Abstract
While numerous studies have assessed the outcomes of methicillin-resistant S. aureus (MRSA) colonization over the short term, little is known about longer-term outcomes after discharge. An assessment of long-term outcomes could provide information about the utility of various MRSA prevention approaches. A matched-cohort study was performed among Veterans Affairs (VA) patients screened for MRSA colonization between the years 2007 and 2009 and followed to evaluate outcomes until 2010. Cox proportional-hazard models were used to evaluate the association between MRSA colonization and long-term outcomes, such as infection-related readmission and crude mortality. A total of 404 veterans were included, 206 of whom were MRSA carriers and 198 of whom were noncarriers. There were no culture-proven MRSA infections on readmission among the noncarriers, but 13% of MRSA carriers were readmitted with culture-proven MRSA infections on readmission (P < 0.01). MRSA carriers were significantly more likely to be readmitted, to be readmitted more than once due to proven or probable MRSA infections, and to be readmitted within 90 days of discharge than noncarriers (P < 0.05). Infection-related readmission (adjusted hazard ratio [HR] = 4.07; 95% confidence interval [CI], 2.16 to 7.67) and mortality (adjusted HR = 2.71; 95% CI, 1.87 to 3.91) were significantly higher among MRSA carriers than among noncarriers after statistically adjusting for potential confounders. Among a cohort of VA patients, MRSA carriers are at high risk of infection-related readmission, MRSA infection, and mortality compared to noncarriers. Noncarriers are at very low risk of subsequent MRSA infection. Future studies should address whether interventions such as nasal or skin decolonization could result in improved outcomes for MRSA carriers.
INTRODUCTION
Staphylococcus aureus frequently causes serious infections involving the bloodstream, lower respiratory tract, and skin and soft tissue (1). Methicillin-resistant S. aureus (MRSA) emerged in the 1960s and has since become a major cause of illness and death in the health care setting (2). In the 2008 National Healthcare Safety Network, MRSA represented over half of all S. aureus health care-associated infections in the United States (3).
Approximately 10 to 40% of people carry S. aureus in their anterior nares (4–6). Nasal carriage of MRSA can be found among 1 to 2% of the general population but in as many as 10 to 15% of patients admitted to acute-care hospitals and intensive care units (ICUs) (7, 8). Several studies have found that MRSA-colonized patients are at higher risk for invasive MRSA infection than noncolonized patients (6, 9–12). In a systematic review of 10 observational studies, nasal colonization by MRSA was associated with a 4-fold increase in the risk of infection (12).
While numerous studies have focused on the impact of MRSA colonization over the short term, such as among inpatient populations during their acute-care stay, little is known about longer-term outcomes after discharge. Only two prior studies have assessed long-term outcomes among MRSA-colonized or infected patients. However, neither study compared these patients to an uninfected, uncolonized control group (13, 14).
The purpose of our study was to examine the long-term (>1-year) risk for MRSA infection among patients found to be MRSA colonized during an acute-care admission, using a non-MRSA-infected and noncolonized control group and adjusting for pertinent comorbidities. Additionally, we aimed to compare rates of infection and death among colonized versus noncolonized patients. With an increasing number of facilities, such as all VA facilities, collecting MRSA surveillance swabs on admission, outcome data concerning patients colonized with MRSA on admission could potentially impact clinical practice and target prevention efforts (15).
MATERIALS AND METHODS
We performed a matched-cohort study of patients admitted to the Iowa City Veterans Affairs Health Care System (VAHCS) between January 2007 and November 2009. This study is nested within a previously described cohort study in which patients were screened for MRSA on admission, subsequently during hospitalization, and at the time of discharge (8). Patients identified as MRSA carriers who had an MRSA-positive nares surveillance swab as measured via PCR at any time during the index admission were frequency matched by month of admission to patients identified as noncarriers. Noncarriers were defined as patients whose nares surveillance screen and any clinical cultures were all negative for MRSA during the index hospitalization.
Detailed chart review was performed for the MRSA carriers and noncarriers by an infectious disease physician fellow (N. M. Quezada Joaquin) to identify MRSA infection-related outcomes. MRSA infections were categorized into culture-proven infections and probable infections. Culture-proven MRSA infections represent all positive clinical cultures for MRSA associated with signs and symptoms of infection. Probable MRSA infections were defined as signs and symptoms of infection, and response to therapy focused on MRSA with either negative cultures or no cultures obtained.
Demographic variables on the index admission were collected, such as sex; age; presence of a central venous catheter or peripherally inserted central catheter (PICC); ICU admission; and health care facility exposure, including admission from home, an outside hospital, or a long-term-care facility, and discharge to a long-term-care facility or home. The Charlson comorbidity index was calculated using discharge International Classification of Diseases, Ninth Revision (ICD-9) codes and included comorbidities, such as heart disease, hypertension, diabetes mellitus, renal disease, liver disease, malignancy, and AIDS (16). The severity of illness on admission to the index hospitalization (i.e., study entry) was measured using the McCabe-Jackson score (17, 18).
All MRSA carriers and noncarriers were followed from the time of enrollment (admission) until death or until November 2010. The primary outcome was hospital readmission due to culture-proven or probable MRSA infection. The secondary outcomes included crude mortality, time to readmission, and diagnosis as infectious at readmission (e.g., osteomyelitis, skin and soft tissue infection, or sepsis). Data were also collected on the source of a positive MRSA culture (e.g., pleural fluid, sputum, or blood).
Bivariable analyses were performed using the chi-square test or Fisher's exact test (for categorical variables) and the t test (for continuous variables) as required. Cox proportional-hazard modeling was performed to statistically adjust for underlying illness between the groups. All variables that were significant in the bivariable analysis (alpha < 0.1) were selected for inclusion in the initial (full) multivariable Cox proportional-hazard model. Variables that were not significantly associated with the outcome (alpha < 0.05) were removed from the full multivariable model in succession. Each variable removed was then reinserted into the model to assess whether the variable altered the regression coefficient of the primary exposure variable by greater than 20%. If so, that variable was included in the model. A priori, we chose the following variables as biologically important: Charlson comorbidity index and McCabe-Jackson severity of illness score. These variables were included in each model irrespective of their statistical significance. The proportional-hazard assumption that the effect of a variable is constant over time was tested for each variable in the final model by assessing the interaction of the variable with a function of time. Crude mortality rates among MRSA carriers and noncarriers ere compared using a Kaplan-Meier survival model. The log-rank test was used to compare survival curves. All analyses were performed using SAS software (SAS Institute, Cary, NC) version 9.3. This study was approved by both the University of Iowa Institutional Review Board and the Iowa City VA Health Care System Research and Development Committee.
RESULTS
A cohort of 404 veterans, 206 of whom were MRSA carriers and 198 of whom were noncarriers, was examined. All 206 MRSA carriers had a positive MRSA nasal surveillance swab during their index admission. In addition, 22 of the 206 MRSA carriers (11%) also had an MRSA infection on the index admission. As expected in an elderly veteran population, over 95% of the patients were male in both groups. Patient age and the prevalences of individual comorbidities (heart disease, renal disease, liver disease, diabetes mellitus, malignancies, and AIDS) were similar in both groups (Table 1). However, the Charlson comorbidity index and the McCabe-Jackson score were significantly higher among the MRSA carriers than among the noncarriers (Table 1).
Table 1.
Comparison of demographics, comorbidities, severity of illness, and health care facility exposure between carriers and noncarriers
| Characteristica | Value |
P value | |
|---|---|---|---|
| MRSA carriers (n = 206) | Noncarriers (n = 198) | ||
| Male [n (%)] | 200 (97) | 195 (98) | 1.0 |
| Age (mean) (yr) | 65 | 64 | 0.27 |
| Comorbidity [n (%)] | |||
| Heart disease | 96 (47) | 86 (44) | 0.55 |
| Diabetes mellitus | 94 (46) | 75 (38) | 0.12 |
| Renal disease | 26 (13) | 26 (13) | 0.86 |
| Liver disease | 20 (10) | 16 (8) | 0.57 |
| Malignancy | 49 (24) | 39 (20) | 0.33 |
| AIDS | 1 (0.5) | 0 | 1.0 |
| Median Charlson score (IQR) | 1 (1,3) | 1 (0,2) | 0.01 |
| McCabe-Jackson score at admission to index hospitalization [n (%)] | <0.01 | ||
| Nonfatal | 36 (18) | 51 (26) | |
| Ultimately fatal | 120 (58) | 139 (70) | |
| Fatal | 49 (24) | 8 (4) | |
| Admitted from [n (%)]: | <0.001 | ||
| Home | 154 (75) | 187 (94) | |
| OSH | 31 (15) | 10 (5) | |
| LTCF | 20 (10) | 1 (0.5) | |
| Discharge to [n (%)]: | <0.01 | ||
| Home | 148 (75) | 184 (93) | |
| LTCF | 47 (23) | 13 (7) | |
| Deceased [n (%)] | 11 (5) | 1 (0.5) | |
| ICU admission [n (%)] | 39 (19) | 20 (10) | 0.01 |
| CVC/PICC line [n (%)] | 31 (14) | 7 (4) | <0.0001 |
Comorbidity data and McCabe-Jackson score were missing for 1 patient. OSH, outside hospital; LTCF, long-term-care facility; CVC, central venous catheter; IQR, interquartile range.
There were 31 (15%) patients admitted from an outside hospital and 20 (10%) patients admitted from a long-term care facility among the MRSA carriers and only 10 (5%) and 1 (0.5%) admitted from outside hospitals and a long-term care facility, respectively, among the noncarriers (P < 0.01). Forty-seven (23%) patients were discharged to a long-term care facility among the MRSA carriers and only 13 (7%) among the noncarriers. Eleven (5%) MRSA carriers and 1 (0.5%) noncarrier died during the index admission (Table 1).
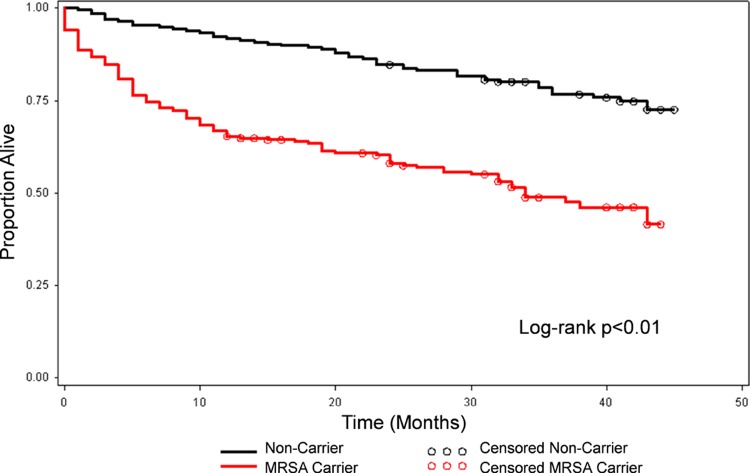
The mean duration of follow-up was 23 months for the MRSA carriers and 34 months in the noncarriers. The shorter mean duration of follow-up was related in large part to the higher crude mortality among carriers (49% versus 23% among the noncarriers). The excess mortality was most prominent during the first year postdischarge (Fig. 1).
Fig 1.
Kaplan-Meier survival plot for MRSA carriers and noncarriers.
There were no culture-proven MRSA infections on readmission among the noncarriers, while 28 (13.6%) MRSA carriers had a total of 35 culture-proven MRSA infections on readmission (P < 0.01). Eleven of these MRSA-positive cultures were from wound cultures, seven from blood cultures, six from sputum cultures, two from bone cultures, and one each from a pleural fluid culture, a cerebrospinal fluid culture, and a joint fluid culture.
Thirteen (6.3%) MRSA carriers had a probable MRSA infection on readmission. Of these, four had osteomyelitis, six had a skin and soft tissue infection, two had pneumonia, and one had osteomyelitis and pneumonia. Fifteen (7.5%) noncarriers were readmitted secondary to a probable MRSA infection, including four who were admitted more than once. Of these, nine had skin and soft tissue infections, three had osteomyelitis, one had meningitis, one had pneumonia, and one had osteomyelitis and pneumonia.
Among the 392 patients who were alive at discharge from the index hospitalization, 45% of MRSA carriers and 23% of noncarriers died during the long-term follow-up. The mortality rate for MRSA carriers was 89 per 389 person years (228 per 1,000 person years), and the mortality rate for noncarriers was 45 per 557 person years (81 per 1,000 person years). In the unadjusted analysis, MRSA carriers had twice the risk of mortality as noncarriers (unadjusted relative risk [RR] = 2.01; 95% confidence interval [CI], 1.49 to 2.71). This significant association remained after statistically adjusting for the McCabe-Jackson score, Charlson comorbidity index, age, and ICU admission on index hospitalization (adjusted hazard ratio [HR] = 2.71; 95% CI, 1.87 to 3.91).
MRSA carriers were significantly more likely to be readmitted, to be readmitted more than once due to proven or probable MRSA infections, and to be readmitted within 90 days of index discharge than noncarriers (Table 2). Among the 392 patients who were alive at discharge from the index hospitalization, MRSA carriers had over four times the risk of infection-related readmission as noncarriers, after statistically adjusting for the Charlson comorbidity index, the McCabe-Jackson score, intensive care unit admission on the index admission, and MRSA infection on the index admission (adjusted HR = 4.07; 95% CI, 2.16 to 7.67).
Table 2.
Infection-related readmission and infection outcomes by MRSA carriage status
| Parameter | Value |
P value | |
|---|---|---|---|
| MRSA carriers (n = 206) | Noncarriers (n = 198) | ||
| Infection-related readmissions [n (% of total)] | |||
| 1 | 28 (13.6) | 11 (5.6) | <0.01 |
| >1 | 13 (6.3) | 4 (2.0) | 0.03 |
| Time to readmission [n (% of readmitted)] | |||
| <90 days | 17 (41.5) | 2 (13.3) | 0.05 |
| 90 days–1 yr | 13 (31.7) | 7 (46.7) | 0.30 |
| 1–2 yr | 5 (12.2) | 4 (26.7) | 0.19 |
| >2 yr | 6 (14.6) | 2 (13.3) | 0.90 |
DISCUSSION
Our study is the first, to our knowledge, to assess the long-term risk of subsequent MRSA infection and mortality and to compare these outcomes with those of a control group of noncarriers. We confirmed that the risks of subsequent infection-related readmission and MRSA infection were elevated among MRSA carriers, with the increased risk concentrated in the first year of follow-up. In addition, the risk of mortality was more than twice as high among MRSA carriers as among noncarriers, even after controlling for patient comorbidities.
Several prior studies have found that MRSA-colonized patients are at higher risk for invasive MRSA infection than noncolonized patients (6, 11, 12). Our findings add to this literature by extending the period of follow-up to over 2 years. Two prior studies assessed long-term outcomes among MRSA carriers but did not include an uninfected control group. One such study, by Huang and Platt, followed 209 patients who had newly acquired MRSA during an acute-care hospitalization and found that 29% developed MRSA infection in the 18 months after discharge, with almost half of the infections becoming apparent after the patients had been discharged from the hospital (13). More recently, another study by Huang et al. reported long-term results from a cohort of 591 newly detected MRSA carriers (14). However, most of those patients (77%) were infected on detection, whereas our population consisted primarily of colonized, uninfected patients at study entry. In that study, almost one-quarter developed postdischarge MRSA infections in the year following detection, most of them more than 90 days postdischarge. Additionally, that study had a large proportion of MRSA carriers who died (46%), which was similar to the proportion of MRSA carriers who died in our study.
It is interesting that none of the noncarrier patients in our cohort had a subsequent culture-proven MRSA infection during the follow-up period. Thus, negative MRSA PCR screening, along with other clinical symptoms, could potentially aid in guiding empirical antibiotic therapy during readmissions. However, further prospective data are needed to confirm these findings.
Our study had several limitations. First, the study is limited by its retrospective observational design. It is also possible that bias may exist due to unmeasured confounding, since we did not include variables such as prior hospitalization or prior antibiotic use in our data set. However, such variables are highly correlated with severity of illness and comorbidities, both of which are included in our statistical analysis. Furthermore, since the MRSA surveillance assay is not 100% sensitive, MRSA carriers may have been misclassified as noncarriers. Readmission due to MRSA infection, our primary outcome, is also subject to misclassification because providers may have been more likely to test MRSA carriers for MRSA infections and because our chart review was not blinded, and thus, we may have inadvertently searched harder for documentation of MRSA infections among MRSA carriers. In addition, all MRSA infections might not be captured using the VA medical records, although most serious infectious should have been captured. Lastly, a predominantly elderly male population limits our ability to extrapolate these findings to the general population.
In conclusion, we demonstrated that among Veterans Affairs patients, MRSA carriers are at high risk of infection-related readmission, MRSA infection, and mortality compared to noncarriers. These associations remained even after statistically adjusting for potential confounders, such as patient comorbidities. In contrast, patients who are not MRSA carriers are at very low risk of subsequent MRSA infection. Future studies should address whether nasal or skin decolonization or other targeted interventions result in improved outcomes for MRSA carriers.
ACKNOWLEDGMENTS
We thank the Iowa City VA Health Care System for its support.
M.L.S. was supported by University of Iowa grant CTSA NIH/NCRR-3KL2 RR024980-04S1. The MRSA screening study was supported by a VA Merit Award to P.L.W.
We have no financial or nonfinancial competing interests.
Authors' contributions: N.M.Q.J. participated in the study design, performed medical chart review, and wrote the manuscript. D.J.D. conceived the study idea, participated in its design and coordination, and helped to draft the manuscript. E.N.P. participated in the study design and helped to draft the manuscript. G.B. created and maintained the databases and helped to draft the manuscript. P.L.W. conceived the study, participated in its design and coordination, and helped to draft the manuscript. M.L.S. participated in the study design and coordination, performed the statistical analysis, and wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 17 December 2012
REFERENCES
- 1. Rubin RJ, Harrington CA, Poon A, Dietrich K, Greene JA, Moiduddin A. 1999. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 5:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall C, Wesselingh S, McDonald M, Spelman D. 2004. Control of endemic MRSA—what is the evidence? A personal view. J. Hosp. Infect. 56:253–268 [DOI] [PubMed] [Google Scholar]
- 3. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities 2008. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 4. Kluytmans JA, Mouton JW, Ijzerman EP, Vandenbroucke-Grauls CM, Maat AW, Wagenvoort JH, Verbrugh HA. 1995. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J. Infect. Dis. 171:216–219 [DOI] [PubMed] [Google Scholar]
- 5. Williams RE. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27:56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 7. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 197:1226–1234 [DOI] [PubMed] [Google Scholar]
- 8. Riedel S, Von Stein D, Richardson K, Page J, Miller S, Winokur P, Diekema D. 2008. Development of a prediction rule for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus carriage in a Veterans Affairs Medical Center population. Infect. Control Hosp. Epidemiol. 29:969–971 [DOI] [PubMed] [Google Scholar]
- 9. Pujol M, Pena C, Pallares R, Ayats J, Ariza J, Gudiol F. 1994. Risk factors for nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 13:96–102 [DOI] [PubMed] [Google Scholar]
- 10. Coello R, Glynn JR, Gaspar C, Picazzo JJ, Fereres J. 1997. Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J. Hosp. Infect. 37:39–46 [DOI] [PubMed] [Google Scholar]
- 11. Garrouste-Orgeas M, Timsit JF, Kallel H, Ben Ali A, Dumay MF, Paoli B, Misset B, Carlet J. 2001. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect. Control Hosp. Epidemiol. 22:687–692 [DOI] [PubMed] [Google Scholar]
- 12. Safdar N, Bradley EA. 2008. The risk of infection after nasal colonization with Staphylococcus aureus. Am. J. Med. 121:310–315 [DOI] [PubMed] [Google Scholar]
- 13. Huang SS, Platt R. 2003. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin. Infect. Dis. 36:281–285 [DOI] [PubMed] [Google Scholar]
- 14. Huang SS, Hinrichsen VL, Datta R, Spurchise L, Miroshnik I, Nelson K, Platt R. 2011. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high risk patients in the year following detection. PLoS One 6:e24340 doi:10.1371/journal.pone.0024340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain R, Kralovic SM, Evans ME, Ambrose M, Simbartl LA, Obrosky DS, Render ML, Freyberg RW, Jernigan JA, Muder RR, Miller LJ, Roselle GA. 2011. Veterans affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N. Engl. J. Med. 364:1419–1430 [DOI] [PubMed] [Google Scholar]
- 16. Deyo RA, Cherkin DC, Ciol A. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45:613–619 [DOI] [PubMed] [Google Scholar]
- 17. McCabe JR, Jackson GG. 1962. Gram-negative bacteremia. I: Etiology and ecology. Arch. Intern. Med. 110:847–855 [Google Scholar]
- 18. McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP, Jr, Miller RR, Furuno JP. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin. Infect. Dis. 45:329–337 [DOI] [PubMed] [Google Scholar]