.4 to 9.42 μM versus 0.03 to 0.4 μM, respectively). Although tenofovir-treated cells displayed significantly lower intracellular drug levels than cells treated with its prodrug, tenofovir disoproxil fumarate, the levels of tenofovir-diphosphate for tenofovir-treated cells were similar in lymphocytes and macrophages. Raltegravir also displayed significantly lower intracellular concentrations in macrophages than in lymphocytes, independent of the activation state, but had similar potencies in resting and activated macrophages. These data underscore the importance of delivering adequate levels of drug to macrophages to reduce and eradicate HIV-1 infection.
INTRODUCTION
Macrophages (Mϕ) are a major target of HIV-1 infection, and infected cells must be selectively destroyed to achieve HIV-1 eradication, since they act as potential long-term HIV reservoirs (1). Macrophages are recruited or already localized to the mucosal site of initial HIV-1 transmission (2–5) and, due to high surface expression of both CD4 and CCR5 (6, 7), represent a target for early establishment and maintenance of latent and chronic HIV-1 infection. These cells are also key mediators of both innate and acquired immunity (5), and thus, HIV-1-orchestrated depletion of macrophages is catastrophic to the global immune response.
Macrophages and macrophage-like cells are found in every tissue and organ (5) and display various activation states, largely dictated by the presence or absence of infection coupled with corresponding autocrine and paracrine cytokine milieus. These factors create many distinct and often concomitant microenvironments with heterogeneous exposures to extracellular drug concentrations relative to systemic CD4+ T lymphocytes. Macrophages are a target for early infection (1, 3, 8) and are present in multiple sites of primary transmission, including the gut and genital tract (1, 2, 4). Infection in macrophages often results in subpopulations of latent infection, which can occur across any tissue or organ containing these cells, such as the brain or lymphoid tissues, rendering them a ubiquitous source for reactivation of virus later in disease progression. Although the antiviral profile of antiretroviral therapy (ART) is partially defined in macrophages (2, 9–11), a direct link between potency and cellular pharmacology had not been reported. The relationship between the cellular pharmacology of ART and macrophages can impact viral loads, emergence of resistant HIV-1, and the long-term survival of infected individuals (8, 12). For these reasons, understanding the dynamics of ART pharmacology in macrophages and subsequent targeted elimination of both the cells and the virus that they harbor are critical to eliminating HIV-1 infection.
Nucleoside-containing regimens are the backbone of standardized treatment for HIV-1, with tenofovir disoproxil fumarate (TDF) and emtricitabine [(−)-FTC] representing two of the three drugs in the most prescribed antiviral combination therapy (Atripla). One of the most widely used integrase inhibitors is raltegravir (RAL) (Merck), although most recently, elvitegravir, as part of the quad pill Stribild (Gilead), was approved and could become an alternative choice for persons infected with HIV-1. As nucleoside-containing regimens will likely remain a sentinel tool in HIV-1 management and treatment for the foreseeable future, and integrase inhibitors provide a novel addition to combination ART (cART), various nucleoside analogs, as well as RAL, were selected for this study. The antiviral potency of the protease inhibitor atazanavir was also evaluated as a control, since it represents a class of antiretroviral agents that do not require metabolic activation and is also known to demonstrate potency in both acutely and chronically infected lymphocytes and macrophages.
Additionally, consideration was given to the fact that lymphocytes and macrophages are found across various activation states in vivo, and the activation state can alter a variety of cellular functions, including levels and concentrations of deoxynucleoside triphosphates (dNTP)/ribonucleoside triphosphates (rNTP) and cellular kinases in these cells. Therefore, it follows that activation state-driven modulation of dNTP/rNTP or kinase levels can alter either the antiviral potency of nucleoside reverse transcriptase inhibitor (NRTI) or NRTI triphosphate (TP) formation because (i) NRTI-TPs compete with endogenous dNTP/rNTP for incorporation into the growing viral DNA strand and (ii) different levels of kinases responsible for phosphorylation of NRTI to NRTI-TP in activated versus resting cells can impact the rate of formation of NRTI-TP. Therefore, it follows that the term “states of activation” in lymphocytes refers to the presence or absence of mitogen/cytokine (phytohemagglutinin [PHA]/interleukin 2 [IL-2]), where the presence of these activators creates a hyperactivated state wherein cellular division, dNTP/rNTP levels, immunologic activation, and a variety of other factors occur. The term “states of activation” in macrophages refers to the presence or absence of macrophage colony-stimulating factor (M-CSF), where M-CSF activates multiple pathways that drive production of proinflammatory, pro-HIV cytokines.
Although previous reports have established different antiviral potencies in acutely infected macrophages versus lymphocytes, we report for the first time a link between antiviral activity and cellular pharmacology in these cells. A previous report by Szebeni et al. (13) measured concentrations of zidovudine monophosphate (AZT-MP), AZT diphosphate (DP), or AZT-TP in lymphocytes and macrophages; however, the data were reported as a sum of AZT-MP plus AZT-DP plus AZT-TP, limiting the ability to delineate concentrations of the active metabolite, AZT-TP. Additionally, antiviral potency was not reported. Although other reports have defined the antiviral potency of NRTI against acute and chronic infection in macrophages and lymphocytes, the data reported here are the first to correlate the concentrations of the active metabolite of NRTI directly with antiviral potency. Together, these data provide a robust foundation for the design of novel therapeutics with greater intracellular concentrations and potency across macrophages of all activation states.
MATERIALS AND METHODS
Cellular pharmacology.
For macrophage cultures, monocytes were isolated from buffy coats of HIV-1-negative, hepatitis B virus/hepatitis C virus (HBV/HCV)-negative donors by density gradient centrifugation coupled with enrichment for CD14+ monocytes with the Rosette Sep antibody cocktail (Stem Cell Technologies, Vancouver, British Columbia, Canada). Wells were seeded at a density of 1.0 × 106 cells/well for 1 h at 37°C and 5% CO2 to allow adherence to the plastic prior to repeated washes with 1× phosphate-buffered saline (PBS). For cellular-pharmacology studies, activated macrophages were maintained in medium containing 100 U/ml M-CSF (R&D Systems, Minneapolis, MN) supplemented with 20% fetal calf serum (heat inactivated for 30 min at 56°C; Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA) for 7 days (37°C; 5% CO2) prior to testing. For resting macrophages, cells were maintained in medium containing M-CSF for 18 h prior to two washes with 1× PBS (to remove M-CSF) and subsequent culture in M-CSF-free medium supplemented with 20% heat-inactivated fetal calf serum and 1% penicillin/streptomycin for six more days prior to cellular-pharmacology studies. For all conditions, macrophages were stained with CD11b-allophycocyanin (APC) (Miltenyi Biotec, Auburn, CA) and subjected to fluorescence-activated cell sorting (FACS) to determine purity of >99%.
Primary human peripheral blood mononuclear (PBM) cells were isolated from buffy coats derived from healthy donors (Lifesouth, Dunwoody, GA). Resting PBM cells were maintained in PHA-free RPMI medium supplemented with 20% heat-inactivated fetal calf serum, 1% penicillin/streptomycin, and 2% l-glutamine (Sigma-Aldrich, San Jose, CA) for 72 h prior to cellular-pharmacology studies. Activated PBM cells were maintained analogously, with the exception that the medium was supplemented with 6 μg/ml phytohemagglutinin (Cape Cod Associates, East Falmouth, MA).
After the preparation of cells as described above, activated or resting cells were exposed to 10 μM AZT, abacavir (ABC), carbovir (CBV), (−)-FTC, TDF, tenofovir (TFV), (−)-beta-d-dioxolane-guanine (DXG), DXG plus AZT (1:1 ratio), or RAL for 4 h at 37°C in a 5% CO2 atmosphere. Extracellular application of the drug at concentrations equal to the 50% effective concentration (EC50) prohibits accumulation of NRTI-TP above the limit of detection of liquid chromatography-tandem mass spectrometry (LC–MS-MS). Therefore, 10 μM NRTI was applied, as it is within the concentration that can be reached in vivo, and an assumption of linearity relative to the extracellular drug concentration/intracellular accumulation of NRTI-TP can be made, providing a mechanistic and physiologically relevant rationale for using that concentration.
Next, the extracellular medium was removed, and the cells were washed twice with ice-cold 1× PBS to remove any residual medium. The cells were resuspended in 60% CH3OH overnight, and extracts were centrifuged at 18,000 × g for 10 min. The supernatants were dried under a flow of air and redissolved in the mobile phase for LC–MS-MS analysis (14). For each condition, experiments were conducted in duplicate 12-well plates (BD Biosciences, San Jose, CA) at a density of 1.0 × 106 cells/well. Duplicate control wells to determine the total number of viable cells (trypan blue exclusion stain) were also prepared. Cell volumes of 0.32 μm3 and 2.66 μm3 for lymphocytes and macrophages, respectively (15), were used to calculate the intracellular concentrations for the active metabolites of each drug.
Antiviral studies.
Resting or activated macrophages were cultured as described above for 7 days. For acute infection, resting or activated cells were serum starved for 18 h prior to infection and cultured for 2 h in media with various concentrations of AZT, ABC, CBV, (−)-FTC, TDF, TFV, DXG, RAL, or atazanavir prior to removal of drug-containing medium and 4-h infection with HIV-1BaL at a multiplicity of infection (MOI) of 0.1 in the absence of drug. Then, the virus was removed and drug-containing medium was returned to each culture. For chronic infections, resting or activated cells were serum starved for 18 h and then infected for 4 h with HIV-1BaL at an MOI of 0.1 prior to removal of virus and culture in drug-containing medium for 7 days. For both acute and chronic infections, supernatants were collected on day 7 after infection, and HIV-1 p24 was quantified by enzyme-linked immunosorbent assay (ELISA) (Zeptometrix Corporation, Buffalo, NY). The EC50 was determined using CalcuSyn software (BioSoft Corporation, Cambridge, United Kingdom). For constitutively activated macrophages, cells were maintained in medium containing M-CSF for 7 days prior to infection and were constitutively exposed to M-CSF prior to and after infection with HIV-1BaL. Supernatants were collected on day 7 after infection, and HIV-1 p24 was quantified by ELISA as described above.
For lymphocytes, testing was performed using at least three independent assays performed with duplicates within each experiment. Cells were incubated in RPMI medium (HyClone, Logan, UT) containing human recombinant interleukin 2 (26.5 units/ml; Chiron Inc., Emeryville, CA) and 20% heat-inactivated fetal calf serum. Infections were performed by adding HIV-1LAI, followed by further incubation at 37°C, 5% CO2, 1 h prior to addition of drugs. Assays were performed in 24-well plates (BD Biosciences, Franklin Lakes, NJ). One milliliter of supernatant was collected after 5 days in culture and then centrifuged at 12,000 rpm for 2 h at 4°C in a Jouan Br43i (Thermo Electron Corp., Marietta, OH). The product of the reverse transcription assay was quantified using a Packard harvester and a direct beta counter, and the data were analyzed as previously described (16).
Statistical methods.
Means and standard deviations were determined and statistical comparisons were performed using unpaired Student t tests with the statistical routines in Microsoft Excel 2007. A P value of < 0.05 was considered statistically significant.
RESULTS
Cellular pharmacology.
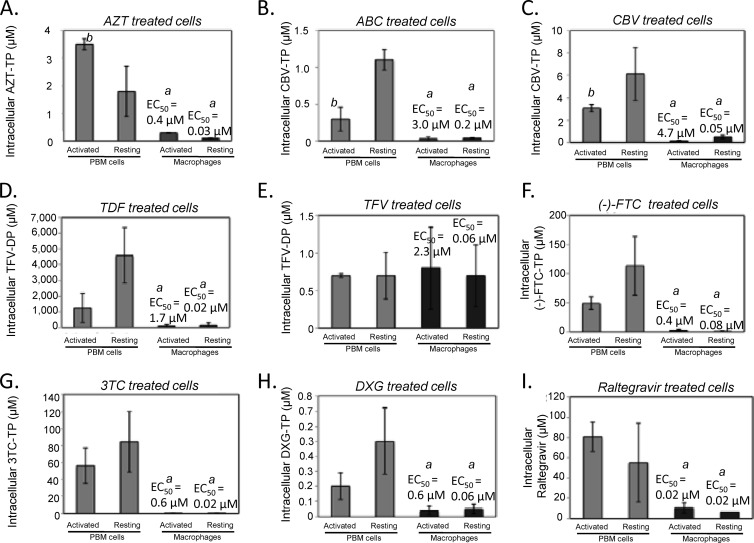
The concentrations of nucleoside analog triphosphate, the active form of the nucleoside (NRTI-TP) in activated and resting macrophages, were significantly lower in macrophages than in activated lymphocytes and were independent of the activation state (P < 0.05), with the exception of TFV (Fig. 1). The intracellular concentrations of AZT-TP were 18- and 35-fold lower in resting macrophages than in resting and activated lymphocytes, respectively (Fig. 1A and Table 1). The intracellular concentration of AZT-TP was 11.6-fold lower in activated macrophages than in lymphocytes. AZT-TP levels were significantly higher (3.5-fold) in activated lymphocytes than in resting lymphocytes (P < 0.05) (Fig. 1A and Table 1).
Fig 1.
Intracellular concentrations of ART drugs are significantly lower in Mϕ than in PBM cells independent of the activation state (A to D and F to I), with the exception of those of TFV, which were similar in both PBM cells and Mϕ. For all NRTIs tested, the EC50 for chronically infected Mϕ was >50 μM (data not shown), and constitutive activation significantly diminished potency versus resting Mϕ (A to I). The potencies of raltegravir and atazanavir (data not shown) and of raltegravir (I) were similar for resting and activated Mϕ. The data represent means and standard deviations calculated from at least 5 independent experiments conducted with duplicates within each experiment. a, statistically significant difference relative to PBM cells (P < 0.01); b, significant difference relative to resting PBM cells (P < 0.05).
Table 1.
Intracellular concentrations and antiviral potencies of ART in resting or activated PBM cells and macrophagesa
| Drug tested | State of cells | Active metabolite measured | Intracellular NTP or drug in PBM cells (μM) (±SD) | Intracellular NTP or drug in PBM cells (pmol/106 cells) (±SD) | EC50 in acutely infected PBM cells (μM) (±SD) | Intracellular NTP or drug in Mϕ (μM) (±SD) | Intracellular NTP or drug in Mϕ (pmol/106 cells) (±SD) | EC50 in acutely infected Mϕ (μM) (±SD) | EC50 in chronically infected Mϕ (μM) |
|---|---|---|---|---|---|---|---|---|---|
| AZT | Activated | AZT-TP | 3.5 ± 0.9b | 1.1 ± 0.3 | 0.004 ± 0.0022 | 0.3 ± 0.3c | 0.8 ± 0.8 | 0.4 ± 0.04d | >50 |
| Resting | AZT-TP | 1.8 ± 0.4 | 0.6 ± 0.1 | NA | 0.1 ± 0.1c | 0.3 ± 0.3c | 0.03 ± 0.007 | >50 | |
| ABC | Activated | CBV-TP | 0.3 ± 0.2b | 0.1 ± 0.06 | 0.3 ± 0.2 | 0.03 ± 0.03c | 0.08 ± 0.08c | 3.0 ± 1.1d | >50 |
| Resting | CBV-TP | 1.1 ± 0.1 | 0.4 ± 0.03 | NA | 0.04 ± 0.01c | 0.1 ± 0.02c | 0.2 ± 0.3 | >50 | |
| CBV | Activated | CBV-TP | 3.1 ± 0.3b | 1.0 ± 0.1 | 0.08 ± 0.08 | 0.1 ± 0.1c | 0.3 ± 0.3c | 4.7 ± 3.2d | >50 |
| Resting | CBV-TP | 7.7 ± 3.7 | 2.5 ± 1.2 | NA | 0.7 ± 0.3c | 1.9 ± 0.8 | 0.05 ± 0.02 | >50 | |
| TDF | Activated | TFV-DP | 1.252 ± 896 | 400.6 ± 286 | 0.01 ± 0.01 | 160 ± 123c | 425.6 ± 327 | 1.7 ± 1.2d | >50 |
| Resting | TFV-DP | 4,589 ± 1,764 | 1,468.0 ± 564 | NA | 107 ± 144c | 284.6 ± 383c | 0.02 ± 0.02 | >50 | |
| TFV | Activated | TFV-DP | 0.7 ± 0.3 | 0.2 ± 0.1 | 1.6 ± 1.2 | 0.7 ± 0.6 | 1.9 ± 1.1 | 2.3 ± 0.9d | >50 |
| Resting | TFV-DP | 0.7 ± 0.3 | 0.2 ± 0.1 | NA | 0.8 ± 0.4 | 2.1 ± 1.5 | 0.06 ± 0.03 | >50 | |
| (−)-FTC | Activated | (−)-FTC-TP | 49.2 ± 41.8 | 15.7 ± 3.4 | 0.008 ± 0.007 | 2.2 ± 2.0c | 5.9 ± 5.0c | 0.4 ± 0.2d | >50 |
| Resting | (−)-FTC-TP | 113 ± 18.8 | 36.2 ± 6.0 | NA | 1.0 ± 0.6c | 2.7 ± 1.6c | 0.08 ± 0.02 | >50 | |
| 3TC | Activated | 3TC-TP | 56 ± 21 | 17.9 ± 6.7 | 0.06 ± 0.04 | 0.6 ± 0.4c | 1.6 ± 1.0c | 0.6 ± 0.3d | >50 |
| Resting | 3TC-TP | 84.5 ± 36 | 27.0 ± 11.5 | NA | 0.8 ± 0.3c | 2.1 ± 0.8c | 0.02 ± 0.01 | >50 | |
| DXG | Activated | DXG-TP | 0.2 ± 0.1 | 0.06 ± 0.03 | 0.3 ± 0.2 | 0.05 ± 0.04c | 0.1 ± 0.1 | 0.6 ± 0.2d | >50 |
| Resting | DXG-TP | 0.5 ± 0.2 | 0.2 ± 0.06 | NA | 0.04 ± 0.03c | 0.1 ± 0.08c | 0.06 ± 0.02 | >50 | |
| RAL | Activated | Raltegravir | 80.9 ± 14.7 | 25.9 ± 4.7 | 0.001 ± 0.002 | 10.5 ± 4.1c | 27.9 ± 11.0 | 0.02 ± 0.02 | >50 |
| Resting | Raltegravir | 55.3 ± 24.1 | 17.7 ± 7.7 | NA | 7.0 ± 0.2c | 18.6 ± 0.5c | 0.02 ± 0.03 | >50 | |
| ATVe | Activated | Atazanavir | ND | ND | 0.007 ± 0.002 | ND | ND | 0.03 ± 0.03 | 0.09 |
| Resting | Atazanavir | ND | ND | NA | ND | ND | 0.03 ± 0.04 | 0.06 |
All data represent at least 5 duplicate independent experiments containing pooled cells from 8 donors. NA, not applicable; ND, not done.
Significant difference relative to resting PBM cells (P < 0.05).
Significant difference relative to PBM cells (P < 0.01).
Significant difference relative to resting macrophages (P < 0.05).
ATV, atazanivir.
For resting cells treated with ABC, the intracellular concentrations of carbovir triphosphate (CBV-TP), the active metabolite of ABC, were 27.5- and 7.5-fold lower in resting macrophages than in activated and resting lymphocytes, respectively (Fig. 1B and Table 1). The intracellular concentration of CBV-TP was 10-fold lower in activated macrophages than in lymphocytes. For both ABC- and CBV-treated cells, the intracellular concentration of CBV-TP was 2-fold lower (P < 0.05) in resting than in activated lymphocytes (Fig. 1B and Table 1). Resting cells treated with CBV demonstrated an intracellular concentration of CBV-TP that was 12.2- and 4.5-fold lower in resting macrophages than in resting and activated lymphocytes, respectively. The intracellular concentration of CBV-TP was 28-fold lower in activated macrophages than in lymphocytes (Fig. 1C and Table 1).
In resting cells treated with (−)-FTC, the intracellular concentration of (−)-FTC-TP was 113-fold and 49.2-fold lower in resting macrophages than in resting and activated lymphocytes, respectively. For activated cells treated with (−)-FTC, the intracellular concentration of (−)-FTC-TP was 22-fold lower in activated macrophages than in activated lymphocytes (Fig. 1F and Table 1).
For resting cells treated with TDF, the intracellular concentration of TFV-DP, the active metabolite of both TDF and TFV, was 28-fold and 11.7-fold lower in resting macrophages than in resting and activated lymphocytes, respectively. For activated cells treated with TDF, the intracellular concentration of TFV-DP was 11.7-fold lower in activated macrophages than in lymphocytes (Fig. 1D and Table 1).
On the other hand, the exception to the overall trend for NRTI was found with TFV. In TFV-treated cells, the intracellular concentration of TFV-DP was not significantly different in macrophages than in lymphocytes, independent of the activation state (Fig. 1E and Table 1). The intracellular concentrations of TFV-DP for TDF-treated cells was not unexpected versus that for TFV-treated cells, as TDF is a prodrug of TFV and is more lipophilic, allowing greater intracellular accumulation.
Lamivudine (3TC)-treated resting cells demonstrated an intracellular concentration of 3TC-TP that was 140-fold and 70-fold lower in resting macrophages than in resting and activated lymphocytes, respectively. For activated cells treated with 3TC, the intracellular concentration of 3TC-TP was 93-fold lower in activated macrophages than in lymphocytes (Fig. 1G and Table 1). Similarly, resting cells treated with DXG displayed an intracellular concentration of DXG-TP that was 10-fold and 5-fold lower in resting macrophages than in resting and activated lymphocytes, respectively. For activated cells treated with DXG, the intracellular concentration of DXG-TP was 4-fold lower in activated macrophages than in lymphocytes (Fig. 1H and Table 1).
Coincubation of cells treated with 10 μM DXG together with AZT (1:1 ratio) was also assessed. The intracellular concentration of AZT-TP was 11-fold lower in resting macrophages than in lymphocytes. For activated cells treated with 10 μM DXG plus AZT (1:1 ratio), the intracellular concentration of AZT-TP was 30-fold lower in activated macrophages than in lymphocytes (Table 2). Quantification of DXG-TP in resting cells treated with 10 μM DXG + AZT (1:1 ratio) demonstrated that the intracellular concentration of DXG-TP was 31-fold lower in resting macrophages than in lymphocytes. For activated cells treated with 10 μM DXG + AZT (1:1 ratio), the intracellular concentration of DXG-TP was 16-fold lower in activated macrophages than in lymphocytes (Table 2).
Table 2.
Intracellular concentrations of AZT-TP or DXG-TP for activated or resting macrophages or PBM cells treated with 10 μM AZT-DXG at a ratio of 1:1a
| Cell type | Drugb | Active metabolite measured | Intracellular NRTI-TP(±SD) |
|
|---|---|---|---|---|
| μM | pmol/106 cells | |||
| PBM cells | ||||
| Activated | AZT | AZT-TP | 3.5 ± 0.9 | 1.1 ± 0.3 |
| Activated | AZT + DXG | AZT-TP | 2.7 ± 0.6 | 0.9 ± 0.2 |
| Activated | DXG | DXG-TP | 0.2 ± 0.1 | 0.06 ± 0.03 |
| Activated | AZT + DXG | DXG-TP | 0.25 ± 0.01 | 0.1 ± 0.003 |
| Resting | AZT | AZT-TP | 1.8 ± 0.4 | 0.6 ± 0.1 |
| Resting | AZT + DXG | AZT-TP | 1.4 ± 0.3 | 0.5 ± 0.1 |
| Resting | DXG | DXG-TP | 0.5 ± 0.2 | 0.2 ± 0.06 |
| Resting | AZT + DXG | DXG-TP | 0.54 ± 0.12 | 0.2 ± 0.03 |
| Macrophages | ||||
| Activated | AZT | AZT-TP | 0.3 ± 0.3 | 0.8 ± 0.8 |
| Activated | AZT + DXG | AZT-TP | 0.1 ± 0.05 | 0.3 ± 0.1 |
| Activated | DXG | DXG-TP | 0.05 ± 0.04 | 0.1 ± 0.1 |
| Activated | AZT + DXG | DXG-TP | 0.02 ± 0.008 | 0.05 ± 0.02 |
| Resting | AZT | AZT-TP | 0.1 ± 0.1 | 0.3 ± 0.3 |
| Resting | AZT + DXG | AZT-TP | 0.1 ± 0.01 | 0.3 ± 0.03 |
| Resting | DXG | DXG-TP | 0.04 ± 0.03 | 0.1 ± 0.08 |
| Resting | AZT + DXG | DXG-TP | 0.02 ± 0.01 | 0.05 ± 0.03 |
The presence of AZT did not antagonize DXG-TP levels in macrophages or PBM cells, independent of the activation state, and the presence of DXG did not antagonize AZT-TP levels in macrophages or PBM cells independent of the activation state.
AZT + DXG at a ratio of 1:1.
RAL-treated resting cells showed an intracellular concentration that was 8-fold lower in resting macrophages than in lymphocytes. For activated cells treated with RAL, the intracellular concentration was 93-fold lower in activated macrophages than in lymphocytes (Fig. 1I and Table 1).
Antiviral potency.
For chronically infected macrophages, the EC50s of all NRTIs were >50 μM. This was expected, as the primary mechanism of action of NRTIs is to inhibit HIV-1 reverse transcriptase (RT)-mediated DNA synthesis, a mechanism that is largely ineffective against chronic, already established infection. Protease inhibitors primarily target virus that is extracellular and affect maturation of the virus, whereas NRTIs target only virus inside cells. Therefore, it follows that protease inhibitors (PI) are able to inhibit chronic infection, which is hallmarked by the presence of integrated provirus.
During acute infection of constitutively activated macrophages, the EC50s of NRTIs ranged from 0.4 to 9.42 μM compared to 0.03 to 0.40 μM in resting macrophages (Table 1). As expected, atazanavir displayed great potency against both acute resting, acute activated, and chronic HIV-1 infection in macrophages (the EC50 values were 0.03, 0.03, and 0.09 μM, respectively), in agreement with the primary mechanism of action of PI, which involves inhibition of viral maturation in already infected cells. RAL displayed similar potency in acutely infected resting and activated macrophages (0.02 and 0.02 μM, respectively) and was inactive even at 50 μM against chronic HIV-1 infection in macrophages. For comparison, the antiviral potencies of NRTIs in activated lymphocytes during acute infection ranged from 0.001 to 0.3 μM (Table 1).
DISCUSSION
The delivery of adequate concentrations of antiretroviral drug to permissive HIV-1 target cells in all viral reservoirs is a primary goal for the development of regimens for eventual viral eradication. As the mechanism of action of NRTI is upstream of integrated provirus found in latently infected cells, therapeutic modalities with novel mechanisms of action designed to eliminate virus across all states of activation, latency, tissue distribution, and localization are necessary to achieve systemic eradication of HIV-1. To that end, delivery of NRTIs at adequate concentrations to inhibit de novo infection across multiple sites remains a significant obstacle and, if achieved, could provide an agent mitigating ongoing de novo replication in concert with novel therapeutic modalities.
Certain organ sites, including the brain and genitourinary tissues, are more shielded from drug delivery; however, antiretroviral agents may also demonstrate nonuniform potency in the various permissive cell types (17–21). Therefore, this mechanistic study was performed to investigate the potencies of antiretroviral agents in macrophages, since the cells become infected early during HIV transmission in the majority of individuals and may be involved in the infection of more shielded organ sites, including tissues protected by the blood brain barrier. The antiretroviral potencies in macrophages were compared to potencies in activated lymphocytes, another primary HIV-1 target cell. These data suggest that certain cells, including macrophages, may require concentrations of certain antiretroviral agents higher than those needed to inhibit infection in activated lymphocytes.
Multiple reports define ongoing chronic inflammation and immune activation in HIV-infected patients, even in those with well-controlled viremia (22–24). Elevated levels of IL-6, tumor necrosis factor alpha (TNF-α), and IL-1α/β largely hallmark these events. Relative to the activation states in these data, IL-6, TNF-α, and IL-1α/β are largely absent in “resting” populations. “Activated” populations as caused by addition of PHA/IL-2 (lymphocytes) or M-CSF (macrophages) (7) trigger intrinsic activation of each population of cells, which, upon infection, as occurs in vivo, cross-activates infected and bystander cells, resulting in the proinflammatory milieu hallmarked by IL-6, TNF-α, and IL-1α/β. Therefore, the “activation” conferred here mirrors a key event in immune activation.
Although it is a long and uncertain leap from in vitro to in vivo studies, these in vitro experiments were designed to recapitulate in vivo dynamics as closely as possible by using primary cells and physiologically relevant concentrations of drug.
Intracellular concentrations of all NRTIs except TFV were 5- to 140-fold lower in macrophages than in lymphocytes independent of the activation state. We further demonstrate that, compared to macrophages maintained in the absence of M-CSF, acutely infected cells that are constitutively maintained in the presence of M-CSF displayed significantly diminished antiviral potency (EC50, 0.03 to 0.40 μM versus 0.4 to 9.42 μM, respectively). These data are confirmed by previous studies (2, 8, 10) on the antiviral potencies of NRTIs in chronically infected macrophages and are also in agreement with reports demonstrating the potencies of protease inhibitors against chronically and acutely infected lymphocytes and macrophages (10). The cellular-pharmacology studies also demonstrated for the first time that the intracellular concentration of RAL was significantly lower in macrophages than in lymphocytes, independent of the activation state. Although RAL is not confined intracellularly as are NRTI-TPs, RAL-mediated export from cells can be caused by the efflux transporters p-glycoprotein/multidrug resistance transporter 1 (p-gp/MDR-1) (25). p-gp/MDR-1 are active at 37°C but inactive at 4°C (25–27). All washes were performed with ice-cold PBS at 4°C, taking care to ensure that RAL could not be effluxed by this mechanism. Additionally, as washing procedures for cells were performed analogously for macrophages and lymphocytes, this allowed direct comparison between data from each cell type without concern for different handling or efflux that could skew the results. Additionally, we demonstrated that RAL was not potent against chronic HIV-1 infection in macrophages, similar to the profile displayed by NRTIs. In contrast to the NRTIs tested, RAL did not display any difference in potency against acute HIV-1 infection in resting versus activated macrophages. These data are in general agreement with those recently reported by Scopelliti et al. (28) for acutely infected macrophages, although the potency of RAL against chronic infection in macrophages was not reported.
A primary mechanism of action for NRTI-TP is through competition with the natural dNTP and rNTP (in PBM cells and macrophages, respectively) for HIV-1 RT (29, 30). Therefore, it is possible that the reduced accumulation of NRTI-TP may be partially compensated for by reduced macrophage concentrations of natural dNTP, namely, dCTP, dTTP, dATP, dGTP, or dUTP, and natural rNTP near the active site of HIV-1 RT in activated lymphocytes relative to macrophages. Activated lymphocytes are considered replicating cells, while macrophages are primarily in a resting G1 state. Therefore, the relative concentrations of dNTP tend to be higher in activated lymphocytes, since they are needed for DNA replication. Recent reports from our group demonstrated that macrophages harbor 22- to 320-fold lower overall dNTP concentrations than dividing target cells, as well as a disparity between dNTP and rNTP concentrations significantly lower than those observed in resting and activated lymphocytes (14, 29, 30).
Here, we demonstrated that the activation state of macrophages does not significantly alter intracellular concentrations of nucleoside analogs and that constitutive exposure to M-CSF (to ensure a constitutively activated phenotype) significantly decreases the potency of NRTIs versus macrophages maintained without constitutive M-CSF exposure. These results could be explained by M-CSF differentially modulating the cellular milieu, resulting in different cytokine-based events that could alter antiviral potency independent of influencing the cellular pharmacology of NRTI. M-CSF is secreted by macrophages upon HIV-1 infection and has been implicated as a factor that promotes infection and triggers a Th2 proinflammatory cytokine milieu, namely, TNF-α and gamma interferon (IFN-γ), that is associated with increased viral loads, decreased CD4 T cell counts, and progression to AIDS (31, 32). A cytokine-based mechanism that impacts viral replication independent of cellular pharmacology could explain the differential potencies of antiretroviral agents in activated versus resting macrophages.
As expected, the accumulation of AZT-TP, a cell cycle-dependent NRTI, was significantly (3.5-fold) higher in activated lymphocytes than in resting lymphocytes (P < 0.05) (33–36). In contrast, ABC- or CBV-treated lymphocytes demonstrated a 2-fold increase in intracellular CBV-TP in resting versus activated lymphocytes (P < 0.01). The metabolism of these agents is complex. For instance, ABC is converted to ABC-MP via adenosine phosphotransferase, which is then converted to CBV-MP by various cytosolic enzymes and then to CBV-TP by cellular kinases (37). An alternate pathway of metabolism is conversion of ABC to 6-amino-CBV and then phosphorylation to 6-amino-CBV-MP by AMP deaminase and subsequent phosphorylation to CBV-TP by cellular kinases. Alternatively, 6-amino-CBV can be converted to CBV by adenosine deaminase and then to CBV-MP by 5′-nucleotidase, an inosine phosphotransferase (37, 38). Therefore, modulation of the activation state of the cell may alter the levels of various enzymes responsible for the complex intracellular metabolism of ABC or CBV to their active metabolite, CBV-TP, in lymphocytes and macrophages.
TFV was the only NRTI tested that did not display significantly lower concentrations of active nucleotide concentrations in macrophages than in lymphocytes. TFV is a weak inhibitor of HIV-1, because it may not penetrate cells efficiently due to the negatively charged phosphate moiety. Therefore, it is possible that the effect of its polarity may be reduced in macrophages, since they demonstrate a high degree of phagocytic activity that could bypass traditional mechanisms of entry. The prodrug TDF is lipid soluble and masks the negative charges of TFV. Therefore, it is not surprising that TFV-DP levels were significantly higher following TDF than following TFV incubation (Fig. 1). TFV is converted to TFV-MP by (5′) AMP-activated protein kinase (AMPK) and to TFV-DP via nucleoside diphosphate kinase (NDPK) or creatine kinase (39, 40). TDF follows the same metabolic pathway but is rapidly converted to TFV by carboxylesterase or phosphodiesterase. TFV circumvents the need for carboxylesterase or phosphodiesterase to convert TDF to TFV; however, since these enzymes are not rate limiting, it is unlikely that they are responsible for the lack of significant difference in TFV-DP across lymphocytes and macrophages. It is more likely that lower levels of TFV, caused by differences in polarity and charge of TFV versus TDF, confer lower enzymatic activity and a lower rate of conversion of TFV to its active metabolite, TFV-DP (39).
Other reports have assessed the concentrations of TFV-DP or (−)-FTC-TP in lymphocytes or CD14+ cells (monocytes) isolated from healthy volunteers orally administered either TDF or (−)-FTC (41). The results indicated that concentrations of both (−)-FTC-TP and TFV-DP were higher in monocytes than in lymphocytes for some time points tested; however, the ranges reported overlapped in some cases. Although it appears that (−)-FTC-TP and TFV-DP concentrations were higher in the monocyte subset, it was difficult to directly correlate these data with ours, as CD14+ monocytes are a markedly different cell type than CD11b+ terminally differentiated macrophages.
Since AZT and DXG are not cross-resistant, AZT can prevent the emergence of the K65R mutation, and in combination, the two drugs are synergistic in vitro and in humans (42). DXG was evaluated in the presence of AZT in macrophages (42, 43). The cellular accumulation of DXG-TP and AZT-TP were unaffected by coincubation (Table 2), supporting similar findings in activated lymphocytes and lending further incentive for the development of this drug combination (44).
In summary, our findings demonstrated that significantly lower levels of NRTI-TP are observed in macrophages than in lymphocytes and demonstrate the relative impact of the activation state upon antiviral potency. We also demonstrated that TFV displays similar intracellular concentrations of drug across both lymphocytes and macrophages and that CBV-treated resting macrophages, TDF- or (−)-FTC-treated resting or activated macrophages, and 3TC-treated resting macrophages displayed EC50s (potencies) that are significantly below the corresponding intracellular concentrations of drug in these cells, necessitating consideration of these drugs in targeting virus in macrophage-derived viral sanctuaries (30). These data underscore the importance of delivering adequate levels of drug to primary HIV-1 target cells and viral reservoirs with antiviral potency of drugs across macrophages in various activation states. Definition of these key facets provides a powerful foundation for better understanding how activation states in vivo can impact the ability to achieve systemic HIV-1 eradication.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants 8R01OD011094 and 5P30-AI-50409 (CFAR) and by the Department of Veterans Affairs.
R. F. Schinazi is the founder and a major shareholder of RFS Pharma, LLC, a clinical company that is developing [(−)-β-d-2,6-diaminopurine dioxolane, DAPD, AMDX]/DXG for the treatment of HIV.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Gavegnano C, Fromentin E, Schinazi RF. 2009. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir. Chem. Chemother. 20:63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aquaro S, Balestra E, Cenci E, Francesconi M, Calio R, Perno CF. 1997. HIV infection in macrophage: role of long-lived cells and related therapeutical strategies. J. Biol. Regul. Homeost Agents 11:69–73 [PubMed] [Google Scholar]
- 3. Balestra E, Perno CF, Aquaro S, Panti S, Bertoli A, Piacentini M, Forbici F, D'Arrigo R, Calio R, Garaci R. 2001. Macrophages: a crucial reservoir for human immunodeficiency virus in the body. J. Biol. Regul. Homeost. Agents 15:272–276 [PubMed] [Google Scholar]
- 4. Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. 2005. Pathogenesis of macrophage tropic HIV-1. Curr. HIV Res. 3:53–60 [DOI] [PubMed] [Google Scholar]
- 5. Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11:723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O'Connor MJ, Doms RW, Gonzalez-Scarano F. 1999. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J. Virol. 73:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958 [DOI] [PubMed] [Google Scholar]
- 8. Aquaro S, Bagnarelli P, Guenci T, De Luca A, Clementi M, Balestra E, Calio R, Perno CF. 2002. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J. Med. Virol. 68:479–488 [DOI] [PubMed] [Google Scholar]
- 9. Perno CF, Aquaro S, Rosenwirth S, Balestra E, Peichl P, Billich A, Villani N, Calio R. 1994. In vitro activity of inhibitors of late stages of the replication of HIV in chronically infected macrophages. J. Leukoc. Biol. 56:381–386 [DOI] [PubMed] [Google Scholar]
- 10. Perno CF, Newcomb FM, Davis DA, Aquaro S, Humphrey RW, Calio R, Yarchoan R. 1998. Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J. Infect. Dis. 178:413–422 [DOI] [PubMed] [Google Scholar]
- 11. Perno CF, Yarchoan R, Balzarini J, Bergamini A, Milanese G, Pauwels R, De Clercq E, Rocchi R, Calio R. 1992. Different pattern of activity of inhibitors of the human immunodeficiency virus in lymphocytes and monocyte/macrophages. Antiviral Res. 17:289–304 [DOI] [PubMed] [Google Scholar]
- 12. Chu H, Wang JJ, Qi M, Yoon JJ, Wen X, Chen X, Ding L, Spearman P. 2012. The intracellular virus-containing compartments in primary human macrophages are largely inaccessible to antibodies and small molecules. PLoS One 7:e35297 doi:10.1371/journal.pone.0035297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szebeni J, Patel SS, Hung K, Wahl LM, Weinstein JN. 1991. Effects of thymidine and uridine on the phosphorylation of 3′-azido-3′-deoxythymidine (zidovudine) in human mononuclear cells. Antimicrob. Agents Chemother. 35:198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fromentin E, Gavegnano C, Obikhod A, Schinazi RF. 2010. Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry. Anal. Chem. 82:1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. 2004. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 279:51545–51553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinstein JN, Bunow B, Weislow OS, Schinazi RF, Wahl SM, Wahl LM, Szebeni J. 1990. Synergistic drug combinations in AIDS therapy. Dipyridamole/3′-azido-3′-deoxythymidine in particular and principles of analysis in general. Ann. N. Y. Acad. Sci. 616:367–384 [DOI] [PubMed] [Google Scholar]
- 17. Best BM, Letendre SL, Brigid E, Clifford DB, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Simpson DM, Ellis R, Capparelli EV, Grant I. 2009. Low atazanavir concentrations in cerebrospinal fluid. AIDS 23:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB, Collier AD, Gelman BB, Mbeo G, McCutchan JA, Simpson DM, Haubrich R, Ellis R, Grant I. 2011. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J. Antimicrob. Chemother. 66:354–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tashima KT, Caliendo AM, Ahmad M, Gormley JM, Fiske WD, Brennan JM, Flanigan TP. 1999. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J. Infect. Dis. 180:862–864 [DOI] [PubMed] [Google Scholar]
- 20. Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D, Collier A, Gelman B, Marra C, Mbeo G, McCutchan A, Morgello S, Simpson D, Way L, Vaida F, Ueland S, Capparelli E, Grant I. 2010. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob. Agents Chemother. 54:5156–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Best BM, Letendre SL, Koopmans P, Rossi SS, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Capparelli EV, Ellis RJ, Grant I. 2012. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J. Acquir. Immune Defic. Syndr. 59:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamat A, Misra V, Cassol E, Ancuta P, Yan Z, Li C, Morgello S, Gabuzda D. 2012. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS One 7:e30881 doi:10.1371/journal.pone.0030881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, Crowe SM, Jaworowski A. 2012. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J. Immunol. 189:1491–1499 [DOI] [PubMed] [Google Scholar]
- 24. Reus S, Portilla J, Sanchez-Paya J, Giner L, Frances R, Such J, Boix V, Merino E, Gimeno A. 2012. Low-level HIV viraemia is associated with microbial translocation and inflammation. J. Acquir. Immune Defic. Syndr. [Epub ahead of print.] doi:10.1097/QAI.0b013e3182745ab0 [DOI] [PubMed] [Google Scholar]
- 25. Moss D, Kwan WS, Liptrott N, Siccardi M, Anstee D, Khoo S, Back D, Owen A. 2010. Raltegravir is a substrate for the influx transporters OAT1 and PEPT1 and the efflux transporter Pgp but is not transported by OATP1A2, OATP1B1, OATP1B3, OCT1, NTCP, or PEPT2, abstr. 613. 17th Conf. Retrovir. Opportunistic Infect.: Progr. Abstr., February 16–19, 2010, Moscone Center West, San Francisco, CA, U. S. A [Google Scholar]
- 26. Ambudkar SV, Kim IW, Sauna ZE. 2006. The power of the pump: mechanisms of action of P-glycoprotein (ABCB1). Eur. J. Pharm. Sci. 27:392–400 [DOI] [PubMed] [Google Scholar]
- 27. Callaghan R, Ford RC, Kerr ID. 2006. The translocation mechanism of P-glycoprotein. FEBS Lett. 580:1056–1063 [DOI] [PubMed] [Google Scholar]
- 28. Scopelliti F, Pollicita M, Ceccherini-Silberstein F, Di Santo F, Surdo M, Aquaro S, Perno CF. 2011. Comparative antiviral activity of integrase inhibitors in human monocyte-derived macrophages and lymphocytes. Antiviral Res. 92:255–261 [DOI] [PubMed] [Google Scholar]
- 29. Gavegnano C, Kennedy EM, Kim B, Schinazi RF. 2012. The impact of macrophage nucleotide pools on HIV-1 reverse transcription, viral replication, and the development of novel antiviral agents. Mol. Biol. Int. 2012:625983 doi:10.1155/2012/625983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennedy EM, Gavegnano C, Nguyen L, Slater R, Lucas A, Fromentin F, Schinazi RF, Kim B. 2010. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J. Biol. Chem. 285:39380–39391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Becker Y. 2004. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers—a review and hypothesis. Virus Genes 28:5–18 [DOI] [PubMed] [Google Scholar]
- 32. Kedzierska K, Crowe SM, Turville S, Cunningham AL. 2003. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 13:39–56 [DOI] [PubMed] [Google Scholar]
- 33. Jacobsson B, Britton S, He Q, Karlsson A, Eriksson S. 1995. Decreased thymidine kinase levels in peripheral blood cells from HIV-seropositive individuals: implications for zidovudine metabolism. AIDS Res. Hum. Retroviruses 11:805–811 [DOI] [PubMed] [Google Scholar]
- 34. Jacobsson B, Britton S, Tornevik Y, Eriksson S. 1998. Decrease in thymidylate kinase activity in peripheral blood mononuclear cells from HIV-infected individuals. Biochem. Pharmacol. 56:389–395 [DOI] [PubMed] [Google Scholar]
- 35. Wurtzer S, Compain S, Benech H, Hance AJ, Clavel F. 2005. Effect of cell cycle arrest on the activity of nucleoside analogues against human immunodeficiency virus type 1. J. Virol. 79:14815–14821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viora M, Di Genova G, Rivabene R, Malorni W, Fattorossi A. 1997. Interference with cell cycle progression and induction of apoptosis by dideoxynucleoside analogs. Int. J. Immunopharmacol. 19:311–321 [DOI] [PubMed] [Google Scholar]
- 37. Faletto MB, Miller WH, Garvey EP, St Clair MH, Daluge SM, Good SS. 1997. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob. Agents Chemother. 41:1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daluge SM, Good SS, Faletto MB, Miller WH, St Clair MH, Boone LR, Tisdale M, Parry NR, Reardon JE, Dornsife RE, Averett DR, Krenitsky TA. 1997. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 41:1082–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schinazi RF, Hernandez-Santiago BI, Hurwitz SJ. 2006. Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses. Antiviral Res. 71:322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma PL, Nurpeisov V, Hernandez-Santiago BI, Beltran T, Schinazi RF. 2004. Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Curr. Top. Med. Chem. 4:895–919 [DOI] [PubMed] [Google Scholar]
- 41. Anderson P, Meditz A, Zheng JH, Predhomme J, Klein B, Guida L, McCallister K, Fernandez C, Kiser J, Bushman L. 2012. Cellular pharmacology of tenofovir and emtricitabine in blood, rectal, and cervical cells from HIV- volunteers, abstr. 587. 19th Annual Conference on Retroviruses and Opportunistic Infections, Seattle, WA, U. S. A [Google Scholar]
- 42. Murphy RL, Kivel NM, Zala C, Ochoa C, Tharnish P, Mathew J, Pascual ML, Schinazi RF. 2010. Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals. Antivir. Ther. 15:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hurwitz SJ, Asif G, Fromentin E, Tharnish PM, Schinazi RF. 2010. Lack of pharmacokinetic interaction between amdoxovir and reduced- and standard-dose zidovudine in HIV-1-infected individuals. Antimicrob. Agents Chemother. 54:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez-Santiago BI, Obikhod A, Fromentin E, Hurwitz SJ, Schinazi RF. 2007. Short communication cellular pharmacology of 9-(beta-d-1,3-dioxolan-4-yl) guanine and its lack of drug interactions with zidovudine in primary human lymphocytes. Antivir. Chem. Chemother. 18:343–346 [DOI] [PubMed] [Google Scholar]