Abstract
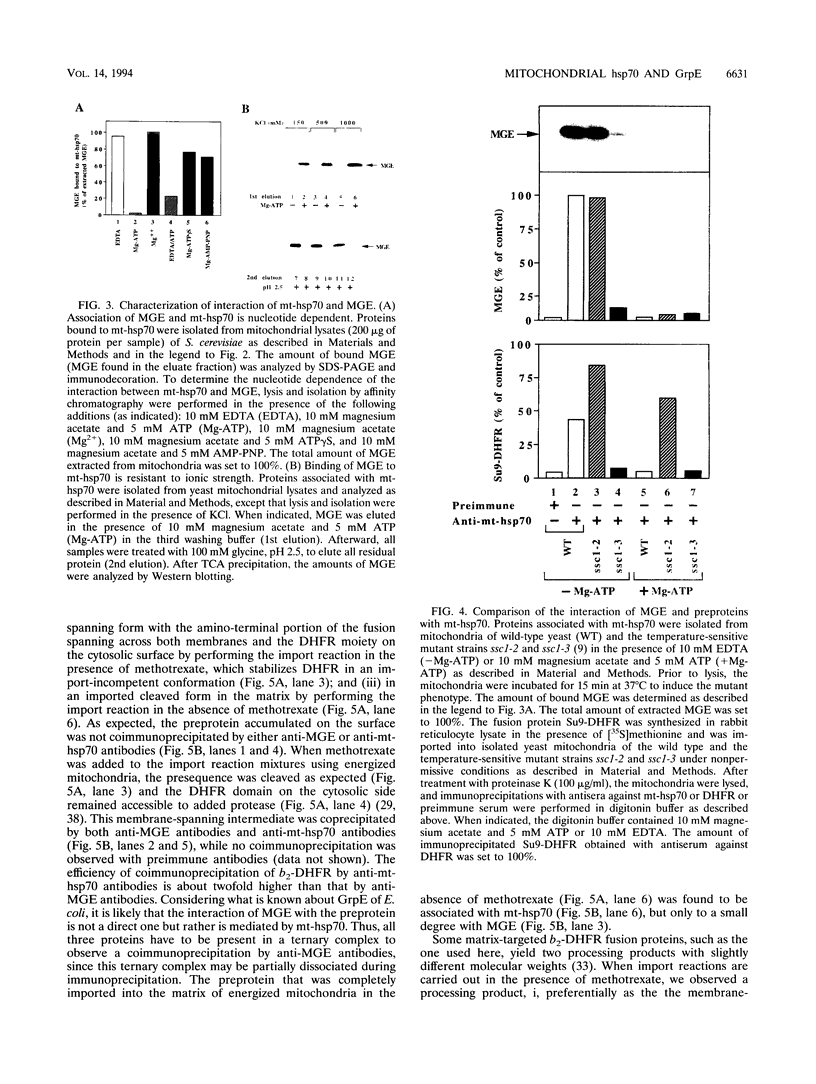
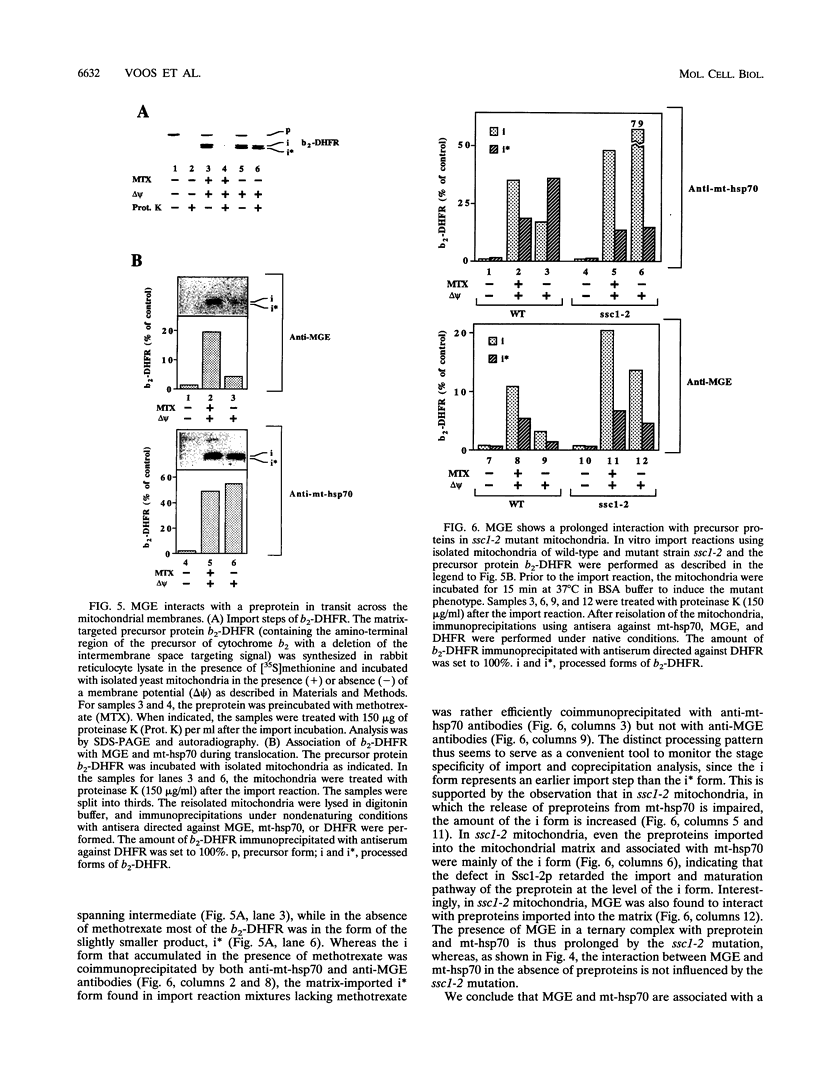
We characterized a 24-kDa protein associated with matrix hsp70 (mt-hsp70) of Neurospora crassa and Saccharomyces cerevisiae mitochondria. By using specific antibodies, the protein was identified as MGE, a mitochondrial homolog of the prokaryotic heat shock protein GrpE. MGE extracted from mitochondria was quantitatively bound to hsp70. It was efficiently released from hsp70 by the addition of Mg-ATP but not by nonhydrolyzable ATP analogs or high salt. A mutant mt-hsp70, which was impaired in release of bound precursor proteins, released MGE in an ATP-dependent manner, indicating that precursor proteins and MGE bind to different sites of hsp70. A preprotein accumulated in transit across the mitochondrial membranes was specifically coprecipitated by either antibodies directed against MGE or antibodies directed against mt-hsp70. The preprotein accumulated at the outer membrane was not coprecipitated by either antibody preparation. After being imported into the matrix, the preprotein could be coprecipitated only by antibodies against mt-hsp70. We propose that mt-hsp70 and MGE cooperate in membrane translocation of preproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Blom J., Kübrich M., Rassow J., Voos W., Dekker P. J., Maarse A. C., Meijer M., Pfanner N. The essential yeast protein MIM44 (encoded by MPI1) is involved in an early step of preprotein translocation across the mitochondrial inner membrane. Mol Cell Biol. 1993 Dec;13(12):7364–7371. doi: 10.1128/mcb.13.12.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H., Silver P. A. A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature. 1991 Feb 14;349(6310):627–630. doi: 10.1038/349627a0. [DOI] [PubMed] [Google Scholar]
- Brodsky J. L., Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993 Dec;123(6 Pt 1):1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Kosic-Smithers J. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4156–4160. doi: 10.1073/pnas.84.12.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr D. M., Lu X., Douglas M. G. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem. 1992 Oct 15;267(29):20927–20931. [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer J., Bujard H., Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell. 1992 May 29;69(5):833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. The emergence of the chaperone machines. Trends Biochem Sci. 1992 Aug;17(8):295–299. doi: 10.1016/0968-0004(92)90439-g. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Hlodan R., Langer T. Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci. 1994 Jan;19(1):20–25. doi: 10.1016/0968-0004(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Ostermann J., Guiard B., Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987 Dec 24;51(6):1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Ikeda E., Yoshida S., Mitsuzawa H., Uno I., Toh-e A. YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett. 1994 Feb 21;339(3):265–268. doi: 10.1016/0014-5793(94)80428-1. [DOI] [PubMed] [Google Scholar]
- Kambouris N. G., Burke D. J., Creutz C. E. Cloning and genetic analysis of the gene encoding a new protein kinase in Saccharomyces cerevisiae. Yeast. 1993 Feb;9(2):141–150. doi: 10.1002/yea.320090205. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Koll H., Guiard B., Rassow J., Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992 Mar 20;68(6):1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
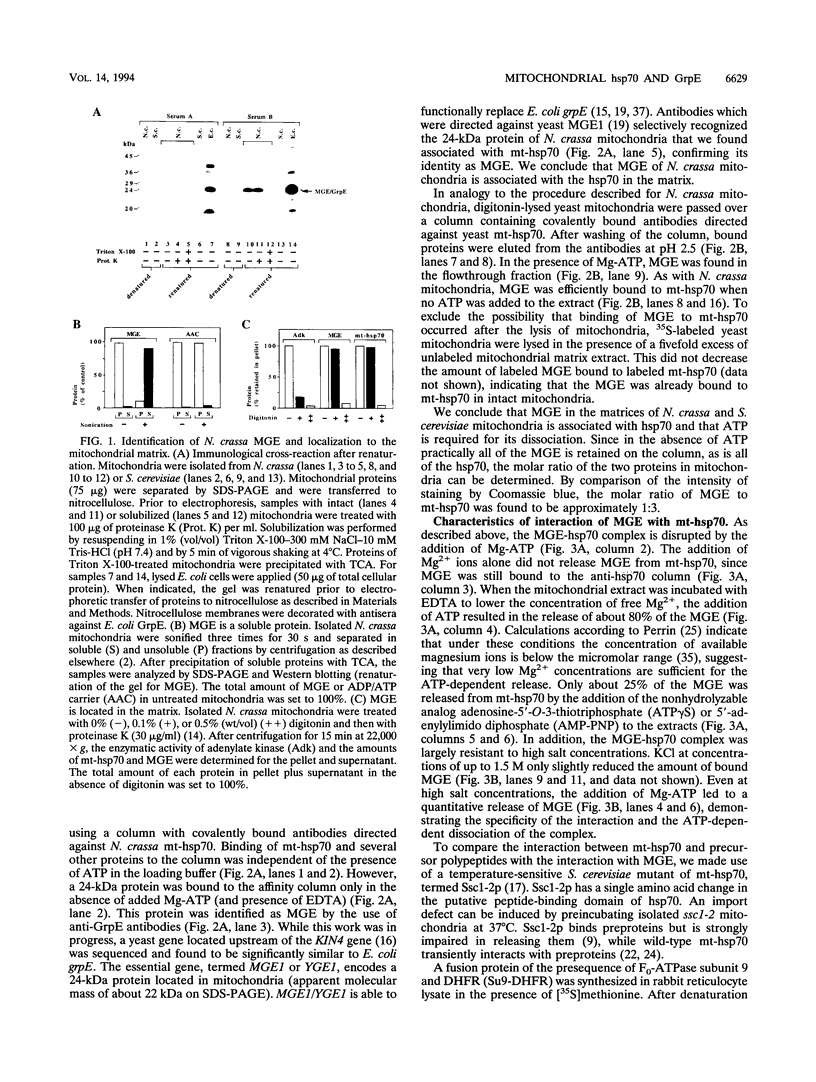
- Laloraya S., Gambill B. D., Craig E. A. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
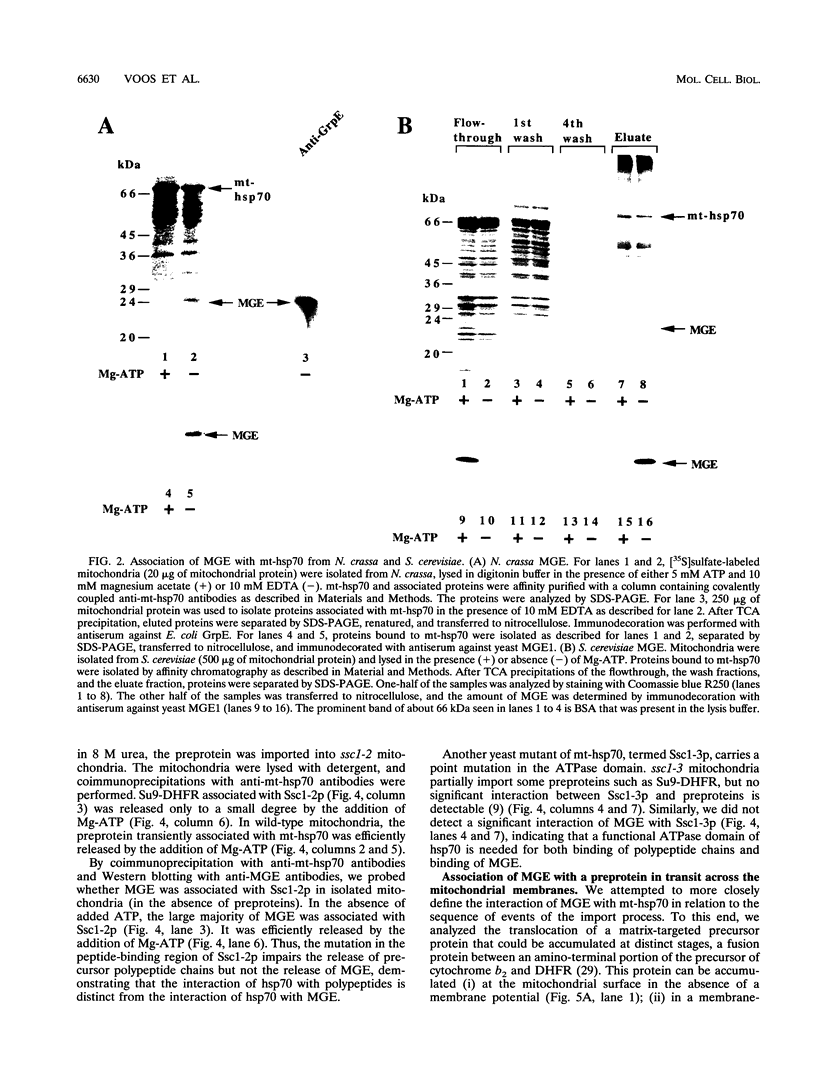
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipiuk J., Georgopoulos C., Zylicz M. Initiation of lambda DNA replication. The Escherichia coli small heat shock proteins, DnaJ and GrpE, increase DnaK's affinity for the lambda P protein. J Biol Chem. 1993 Mar 5;268(7):4821–4827. [PubMed] [Google Scholar]
- Ostermann J., Voos W., Kang P. J., Craig E. A., Neupert W., Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990 Dec 17;277(1-2):281–284. doi: 10.1016/0014-5793(90)80865-g. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985 Nov;4(11):2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Rassow J., Guiard B., Wienhues U., Herzog V., Hartl F. U., Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J Cell Biol. 1989 Oct;109(4 Pt 1):1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Schwaiger M., Herzog V., Neupert W. Characterization of translocation contact sites involved in the import of mitochondrial proteins. J Cell Biol. 1987 Jul;105(1):235–246. doi: 10.1083/jcb.105.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Seytter T., Guiard B., Neupert W. Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 1993 Jun;12(6):2295–2302. doi: 10.1002/j.1460-2075.1993.tb05883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. Y., Goldberg A. L. Formation in vitro of complexes between an abnormal fusion protein and the heat shock proteins from Escherichia coli and yeast mitochondria. J Bacteriol. 1991 Nov;173(22):7249–7256. doi: 10.1128/jb.173.22.7249-7256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D., Wickner S. The interplay of the GrpE heat shock protein and Mg2+ in RepA monomerization by DnaJ and DnaK. J Biol Chem. 1993 Dec 5;268(34):25296–25301. [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Voos W., Gambill B. D., Guiard B., Pfanner N., Craig E. A. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol. 1993 Oct;123(1):119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Ang D., Georgopoulos C. The grpE protein of Escherichia coli. Purification and properties. J Biol Chem. 1987 Dec 25;262(36):17437–17442. [PubMed] [Google Scholar]
- Zylicz M. The Escherichia coli chaperones involved in DNA replication. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):271–278. doi: 10.1098/rstb.1993.0025. [DOI] [PubMed] [Google Scholar]