Abstract
Prothioconazole is a new triazolinthione fungicide used in agriculture. We have used Candida albicans CYP51 (CaCYP51) to investigate the in vitro activity of prothioconazole and to consider the use of such compounds in the medical arena. Treatment of C. albicans cells with prothioconazole, prothioconazole-desthio, and voriconazole resulted in CYP51 inhibition, as evidenced by the accumulation of 14α-methylated sterol substrates (lanosterol and eburicol) and the depletion of ergosterol. We then compared the inhibitor binding properties of prothioconazole, prothioconazole-desthio, and voriconazole with CaCYP51. We observed that prothioconazole-desthio and voriconazole bind noncompetitively to CaCYP51 in the expected manner of azole antifungals (with type II inhibitors binding to heme as the sixth ligand), while prothioconazole binds competitively and does not exhibit classic inhibitor binding spectra. Inhibition of CaCYP51 activity in a cell-free assay demonstrated that prothioconazole-desthio is active, whereas prothioconazole does not inhibit CYP51 activity. Extracts from C. albicans grown in the presence of prothioconazole were found to contain prothioconazole-desthio. We conclude that the antifungal action of prothioconazole can be attributed to prothioconazole-desthio.
INTRODUCTION
Sterol 14α-demethylase inhibitors (DMIs) are a class of antifungal compounds used to treat both agricultural and medical fungal infections (1). They inhibit ergosterol biosynthesis by directly binding to CYP51 (sterol 14α-demethylase), therefore resulting in the depletion of ergosterol and the concomitant increase in 14α-methylated sterols (1). These compounds are classically imidazole or triazole compounds, where the N-3 of imidazole and N-4 of triazole compounds form a sixth ligand with the heme of the CYP51 and inhibit the enzyme activity. This interaction is reflected in a type II binding spectrum formed when the azoles become ligands of low-spin CYP51 (2). The selectivity of DMIs is defined by the interaction of the N-1 substituent groups of the azole and the CYP51 structure (3, 4).
The emergence of new compounds inhibiting CYP51 is desirable to give increased selectivity of action and broader pathogen efficacy and to combat resistance. Prothioconazole is a systemic triazolinthione that we previously showed inhibited CYP51 in Mycosphaerella graminicola (anamorph: Septoria tritici), a plant-pathogenic fungus causing septoria leaf blotch of wheat (5) that has developed widespread resistance to DMI fungicides (6). We showed previously that the mode of action of prothioconazole is through inhibition of CYP51 (5), but the antifungal effect of prothioconazole seen in vivo was unlikely to be attributable to the binding of prothioconazole to CYP51.
We were interested in the potential of this compound type in treating medically relevant fungi and especially to see if the prothioconazole-desthio (Fig. 1), which is known to be a major product of metabolism of prothioconazole in both plants and animals (7), was responsible for antifungal activity. Prothioconazole-desthio is important in the toxicology of prothioconazole (8) since it is the major breakdown metabolite and is itself a triazole compound. In this study, we utilized the established C. albicans enzyme systems previously used for in vitro studies (9) to elucidate the effects observed in whole-cell treatments of C. albicans. We compared the inhibitory properties of voriconazole, used to treat both Aspergillus and Candida infections (10), to those of prothioconazole and prothioconazole-desthio using C. albicans CYP51 (CaCYP51) as a model system. This provided a comparison of the effects of prothioconazole and prothioconazole-desthio and revealed that triazolinthiones should be considered for use in candidiasis.
Fig 1.
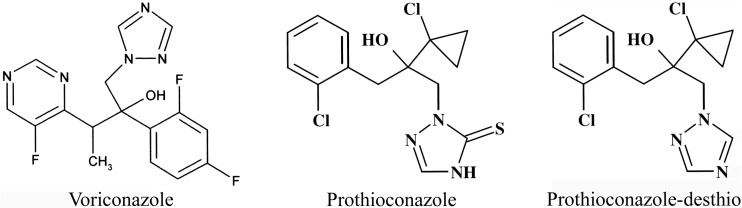
Chemical structures of antifungals. The chemical structures of voriconazole (molecular weight [MW], 349), prothioconazole (MW, 344), and prothioconazole-desthio (MW, 312) are shown.
MATERIALS AND METHODS
Abbreviations.
5-ALA, 5-aminolevulinic acid; BSTFA, N,O-bis(trimethylsilyl)trifluoroacetamide; DMF, dimethyl formamide; DMSO, dimethyl sulfoxide; ESI-MS/MS, electrospray ionization-tandem mass spectrometry; GC, gas chromatography; IC50, 50% inhibitory concentration; IPTG, isopropyl-β-d-thiogalactopyranoside; MCP, microchannel plate detector; NTA, nitrilotriacetic acid; TMCS, trimethylchlorosilane; TMS, trimethylsilyl.
Chemicals, media, and strains.
All chemicals, unless otherwise stated, were obtained from Sigma Chemical Company (Poole, United Kingdom). Voriconazole was supplied by Discovery Fine Chemicals (Bournemouth, United Kingdom). Growth media, sodium ampicillin, IPTG, and 5-aminolevulenic acid were obtained from Foremedium Ltd. (Hunstanton, United Kingdom). A Ni2+-NTA agarose affinity chromatography matrix was obtained from Qiagen (Crawley, United Kingdom). Candida albicans ATCC SC5314 was obtained from the American Type Culture Collection.
Heterologous expression of CaCYP51.
Heterologous expression (performed in Escherichia coli DH5α supplemented with the heme precursor 5-ALA), protein isolation and purification, and spectral characterization of CaCYP51 (UniProt P10613) were performed as previously described (9). Purified CaCYP51 was dialyzed against 50 mM Tris-HCl (pH 8.1) and 10% (wt/vol) glycerol prior to use in CYP51 reconstitution assays and ligand binding studies.
Antifungal binding determinations.
The chemical structures of the azoles used in this study are shown in Fig. 1. Binding of azole to CaCYP51 was performed as previously described (5). A stock 2 mg · ml−1 solution of prothioconazole, a 0.2 mg · ml−1 solution of prothioconazole-desthio, and a 0.1 mg · ml−1 solution of voriconazole were prepared in DMF. Azoles were progressively titrated against 5 μM CaCYP51 in 0.1 M Tris-HCl (pH 8.1) and 25% (wt/vol) glycerol, with the difference spectra between 500 and 350 nm determined after each addition. Azole binding determinations were performed in triplicate for each compound. Binding saturation curves were constructed from ΔApeak-trough against the azole concentration. A rearrangement of the Morrison equation (11, 12) was used to determine the dissociation constant (Kd) values when ligand binding was “tight.” Tight binding is observed when the Kd for azole is similar to or lower than the concentration of CYP51 present (13). The Michaelis-Menten equation was used when the ligand binding was not tight. The Kd values reported are the mean values from three replicates along with the associated standard deviations.
Substrate binding studies.
A 0.1% (wt/vol) aqueous solution of lanosterol in 0.5% (vol/vol) Tween 80 was prepared as previously described (9). Lanosterol was progressively titrated against 10 μM CaCYP51 in the sample cuvette with equivalent amounts of 0.5% (vol/vol) Tween 80 added to the P450-containing reference cuvette. The absorbance difference spectrum between 500 and 350 nm was determined after each incremental addition of lanosterol, and binding saturation curves were constructed from the ΔA385–419, including corrections for changes in sample volume. The substrate binding constant (Ks) was determined by nonlinear regression (Levenberg-Marquardt algorithm) using the Michaelis-Menten equation. Ks values reported for lanosterol are the mean values from three replicates along with the associated standard deviations. The spin-state change of CaCYP51 was calculated from the ΔA385–419 value using an extinction coefficient of 118 mM−1 · cm−1 derived for the type I difference spectrum of CYP164A2 which was modulated from 100% low spin to nearly 100% high spin by physicochemical means (14).
Lanosterol binding difference spectra were determined with 10 μM CaCYP51 in the presence and absence of 4 μM voriconazole, 100 μM prothioconaozle, and 4 μM prothioconazole-desthio. Negative-control determinations were made in the presence of 1.25% (vol/vol) DMF. Determinations were performed in triplicate, and Lineweaver-Burk plots were constructed from resultant CaCYP51 substrate binding spectra.
CYP51 reconstitution assays and IC50 determinations.
The CYP51 enzyme reconstitution system previously described (9) containing 2.5 μM CaCYP51 and 10 μM truncated yeast cytochrome P450 reductase (15) was used. The reaction was terminated by the addition of 2 ml 15% (wt/vol) KOH in ethanol followed by incubation at 85°C for 90 min. IC50 determinations were performed by adding various concentrations of voriconazole, prothioconazole, and prothioconazole-desthio in 5 μl of DMF prior to incubation at 37°C and the addition of β-NADPH-Na4. Sterol substrates and products were extracted and analyzed by GC/MS as described below.
Antifungal treatment of cells.
C. albicans cells were grown overnight in RPMI 1640 l-glutamine (Gibco, Life Technologies, Paisley, United Kingdom). A starting concentration of 1 × 103 cells/ml was used to inoculate RPMI medium containing 4 μg · ml−1 prothioconazole, 4 μg · ml−1 prothioconazole-desthio, or 1 μg · ml−1 voriconazole (all with a final concentration of 1% DMSO) and a control containing 1% DMSO (untreated). Cultures were incubated at 37°C and 200 rpm overnight and cells harvested and washed twice with deionized water prior to sterol extraction.
Sterol extraction and analysis.
Nonsaponifiable lipids were extracted as reported previously (16). Samples were dried in a vacuum centrifuge and were derivatized by the addition of 100 μl 99:1 BSTFA:TMCS and 100 μl anhydrous pyridine and heating for 2 h at 80°C. TMS-derivatized sterols were analyzed and identified using GC/MS (Agilent 5975C Inert XL GC/MSD) with reference to retention times and fragmentation spectra for known standards. GC/MS data files were analyzed using Agilent software (MSD Enhanced ChemStation; Agilent Technologies Ltd., Stockport, United Kingdom) to determine sterol profiles for all isolates and for integrated peak areas.
Solid-phase extraction of antifungals from Candida albicans cells and growth media.
A 100-μl aliquot of 1 × 107 cells/ml from overnight cultures of C. albicans (ATCC SC5314) was subcultured into 250 ml RPMI l-glutamine and yeast extract-peptone-dextrose (YPD) broth containing 8 μg · ml−1 prothioconazole with a final concentration of 1% (vol/vol) DMSO (and controls without any prothioconazole) and incubated at 37°C and 200 rpm for 24 h. Cells were harvested and washed three times with deionized water before sonication (60-s bursts and 60-s rests for 10 min) in 99:1 methanol:acetic acid. Samples were then dried in a vacuum centrifuge and resuspended in 20% (vol/vol) methanol. Sep-Pak cartridges (tC18; Waters, Elstree, United Kingdom) (Vac 3 cc, 200 mg) were prepared by washing with 6 ml of methanol and subsequently 6 ml of 20% (vol/vol) methanol. Extracts were each applied to a cartridge which was then washed with 4 sequential 1-ml volumes of 40%, 60%, 80%, and 100% (vol/vol) methanol. The eluent was collected for each wash.
Extracts from the media used for the growth of C. albicans with prothioconazole, control media containing prothioconazole and no cells, and sterile deionized water with prothioconazole and no cells were prepared in a manner similar to that described for the cell extractions. Methanol was added to samples to reach a final concentration of 20% (vol/vol) (standards were diluted in 20% [vol/vol] methanol) prior to solid-phase extraction.
Identification of prothioconazole and prothioconazole-desthio.
Samples were analyzed by ESI-MS and -MS/MS on a Q-Tof Ultima spectrometer (Micromas, Manchester, United Kingdom) in positive-ion mode. Samples were electrosprayed from nano-ESI metal-coated capillaries, and collision-induced dissociation was achieved with argon gas. The following instrument parameters were used: capillary voltage, 1.8 kV; collision voltage, 8 V for MS (25 V for MS/MS); m/z range, 50 to 1,000; scan time, 2.4 s/scan; MCP detector voltage, 2,100 V. The instrument was calibrated immediately prior to use with 1 pmol/μl Glu-1-Fibrinopeptide B. The data obtained were processed using the MassLynx V4.1 software package (Micromass, Manchester, United Kingdom).
Prothioconazole and prothioconazole-desthio were identified by their measured mass and by their fragmentation patterns obtained through MS/MS. Both prothioconazole and prothioconazole-desthio standards were found to be most abundant in the 80% (vol/vol) methanol eluent; therefore, analysis of extractions was performed by comparing the 80% (vol/vol) methanol eluents of all samples.
Data analysis.
Curve fitting of numerical data was performed using the computer program ProFit 6.1.12 (QuantumSoft, Zurich, Switzerland).
RESULTS
Prothioconazole inhibits CYP51 in vivo.
Prothioconazole and prothioconazole-desthio inhibited growth of C. albicans. In order to confirm the mechanism of action, the sterol contents of treated cells were investigated. Cells treated with prothioconazole, prothioconazole-desthio, or voriconazole all exhibited similar sterol profiles with an accumulation of 14α-methylated sterols and a depletion of ergosterol (see Table 1). All three compounds therefore inhibit CYP51 (14α-demethylase) in vivo.
Table 1.
Total sterol content of C. albicansa
| Sterol | % (± SD) sterol for C. albicans SC5314 with indicated treatment |
|||
|---|---|---|---|---|
| Untreated | Prothioconazole | Prothioconazole-desthio | Voriconazole | |
| 14-Methylated sterols | ||||
| 14-Methyl fecosterol | ND | 7.3 ± 0.42 | 6.9 ± 0.32 | 10.5 ± 0.31 |
| 14-Methyl ergosta-8,24(28)-dien-3,6-diol | ND | 25.5 ± 0.93 | 49.6 ± 1.22 | 31.3 ± 2.40 |
| Lanosterol | 10.47 ± 0.72 | 36.2 ± 0.89 | 24.7 ± 1.39 | 32.6 ± 1.86 |
| Eburicol | ND | 18.1 ± 0.35 | 10.0 ± 0.87 | 14.0 ± 0.72 |
| 4,14-Dimethyl zymosterol | ND | 5.1 ± 0.38 | 3.6 ± 0.67 | 6.10 ± 0.67 |
| 14-Demethylated sterols | ||||
| Ergosterol | 74.4 ± 1.14 | 5.0 ± 0.15 | 1.1 ± 0.26 | 2.3 ± 0.67 |
| Ergosta-5,7,22,24(28)-tetraenol | 3.0 ± 0.50 | ND | ND | ND |
| Fecosterol | 5.9 ± 0.15 | ND | ND | ND |
| Ergosta-5,7-dienol | 2.2 ± 0.40 | ND | ND | ND |
| 4,4-Dimethyl zymosterol | 2.5 ± 0.55 | ND | ND | ND |
| Zymosterol | 1.0 ± 0.40 | ND | ND | ND |
The percentages of total sterols for C. albicans SC5314 untreated or in the presence of 4 μg · ml−1 prothioconazole, 4 μg · ml−1 prothioconazole-desthio, or 1 μg · ml−1 voriconazole are shown. Sterols comprising <1% total sterols were omitted. ND, not detected.
Prothioconazole-desthio binds as a type II inhibitor of CaCYP51.
Inhibitor binding studies were used to investigate the in vitro binding interaction of prothioconazole, prothioconazole-desthio, and voriconazole. Spectra induced on binding of antifungals to CaCYP51 give insight into whether they form heme ligands. Azole inhibition of CYP51 occurs via direct interaction of the lone pair of electrons of an available nitrogen with the heme of the enzyme. Interactions of this type elicit a type II inhibitor binding spectrum (with a peak at 423 to 429 nm and trough at 406 to 409 nm) (17).
Voriconazole and prothioconazole-desthio bound tightly to CaCYP51, producing strong type II difference spectra indicative of an azole-bound low-spin CYP51 complex (Fig. 2A and C). The Morrison equation (11) was used to fit the curve (Fig. 2D), yielding apparent Kd values of 6.8 ± 2.2 nM and 36.4 ± 10.7 nM, respectively. This is indicative of direct coordination of the triazole N-4 as the sixth ligand of the heme ferric ion (17).
Fig 2.
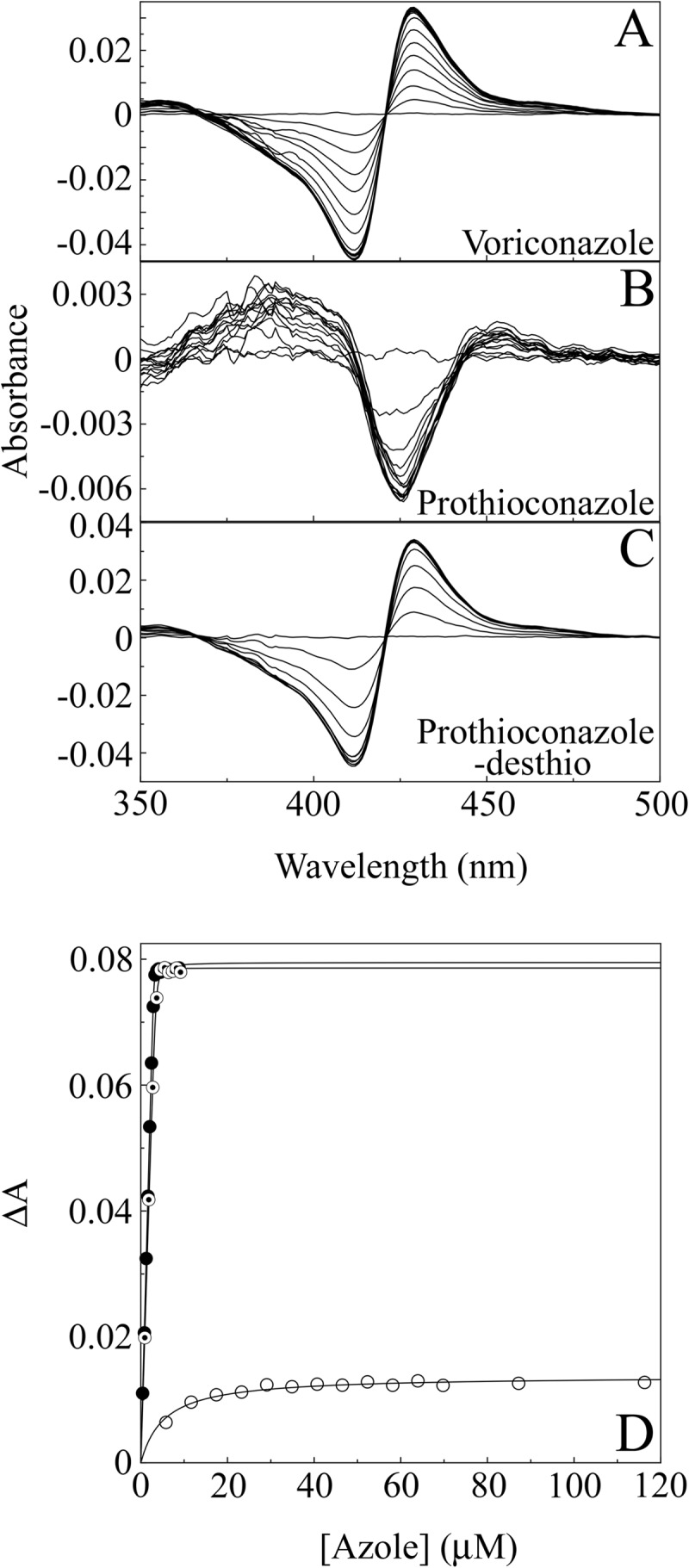
Azole binding difference spectra of CaCYP51. (A to C) Absorbance difference spectra were measured during the progressive titration of 5 μM CaCYP51 with voriconazole (A), prothioconazole (B), and prothioconazole-desthio (C). (D) Azole binding saturation curves were constructed from the change in absorbance (ΔApeak-trough) against azole concentration for voriconazole (solid circles), prothioconazole (hollow circles), and prothioconazole-desthio (bullets). One representative example of each experiment is shown, although all experiments were performed in triplicate.
Prothioconazole, in contrast, bound weakly to CaCYP51 (Fig. 2B), and the best fit was calculated using the Michaelis-Menten equation, yielding an apparent Kd of 6.3 ± 1.5 μM. Binding of prothioconazole produced a weak type I difference spectrum of the type more usually associated with substrate binding and indicative of the displacement of a water molecule as the sixth ligand of the heme.
Prothioconazole-desthio binds to CaCYP51 as a noncompetitive inhibitor.
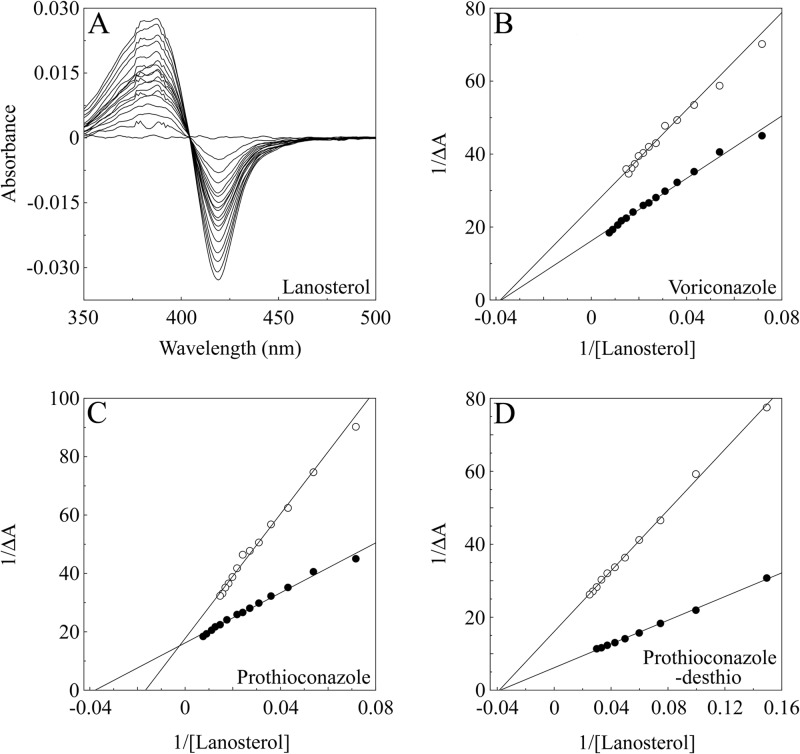
The inhibition of substrate (lanosterol) binding to CaCYP51 by prothioconazole, prothioconazole-desthio, and voriconazole was investigated to determine the mode of inhibition of CaCYP51. Progressive titration of CaCYP51 with lanosterol gave type I difference spectra (Fig. 3A) as expected. CaCYP51 had an apparent Ks value for lanosterol of 26.7 ± 6.1 μM in the absence of azole antifungals, with an 11.3% change in spin state from low to high spin being obtained in the presence of 90 μM lanosterol.
Fig 3.
Azole inhibition of lanosterol binding to CaCYP51. Absorbance difference spectra were measured during the progressive titration of 10 μM CaCYP51 with lanosterol in the absence and presence of azole antifungals. (A) An example of the type I difference spectrum obtained for lanosterol titration against 10 μM CaCYP51 in the absence of azole is shown. (B to D) Lineweaver-Burk plots were constructed from the type I binding spectra in order to compare lanosterol binding in the absence (filled circles) and presence (hollow circles) of 4 μM voriconazole (B), 100 μM prothioconazole (C) and 4 μM prothioconazole-desthio (D). One representative example of each experiment is shown, although all experiments were performed in triplicate.
The addition of 4 μM voriconazole or 4 μM prothioconazole-desthio caused a reduction in the intensity (ΔAmax) of the lanosterol binding spectra, although the apparent Ks values for lanosterol remained effectively constant at 20.9 ± 2.5 μM and 22.3 ± 5.3 μM. In contrast, the addition of 100 μM prothioconazole caused the apparent Ks value for lanosterol to increase 2.5-fold to 62.2 ± 2.8 μM along with a reduction in the spectral intensity. The Lineweaver-Burk plot of lanosterol binding in the presence and absence of 4 μM voriconazole (Fig. 3B) and the presence and absence of 4 μM prothioconazole-desthio (Fig. 3D) converged at a common Ks value with a reduction in the ΔAmax value which is indicative of noncompetitive inhibition of ligand binding (13). However, the Lineweaver-Burk plots of lanosterol binding in the presence and absence of 100 μM prothioconazole (Fig. 3C) converged at a common ΔAmax value and showed an apparent 2.5-fold increase in the Ks value for lanosterol, indicating competitive inhibition of lanosterol binding (13) and suggesting binding to the substrate recognition site(s) of CaCYP51.
Prothioconazole-desthio, unlike prothioconazole, is a potent inhibitor of CYP51 in vitro.
An in vitro, cell-free enzyme assay was used to investigate the inhibition of CaCYP51 by prothioconazole, prothioconazole-desthio, and voriconazole. As expected, voriconazole and prothioconazole-desthio strongly inhibited CaCYP51 activity, with IC50s approximately half the CaCYP51 concentration at 1.6 and 1.9 μM (Fig. 4). In contrast, prothioconazole weakly inhibited CaCYP51 activity, with an apparent IC50 of ∼120 μM and only 45% inhibition of CaCYP51 activity being observed with 100 μM prothioconazole. These results therefore confirmed that prothioconazole is a weak inhibitor of CaCYP51 activity, whereas both the desthio analog and voriconazole are strong inhibitors.
Fig 4.
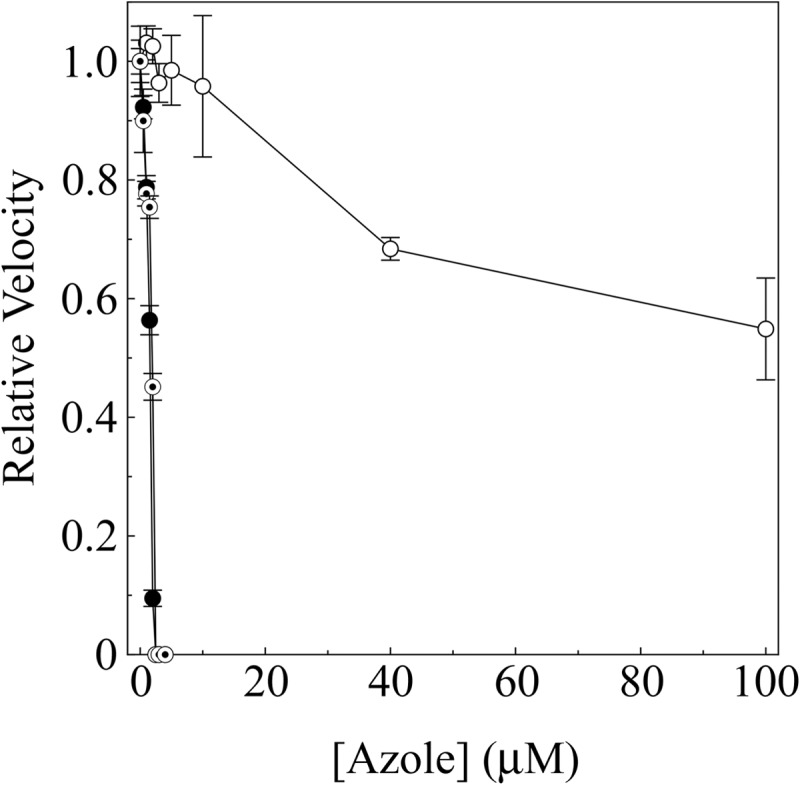
IC50 determinations for azole antifungals. CYP51 reconstitution assays contained 2.5 μM CaCYP51. Voriconazole (filled circles) and prothioconazole-desthio (bullets) concentrations ranged from 0 to 4 μM, and prothioconazole (hollow circles) concentrations ranged from 0 to 100 μM, with the DMF concentration kept constant at 0.5% (vol/vol). Mean values from three replicates are shown along with associated standard error bars. Relative velocities of 1.0 equate to actual velocities of 0.080, 0.098, and 0.087 nmol min−1 for the IC50 determinations with voriconazole, prothioconazole, and prothioconazole-desthio, respectively.
Prothioconazole-desthio is present in cells treated with prothioconazole.
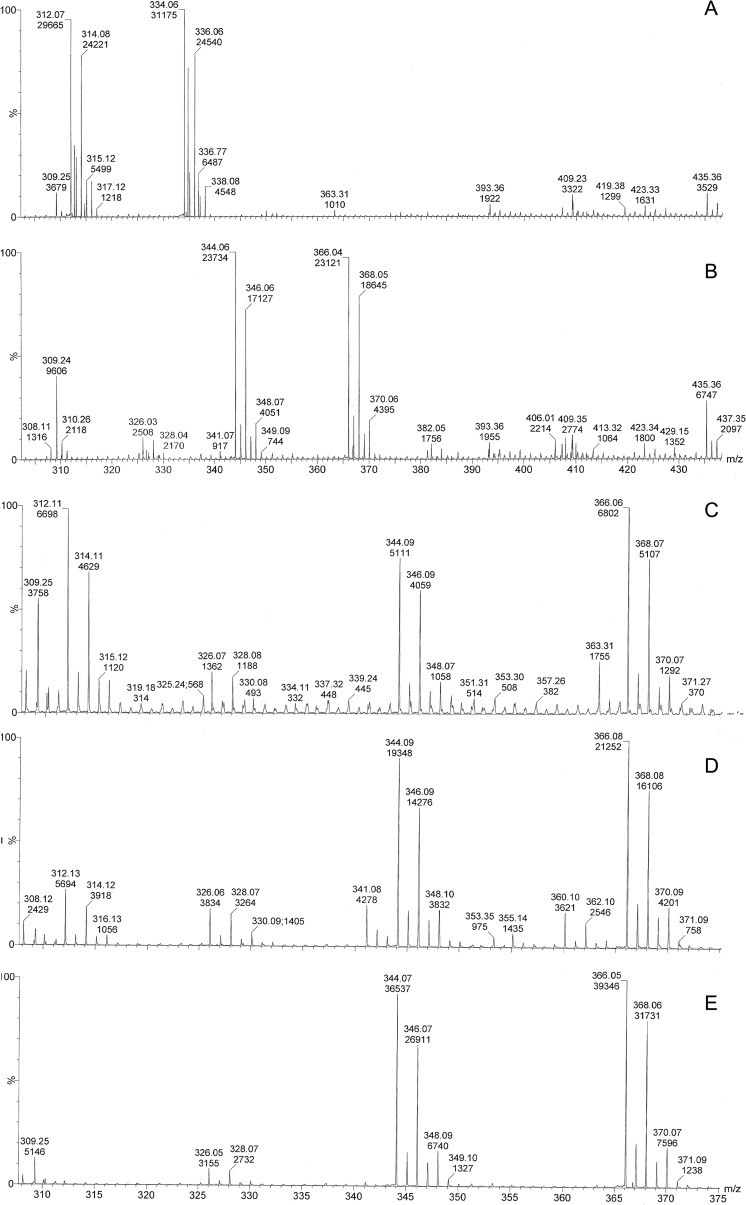
The presence of prothioconazole and prothioconazole-desthio in media and cell extracts (after solid-phase extraction) was determined by ESI-MS and ESI-MS/MS in order to deduce whether the active compound (prothioconazole-desthio) was present after treatment with prothioconazole. Standards of prothioconazole and prothioconazole-desthio showed [M+H]+ ions of the expected m/z (344 and 312, respectively), as well as [M+Na]+ ions at m/z 366 and 334 (Fig. 5A and B). Neither prothioconazole nor prothioconazole-desthio was identified in cell and medium negative controls. Prothioconazole and prothioconazole-desthio were both identified in cell extracts and media from cultures of C. albicans grown in either YPD containing prothioconazole or RPMI medium containing prothioconazole (Fig. 5C). In addition, prothioconazole and prothioconazole-desthio were isolated from control media containing no cells (YPD containing prothioconazole and RPMI medium containing prothioconaozle) incubated at 37°C for 24 h (Fig. 5D). Control samples of sterile deionized water containing prothioconazole (incubated at 37°C for 24 h) contained only prothioconazole, and no prothioconazole-desthio was present (Fig. 5E).
Fig 5.
Q-Tof analysis of cell extracts of antifungal-treated Candida albicans. Solid-phase extraction of prothioconazole and prothioconazole-desthio standards contained peaks corresponding to [M+H]+ ions of m/z 344 and 312, respectively; [M+Na]+ ions were also observed at 366 and 334 (A and B). Cell extracts of C. albicans treated with prothioconazole contained both prothioconazole and prothioconazole-desthio (an example from cells grown in RPMI medium for 24 h at 37°C and 200 rpm is shown in panel C). RPMI and YPD media containing prothioconazole incubated at 37°C for 24 h contained both prothioconazole and prothioconazole-desthio (an example from RPMI medium is shown in panel D), and sterile deionized water containing prothioconazole incubated at 37°C for 24 h contained only prothioconazole (E). Each spectrum represents the sum of 10 scans.
DISCUSSION
We have previously reported that prothioconazole inhibition of the CYP51 of Mycosphaerella graminicola (5) is the mode of action of this new triazolinthione antifungal. We were interested in assessing the potential of triazolinthiones for therapeutic use as a new source of antimycotic agents. In mammals and plants, prothioconazole is metabolized primarily to the desthio analog (7, 8, 18), so we investigated whether the antifungal effects observed were indeed due to the presence of prothioconazole-desthio rather than to direct interaction of prothioconazole with CYP51. The use of a reconstituted lanosterol 14α-demethylase system with CaCYP51 enabled us to further investigate the mode of action of this antifungal compound.
Prothioconazole has efficacy comparable to that of prothioconazole-desthio when C. albicans cultures are treated. Voriconazole, prothioconazole, and prothioconazole-desthio treatments of C. albicans have the same effect on the sterol composition of cells, indicating that, as with M. graminicola (5), treatment of C. albicans with prothioconazole inhibits sterol biosynthesis by inhibiting CYP51.
However, CaCYP51 does not bind prothioconazole as a type II inhibitor typical of azole antifungals but induces a weak type I binding spectrum with a maximum at ∼425 nm and a minimum at 440 nm (Fig. 2B). Type I difference spectra are often associated with, but not exclusively caused by, substrate or substrate analog binding (17). Type I difference spectra are indicative of a change in the CYP spin state from low spin (hexa coordinated) to high spin (penta coordinated) and can also be induced by physicochemical means such as cations, pH, and the dielectric constant of the solvent altering the CYP spin-state equilibrium (14). The associated change in spin state from low spin to high spin confirms that prothioconazole does not coordinate directly with the heme ferric ion as expected for azole antifungal agents.
The observation that prothioconazole-desthio bound tightly to CaCYP51 while prothioconazole bound weakly indicates that a triazole N-4 nitrogen atom, which is sterically unhindered, is required for strong coordination as the sixth ligand to the CaCYP51 heme ferric ion in combination with interactions of the N-1 substituent group with the protein.
The binding of lanosterol to CaCYP51 was shown to be inhibited noncompetitively by both voriconazole and prothioconazole-desthio, as is expected for the inhibition of CYP51 substrate binding by a tight-binding azole antifungal compound. However, the Lineweaver-Burk plot (Fig. 3C) indicated that prothioconazole competitively inhibited CaCYP51, suggesting binding to the substrate recognition site(s) of CaCYP51 (19). This observation is similar to that obtained previously with M. graminicola CYP51 (5). It can be concluded that the “bulky” sulfur atom covalently attached adjacent to the triazole nitrogen atom (Fig, 1) prevented direct coordination to the heme ferric ion.
In this study, we were also able to examine the effect of prothioconazole inhibition on CYP51 activity and compare this with the data from spectrophotometric studies. IC50 determinations (Fig. 4) revealed differences between prothioconazole and voriconazole/prothiconazole-desthio potencies against CaCYP51. Voriconazole and prothioconazole-desthio exhibited low IC50s in this cell-free enzyme assay, inhibiting activity at concentrations similar to the CaCYP51 concentration. This was as expected for tight-binding CYP51 inhibitors, and the voriconazole IC50 result agrees with previous in vitro CYP51 activity studies using purified CaCYP51 and microsomal protein fractions (9, 20). The IC50 of prothioconazole is around 100 times higher than those of voriconazole and prothioconazole-desthio at >100 μM.
The poor in vitro CaCYP51 inhibition properties of prothioconazole relative to prothioconazole-desthio stimulated further investigation of the mode of action. Prothioconazole-desthio was found to be present in both the cells and the media of C. albicans cultures treated with prothioconazole. Therefore, the antifungal effect seen during treatment with prothioconazole is due to the presence of the desthio product. Our results also showed that the desthio analog was present in both YPD media containing prothioconazole and RPMI media containing prothioconazole, but not in sterile deionized water containing prothioconazole after incubation at 37°C for 24 h. Therefore, prothioconazole is readily converted to the desthio form and accounts for the antifungal effect. It is conceivable that the high potency of prothioconazole as an agricultural fungicide is also enhanced due to intracellular metabolism of the relatively inactive prothioconazole in the pathogenic fungi to the highly active desthio form and in the host. Additional work on triazolinthiones may further the development of new antifungal compounds of this type for therapeutic use in the clinic, or as more effective compounds in crop protection, and stimulate consideration of the value of a profungicide or prodrug approach.
ACKNOWLEDGMENTS
We are grateful to the European Union for support by the FP6 EURESFUN project and to the Biotechnology and Biological Science Research Council (project numbers BBE02257X1 and BBE0218321) of the United Kingdom for supporting this work.
We are also grateful to the Engineering and Physical Sciences Research Council National Mass Spectrometry Service Centre at Swansea University for assistance in sterol analysis.
Footnotes
Published ahead of print 28 December 2012
REFERENCES
- 1. Kelly SL, Lamb DC, Jackson CJ, Warrilow AG, Kelly DE. 2003. The biodiversity of microbial cytochromes P450. Adv. Microb. Physiol. 47:131–186 [DOI] [PubMed] [Google Scholar]
- 2. Jefcoate CR, Gaylor JL, Calabrese RL. 1969. Ligand interactions with cytochrome P450. I. Binding of primary amines. Biochemistry 8:3455–3463 [DOI] [PubMed] [Google Scholar]
- 3. Lepesheva GI, Park HW, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, Sundaramoorthy M, Nes WD, Pays E, Chaudhuri M, Villalta F, Waterman MR. 2010. Crystal structures of Trypanosoma brucei sterol 14α-demethylase and implications for selective treatment of human infections. J. Biol. Chem. 285:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strushkevich N, Usanov SA, Park HW. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J. Mol. Biol. 397:1067–1078 [DOI] [PubMed] [Google Scholar]
- 5. Parker JE, Warrilow AG, Cools HJ, Martel CM, Nes WD, Fraaije BA, Lucas JA, Kelly DE, Kelly SL. 2011. Mechanism of binding of prothioconazole to Mycosphaerella graminicola CYP51 differs from that of other azole antifungals. Appl. Environ. Microbiol. 77:1460–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cools HJ, Fraaije BA. 26 April 2012. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag. Sci. [Epub ahead of print.] doi:10.1002/ps.3348 [DOI] [PubMed] [Google Scholar]
- 7.Australian Pesticides and Veterinary Medicines Authority 2007. Evaluation of the new active prothioconazole in the product redigo fungicidal seed treatment. ISSN 1443-1335 Australian Pesticides and Veterinary Medicines Authority, Symonston, Australian Capital Territory, Australia [Google Scholar]
- 8.United States Environmental Protection Agency 2007. Pesticide fact sheet: prothioconazole (7501 P). Office of Prevention, Pesticides and Toxic Substances, United States Environmental Protection Agency, Washington, DC [Google Scholar]
- 9. Warrilow AG, Martel CM, Parker JE, Melo N, Lamb DC, Nes WD, Kelly DE, Kelly SL. 2010. Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51). Antimicrob. Agents Chemother. 54:4235–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson LB, Kauffman CA. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630–637 [DOI] [PubMed] [Google Scholar]
- 11. Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JE, Nelson WL, Isoherranen N. 2009. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem. Pharmacol. 77:258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morrison JF. 1969. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim. Biophys. Acta 185:269–286 [DOI] [PubMed] [Google Scholar]
- 13. Copeland RA. 2005. Evaluation of enzyme inhibitors in drug discovery: a guide for medicinal chemists an pharmacologists, p 178–213 Wiley-Interscience, New York, NY: [PubMed] [Google Scholar]
- 14. Warrilow AG, Jackson CJ, Parker JE, Marczylo TH, Kelly DE, Lamb DC, Kelly SL. 2009. Identification, characterization, and azole-binding properties of Mycobacterium smegmatis CYP164A2, a homolog of ML2088, the sole cytochrome P450 gene of Mycobacterium leprae. Antimicrob. Agents Chemother. 53:1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venkateswarlu K, Lamb DC, Kelly DE, Manning NJ, Kelly SL. 1998. The N-terminal membrane domain of yeast NADPH-cytochrome P450 (CYP) oxidoreductase is not required for catalytic activity in sterol biosynthesis or in reconstitution of CYP activity. J. Biol. Chem. 273:4492–4496 [DOI] [PubMed] [Google Scholar]
- 16. Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14 alpha-methylergosta-8,24(28)-dien-3 β,6α-diol. Biochem. Biophys. Res. Commun. 207:910–915 [DOI] [PubMed] [Google Scholar]
- 17. Jefcoate CR. 1978. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol. 52:258–279 [DOI] [PubMed] [Google Scholar]
- 18.Food and Agriculture Organization (FAO) of the United Nations/World Health Organization (WHO) 2008. Pesticide residues in food 2008: joint FAO/WHO meeting on pesticide residues, New York, NY, p 265–292 FAO plant production and protection paper 193. ISSN 0259-2517 Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 19. Podust LM, Stojan J, Poulos TL, Waterman MR. 2001. Substrate recognition sites in 14α-sterol demethylase from comparative analysis of amino acid sequences and X-ray structure of Mycobacterium tuberculosis CYP51. J. Inorg. Biochem. 87:227–235 [DOI] [PubMed] [Google Scholar]
- 20. Lamb DC, Kelly DE, Schunck WH, Shyadehi AZ, Akhtar M, Lowe DJ, Baldwin BC, Kelly SL. 1997. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J. Biol. Chem. 272:5682–5688 [DOI] [PubMed] [Google Scholar]