Abstract
Urban, natural, and pasture areas were investigated for prevalences and 16S rRNA gene variants of Anaplasma phagocytophilum in questing Ixodes ricinus ticks. The prevalences differed significantly between habitat types, and year-to-year variations in prevalence and habitat-dependent occurrence of 16S rRNA gene variants were detected.
TEXT
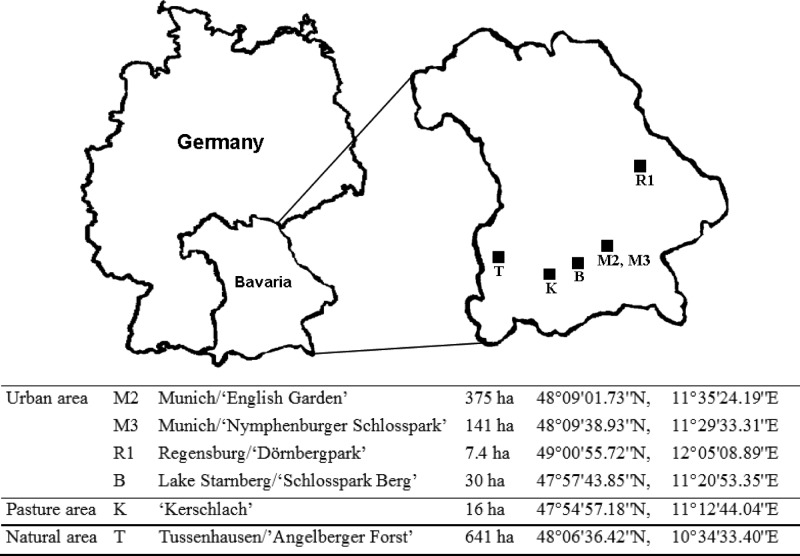
The obligate intracellular bacterium Anaplasma phagocytophilum is transmitted by Ixodes ricinus ticks in Europe (1) and causes granulocytic anaplasmosis in humans and several other mammalian species (2–5). Reservoir hosts are necessary to maintain the endemic cycle of A. phagocytophilum (transstadial but not transovarial transmission [6]). Different host species seem to be susceptible to different genetic variants of this pathogen, with potentially differing pathogenicity (1, 7). The aim of this study was to compare the prevalences and the variability in 16S rRNA gene variants of A. phagocytophilum in I. ricinus ticks, as well as the densities of questing I. ricinus ticks, collected in 2011 and 2012 from six study sites in Bavaria, Germany (structured in three different habitats—urban, pasture, and natural), with regard to the occurrence of various potential reservoir hosts (Fig. 1; further description of study sites will be published elsewhere [E. Overzier, K. Pfister, C. Thiel, I. Herb, M. Mahling, and C. Silaghi, submitted for publication]).
Fig 1.
Locations of sampling sites.
In April, May, and June of 2011 and 2012, questing ticks were collected with the flagging method on 300 m2 transects on each study site. DNA was extracted automatically with the Maxwell 16 system (Promega, Mannheim, Germany). The DNA concentration was measured spectrophotometrically (NanoDrop ND-1000; PeqLab, Erlangen, Germany). The msp2 gene of A. phagocytophilum was detected by SYBR Green real-time PCR in the AB 7500 real-time PCR system (Applied Biosystems, Darmstadt, Germany) as described previously (8). An assortment of positive samples was investigated with a nested PCR targeting the 16S rRNA gene to detect different gene variants (9, 10). Positive PCR products were purified, sequenced, and analyzed as described previously (8). All sequences were compared with sequences from previous studies by our group (4, 8, 10–13).
Exact 95% confidence intervals (95% CI) of prevalences in ticks were computed with the Clopper and Pearson method (14). Two logistic regression models were estimated to investigate the effects of gender, month, and year on the probability of ticks being positive for A. phagocytophilum. One model also included habitat, and another one the different sites. A simultaneous test for general linear hypotheses based on multiple comparisons of means with Tukey contrasts (15) was used to test for differences between gender, month, habitat, and site, respectively. Statistical analysis was performed with R, version 2.15.1 (16).
Overall 9,672 I. ricinus ticks were collected, with average tick densities in the urban area (ticks/100 m2) ranging from 21 to 97 ticks per 100 m2 in 2011 and 15 to 51 ticks per 100 m2 in 2012. On the pasture, the tick densities were 50 and 38 ticks per 100 m2 in 2011 and 2012, and in the natural area, the densities were 73 and 34 ticks per 100 m2 (Table 1). The mean values of the ratio of adults to nymphs were 1:2.3 (2011) and 1:0.9 (2012) (Table 1).
Table 1.
Absolute and average tick density (Ixodes ricinus: adults and nymphs/100 m2) per site in April, May, and June and relationship of stages in 2011 and 2012
| Habitat | Site | Tick density/100 m2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| April |
May |
June |
Mean |
2011 |
2012 |
||||||||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | Adults | Nymphs | Ratio | Adults | Nymphs | Ratio | ||
| Urban | English Garden (M2) | 17 | 6 | 89 | 35 | 69 | 28 | 58 | 19 | 25 | 33 | 1:1.3 | 17 | 6 | 1:0.4 |
| Nymphenburger Schlosspark (M3) | 22 | 14 | 31 | 21 | 10 | 10 | 21 | 15 | 7 | 14 | 1:2.0 | 8 | 7 | 1:0.9 | |
| Dörnbergpark (R1) | 93 | 15 | 91 | 114 | 108 | 23 | 97 | 51 | 56 | 41 | 1:0.7 | 33 | 18 | 1:0.5 | |
| Berg (B) | 31 | 13 | 105 | 21 | 134 | 20 | 90 | 18 | 10 | 80 | 1:8.0 | 14 | 4 | 1:0.3 | |
| Mean | 41 | 12 | 79 | 48 | 80 | 20 | 67 | 26 | 25 | 42 | 1:1.7 | 18 | 9 | 1:0.5 | |
| Pasture | Kerschlach (K) | 68 | 26 | 70 | 53 | 12 | 33 | 50 | 38 | 14 | 36 | 1:2.6 | 16 | 22 | 1:1.4 |
| Natural | Angelberger Forst (T) | 65 | 28 | 74 | 38 | 80 | 36 | 73 | 34 | 5 | 68 | 1:13.6 | 9 | 25 | 1:2.8 |
| All sites | Mean | 49 | 17 | 77 | 47 | 69 | 25 | 65 | 29 | 20 | 45 | 1:2.3 | 16 | 14 | 1:0.9 |
A total of 214 of 4,064 questing-tick samples were positive for DNA of A. phagocytophilum, with mean prevalences in 2011 and 2012, respectively, as follows: urban, 4.9% and 7.4%; pasture, 1.1% and 2.8%; and natural area, 4.0% and 5.8% (Tables 2 and 3). No significant difference was detected between collection months, but the overall prevalence of A. phagocytophilum was significantly higher in 2012 than in 2011 (P < 0.01) (Tables 2 and 3). Adults showed a significantly higher prevalence than nymphs (P < 0.001). The prevalence was significantly lower on the pasture than in the urban (P < 0.001) and natural (P < 0.01) areas. Study site R1 showed a significantly higher prevalence than all other study sites (P < 0.001). Study site B showed a significantly lower prevalence than study sites M2 (P < 0.05) and T (natural area) (P < 0.01).
Table 2.
Prevalence and 95% confidence interval of Anaplasma phagocytophilum in Ixodes ricinus ticks per site for 2011 and 2012a
| Habitat | Site | Yr | Adult ticks |
Females |
Males |
Nymphs |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. pos/total no. | % | No. pos/total no. | % | 95% CI | No. pos/total no. | % | 95% CI | No. pos/total no. | % | 95% CI | |||
| Urban | M2 | 2011 | 9/240 | 3.8 | 2/120 | 1.7 | 0.2–5.9 | 7/120 | 5.8 | 2.4–11.6 | 1/120 | 0.8 | 0.0–4.6 |
| 2012 | 20/240 | 8.3 | 8/120 | 6.7 | 2.9–12.7 | 12/120 | 10.0 | 5.3–16.8 | 5/118 | 4.2 | 1.4–9.6 | ||
| M3 | 2011 | 14/259 | 5.4 | 9/135 | 6.7 | 3.1–12.3 | 5/124 | 4.0 | 1.3–9.2 | 0/140 | 0.0 | 0.0–2.6 | |
| 2012 | 10/232 | 4.3 | 6/115 | 5.2 | 1.9–11.0 | 4/117 | 3.4 | 0.9–8.5 | 2/120 | 1.7 | 0.2–5.9 | ||
| R1 | 2011 | 39/240 | 16.3 | 16/120 | 13.3 | 7.8–20.7 | 23/120 | 19.2 | 12.6–27.4 | 2/120 | 1.7 | 0.2–5.9 | |
| 2012 | 54/235 | 23.0 | 26/115 | 22.6 | 15.3–31.3 | 28/120 | 23.3 | 16.1–31.9 | 7/120 | 5.8 | 2.4–11.6 | ||
| B | 2011 | 2/150 | 1.3 | 2/79 | 2.5 | 0.3–8.8 | 0/71 | 0.0 | 0.0–5.1 | 1/120 | 0.8 | 0.0–4.6 | |
| 2012 | 6/240 | 2.5 | 3/120 | 2.5 | 0.5–7.1 | 3/120 | 2.5 | 0.5–7.1 | 0/106 | 0.0 | 0.0–3.4 | ||
| Total | 2011 | 64/889 | 7.2 | 29/454 | 6.4 | 4.3–9.1 | 35/435 | 8.0 | 5.7–11.0 | 4/500 | 0.8 | 0.2–2.0 | |
| 2012 | 90/947 | 9.5 | 43/470 | 9.1 | 6.7–12.1 | 47/477 | 9.9 | 7.3–12.9 | 14/464 | 3.0 | 1.7–5.0 | ||
| Pasture | K | 2011 | 4/225 | 1.8 | 2/93 | 2.2 | 0.3–7.6 | 2/132 | 1.5 | 0.2–5.4 | 0/140 | 0.0 | 0.0–2.6 |
| 2012 | 10/234 | 4.3 | 7/114 | 6.1 | 2.5–12.2 | 3/120 | 2.5 | 0.5–7.1 | 0/120 | 0.0 | 0.0–3.0 | ||
| Natural | T | 2011 | 7/79 | 8.9 | 2/33 | 6.1 | 0.7–20.2 | 5/46 | 10.9 | 3.6–23.6 | 1/120 | 0.8 | 0.0–4.6 |
| 2012 | 17/226 | 7.5 | 11/109 | 10.1 | 5.1–17.3 | 6/117 | 5.1 | 1.9–10.8 | 3/120 | 2.5 | 0.5–7.1 | ||
| All sites | Total | 2011 | 75/1,193 | 6.3 | 33/580 | 5.7 | 3.9–7.9 | 42/613 | 6.9 | 5.0–9.1 | 5/760 | 0.7 | 0.2–1.5 |
| 2012 | 117/1,407 | 8.3 | 61/693 | 8.8 | 6.8–11.2 | 56/714 | 7.8 | 6.0–10.1 | 17/704 | 2.4 | 1.4–3.8 | ||
pos, positive for A. phagocytophilum; 95% CI, 95% confidence interval.
Table 3.
Prevalence and 95% confidence interval of Anaplasma phagocytophilum in Ixodes ricinus ticks per month for 2011 and 2012a
| Month | Yr | Site | Adults |
Female |
Male |
Nymph |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. pos/total no. | % | No. pos/total no. | % | 95% CI | No. pos/total no. | % | 95% CI | No. pos/total no. | % | 95% CI | |||
| April | 2011 | Urban | 18/283 | 6.4 | 8/141 | 5.7 | 2.5–10.9 | 10/142 | 7.0 | 3.4–12.6 | 3/160 | 1.9 | 0.4–5.4 |
| Pasture | 2/54 | 3.7 | 1/21 | 4.8 | 0.1–23.8 | 1/33 | 3.0 | 0.1–15.8 | 0/40 | 0.0 | 0.0–8.8 | ||
| Natural | 3/29 | 10.3 | 2/13 | 15.4 | 1.9–45.4 | 1/16 | 6.3 | 0.2–30.2 | 0/40 | 0.0 | 0.0–8.8 | ||
| Total | 23/366 | 6.3 | 11/175 | 6.3 | 3.2–11.0 | 12/191 | 6.3 | 3.3–12.2 | 3/240 | 1.3 | 0.3–3.6 | ||
| 2012 | Urban | 42/312 | 13.5 | 22/155 | 14.2 | 9.1–20.7 | 20/157 | 12.7 | 8.0–19.0 | 2/146 | 1.4 | 0.2–4.9 | |
| Pasture | 0/79 | 0.0 | 0/39 | 0.0 | 0.0–9.0 | 0/40 | 0.0 | 0.0–8.8 | 0/40 | 0.0 | 0.0–8.8 | ||
| Natural | 10/74 | 13.5 | 6/34 | 17.6 | 6.8–34.5 | 4/40 | 10.0 | 2.8–23.7 | 0/40 | 0.0 | 0.0–8.8 | ||
| Total | 52/465 | 11.2 | 28/228 | 12.3 | 8.3–17.3 | 24/237 | 10.1 | 6.6–14.7 | 2/226 | 0.9 | 0.1–3.2 | ||
| May | 2011 | Urban | 28/317 | 8.8 | 13/160 | 8.1 | 4.4–13.5 | 15/157 | 9.6 | 5.5–15.3 | 0/160 | 0.0 | 0.0–2.3 |
| Pasture | 2/79 | 2.5 | 1/39 | 2.6 | 0.1–13.5 | 1/40 | 2.5 | 0.1–13.2 | 0/40 | 0.0 | 0.0–8.8 | ||
| Natural | 1/28 | 3.6 | 0/8 | 0.0 | 0.0–36.9 | 1/20 | 5.0 | 0.1–24.9 | 0/40 | 0.0 | 0.0–8.8 | ||
| Total | 31/424 | 7.3 | 14/207 | 6.8 | 3.8–11.1 | 17/217 | 7.8 | 4.6–12.2 | 0/240 | 0.0 | 0.0–1.5 | ||
| 2012 | Urban | 23/320 | 7.2 | 8/160 | 5.0 | 2.2–9.6 | 15/160 | 9.4 | 5.3–15.0 | 4/158 | 2.5 | 0.7–6.3 | |
| Pasture | 3/80 | 3.8 | 2/40 | 5.0 | 0.6–16.9 | 1/40 | 2.5 | 0.1–13.2 | 0/40 | 0.0 | 0.0–8.8 | ||
| Natural | 3/78 | 3.8 | 2/38 | 5.3 | 0.6–17.7 | 1/40 | 2.5 | 0.1–13.2 | 3/40 | 7.5 | 1.6–20.4 | ||
| Total | 29/478 | 6.1 | 12/238 | 5.0 | 2.6–8.6 | 17/240 | 7.1 | 4.2–11.1 | 7/238 | 2.9 | 1.2–6.0 | ||
| June | 2011 | Urban | 18/289 | 6.2 | 8/153 | 5.2 | 2.3–10.0 | 10/136 | 7.4 | 3.6–13.11 | 1/180 | 0.6 | 0.0–3.1 |
| Pasture | 0/92 | 0.0 | 0/33 | 0.0 | 0.0–10.6 | 0/59 | 0.0 | 0.0–6.1 | 0/60 | 0.0 | 0.0–6.0 | ||
| Natural | 3/22 | 13.6 | 0/12 | 3.0 | 0.0–26.5 | 3/10 | 30.0 | 6.7–65.2 | 1/40 | 2.5 | 0.0–13.2 | ||
| Total | 21/403 | 5.2 | 8/198 | 4.0 | 1.8–7.8 | 13/205 | 6.3 | 3.4–10.6 | 2/280 | 0.7 | 0.1–2.6 | ||
| 2012 | Urban | 25/315 | 7.9 | 13/155 | 8.4 | 4.5–13.9 | 12/160 | 7.5 | 3.9–12.7 | 8/160 | 5.0 | 2.2–9.6 | |
| Pasture | 7/75 | 9.3 | 5/35 | 14.3 | 4.8–30.3 | 2/40 | 5.0 | 0.6–16.9 | 0/40 | 0.0 | 0.0–8.8 | ||
| Natural | 4/74 | 5.4 | 3/37 | 8.1 | 1.7–21.9 | 1/37 | 2.7 | 0.1–14.2 | 0/40 | 0.0 | 0.0–8.8 | ||
| Total | 36/464 | 7.8 | 21/227 | 9.3 | 5.8–13.8 | 15/237 | 6.3 | 3.6–10.2 | 8/240 | 3.3 | 1.4–6.5 | ||
pos, positive for A. phagocytophilum; 95% CI, 95% confidence interval.
A total of 116 of 214 positive samples were sequenced. Alignment of 497 bp of the partial 16S rRNA gene sequences revealed 9 variants with 99 to 100% identity to each other and to sequences previously deposited in GenBank (Table 4). Nucleotide sequence accession numbers: The partial 16S rRNA gene sequences found in this study were submitted to GenBank under the accession numbers given in Table 4.
Table 4.
16S rRNA gene variants of Anaplasma phagocytophilum in 116 samples of questing ticks with single-nucleotide substitutions in the 497-bp sequence compared with GenBank sequences
| Sequence varianta | Total no. of samples with variant | Habitatb | Sampling site (no. of samples with variant) | Hosts found in other studies (GenBank accession no.)c | GenBank accession no. of sequence found in this study | Nucleotide at indicated positiond |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 74 | 76 | 78 | 80 | 84 | 170 | 376 | ||||||
| A | 80 | U | M2 (16), M3 (9), R (55) | Ixodes ricinus (JN181064), Dermacentor reticulatus (JN181063), dog (Canis lupus familiaris) (FJ829761), European hedgehog (Erinaceus europaeus) (JN571156), cat (Felis domesticus) (HM138366), human (GU236655) | JX909353 | A | A | A | A | G | C | A |
| P | 1 | U | M2 | NMe | JX909354 | G | A | A | A | G | C | A |
| Z | 1 | U | M3 | Ixodes ricinus (EU490523), goat (FJ538290) | JX909355 | A | A | A | A | G | T | A |
| W | 1 | P | K | Ixodes ricinus (JN181071), Ixodes persulcatus (HM366582), northern red-backed vole (Myodes rutilus) (HQ630622), bank vole (Myodes glareolus) (AY094353), common shrew (Sorex araneus) (HQ630623), hedgehog (JN571163), coyote (Canis latrans) (AF170728), dog (AY741098), horse (Equus caballus) (AF172167), cattle (Bos taurus) (JQ026308), lama (Lama glama) (AF241532), mouflon (Ovis musimon) (EU839851), chamois (Rupicapra rupicapra) (FJ812399), red deer (Cervus elaphus) (GQ428331), sheep (Ovis aries) (GQ428333), roe deer (Capreolus capreolus) (JX627363), wild boar (Sus scrofa) (GU391313), human (Homo sapiens) (AF093789) | JX909356 | A | A | A | A | A | C | G |
| O | 1 | N | T | Roe deer (GU236538) | JX627370 | A | G | G | A | G | C | G |
| V | 3 | U, N | M3 (1), T (2) | Ixodes ricinus (FJ788512), dog (JN656381), hedgehog (JN571162), roe deer (JX627362) | JX909357 | A | A | A | G | A | C | G |
| B | 3 | P, N | K (2), T (1) | Ixodes ricinus (HQ629917), Ixodes ovatus (AY969015), Hemaphysalis longicornis (GU064899), vulture (Falconiformes) (JN217095), cottontail rabbit (Sylvilagus floridanus) (AY144728), white-footed mouse (Peromyscus leucopus) (U72878), European hedgehog (JN571159), dog (FJ829787), horse (JF893938), red deer (EU839850), roe deer (EU839848), human (AY886761, U02521) | JX909358 | A | A | A | A | G | C | G |
| X | 8 | U, P, N | B (3), K (2), T (3) | Ixodes ricinus (HQ629923), Ixodes scapularis (AF311343), goat (Capra aegagrus hircus) (FJ538288), roe deer (HM480381) | JX909359 | A | G | A | A | A | C | G |
| Y | 18 | U, P, N | M3 (7), B (2), K (3), T (6) | Ixodes ricinus (JN181069), cotton rat (Sigmodon hispidus) (JQ063025), goat (FJ538289), mouflon (FJ812409), roe deer (HM480385) | JX909360 | A | G | A | A | G | C | G |
| Anaplasma phagocytophilum HZ complete genome (NC_007797) | A | A | A | A | G | C | G | |||||
U, urban area; P, pasture area; N, natural area.
The list is not exhaustive.
Anaplasma phagocytophilum HZ (complete genome, GenBank accession no. NC_007797) was used as the reference strain; nucleotide positions indicate the position relative to bp 1433 of the rrsA 16S rRNA gene (Gene ID 3930754). Bold indicates nucleotide differences compared to the complete genome.
NM, no match with sequences in GenBank.
Our results support the hypothesis that the prevalence and genetic variants of A. phagocytophilum vary depending on habitat structures and the occurrence of different potential reservoir hosts. In a comparison of the results from the urban sites with the results from the same sites from a previous study during 2009 and 2010 (8), an overall continuous decrease followed by an increase in prevalence was detected. This might depend on more global factors affecting all sites, such as the weather conditions during those years, the overall appearance of the vector I. ricinus, and/or the appearance of common reservoir hosts, and not on habitat structure or other factors on single sites. The prevalence of A. phagocytophilum in ticks (which had their last blood meal 1 or 2 years prior to this investigation) depends on its prevalence in the reservoir hosts. Consequently, changes of the A. phagocytophilum prevalence in reservoir hosts are detected with a temporal delay by the prevalence in questing ticks.
We detected 9 different 16S rRNA gene variants of A. phagocytophilum with variations in study sites. In urban sites R1 and M2, variant A was found most frequently. This confirms the 16S rRNA gene variants found on these study sites in 2009 and 2010 (8). Variant A has not yet been detected in wild ungulates in our investigations (12, 24). Furthermore, as wild ungulates are rare to nonexistent on study sites R1 and M2, there must be other reservoir hosts present, such as foxes, small rodents, hedgehogs, squirrels, or birds (13, 17–21). Variant A has also been detected in a human patient (22) and in granulocytic anaplasmosis cases in horses and dogs (3, 4). It has been discussed previously whether this variant may be less pathogenic (3). This might explain the discrepancy between the high prevalence in questing ticks in urban areas and the lack of clinical human cases in Germany. More in-depth and experimental studies on the pathogenic potential of this variant are needed to elucidate this hypothesis. Further efforts to find the main reservoir host for A. phagocytophilum in urban areas are necessary. In the urban site M3, wild game exists, and besides variant A, variant Y, previously found in roe deer, was detected (12, 22). Variant Y was also detected in the other study sites where wild ungulates exist, whereas it was not detected in those city parks without large ungulate species. These findings confirm results from 2009 and 2010 (8). On the pasture, where mainly cattle are kept, variants B and W were additionally found. Variant B is identical to the prototype variant of A. phagocytophilum from human clinical cases in the amplified part of the 16S rRNA gene and is also frequently detected in horses and dogs with granulocytic anaplasmosis (3, 4). Variant W was evidenced mainly in sheep and cattle with tick-borne fever in previous studies (5, 23). In the natural and pasture areas, as well as in the forest-like park (site B), variant X was also found. Furthermore, it was detected in 44% of sequenced A. phagocytophilum-positive roe deer samples in the natural area in 2010 and 2011 (24).
In conclusion, the prevalence rates and the occurrence of partial 16S rRNA gene variants of A. phagocytophilum differed in all habitats investigated in our study, most likely depending on the habitat structure and, therefore, the appearance and availability of typical reservoir hosts. Furthermore, a year-to-year variation could be detected that was unaffected by the habitat structure, suggesting the involvement of more global factors in the occurrence of A. phagocytophilum in ticks.
Nucleotide sequence accession numbers.
The partial 16S rRNA gene sequences found in this study (except for JX627370) were submitted to GenBank under the accession numbers given in Table 4.
ACKNOWLEDGMENTS
We especially thank Tim Tiedemann for his assistance in the laboratory work.
This study was partially funded by EU grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext079 (http://www.edenext.eu/).
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Woldehiwet Z. 2010. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 167:108–122 [DOI] [PubMed] [Google Scholar]
- 2. Heikkilä HM, Bondarenko A, Mihalkov A, Pfister K, Spillmann T. 2010. Anaplasma phagocytophilum infection in a domestic cat in Finland: case report. Acta Vet. Scand. 52:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silaghi C, Kohn B, Chirek A, Thiel C, Nolte I, Liebisch G, Pfister K. 2011. Relationship of molecular and clinical findings on Anaplasma phagocytophilum involved in natural infections of dogs. J. Clin. Microbiol. 49:4413–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silaghi C, Liebisch G, Pfister K. 2011. Genetic variants of Anaplasma phagocytophilum from 14 equine granulocytic anaplasmosis cases. Parasit. Vectors 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nieder M, Silaghi C, Hamel D, Pfister K, Schmäschke R, Pfeffer M. 2012. Tick-borne fever caused by Anaplasma phagocytophilum in Germany. First laboratory confirmed case in a dairy cattle herd. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 40:101–106 [PubMed] [Google Scholar]
- 6. Ogden NH, Bown K, Horrocks BK, Woldehiwet Z, Bennett M. 1998. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med. Vet. Entomol. 12:423–429 [DOI] [PubMed] [Google Scholar]
- 7. Foley JE, Nieto NC, Massung R, Barbet A, Madigan J, Brown RN. 2009. Distinct ecologically relevant strains of Anaplasma phagocytophilum. Emerg. Infect. Dis. 15:842–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schorn S, Pfister K, Reulen H, Mahling M, Manitz J, Thiel C, Silaghi C. 2011. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus in Bavarian public parks, Germany. Ticks Tick Borne Dis. 2:196–203 [DOI] [PubMed] [Google Scholar]
- 9. Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, Olson JG. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silaghi C, Scheuerle MC, Friche Passos LM, Thiel C, Pfister K. 2011. PCR detection of Anaplasma phagocytophilum in goat flocks in an area endemic for tick-borne fever in Switzerland. Parasite 18:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silaghi C, Gilles J, Höhle M, Fingerle V, Just FT, Pfister K. 2008. Anaplasma phagocytophilum infection in Ixodes ricinus, Bavaria, Germany. Emerg. Infect. Dis. 14:972–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silaghi C, Hamel D, Thiel C, Pfister K, Passos LM, Rehbein S. 2011. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the Alps in Tyrol, Austria. Vector Borne Zoonotic Dis. 11:355–362 [DOI] [PubMed] [Google Scholar]
- 13. Silaghi C, Skuballa J, Thiel C, Pfister K, Petney T, Pfäffle M, Taraschewski H, Passos LM. 2012. The European hedgehog (Erinaceus europaeus)—a suitable reservoir for variants of Anaplasma phagocytophilum? Ticks Tick Borne Dis. 3:49–54 [DOI] [PubMed] [Google Scholar]
- 14. Clopper CJ, Pearson ES. 1934. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404–413 [Google Scholar]
- 15. Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometric J. 50:346–363 [DOI] [PubMed] [Google Scholar]
- 16. R Development Core Team 2012. R: a language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/ [Google Scholar]
- 17. Liz JS, Anderes L, Sumner JW, Massung RF, Gern L, Rutti B, Brossard M. 2000. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J. Clin. Microbiol. 38:1002–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrovec M, Sixl W, Schweiger R, Mikulasek S, Elke L, Wüst G, Marth E, Strasek K, Stünzner D, Avsic-Zupanc T. 2003. Infections of wild animals with Anaplasma phagocytophila in Austria and the Czech Republic. Ann. N. Y. Acad. Sci. 990:103–106 [DOI] [PubMed] [Google Scholar]
- 19. Franke J, Fritzsch J, Tomaso H, Straube E, Dorn W, Hildebrandt A. 2010. Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in Central Germany. Appl. Environ. Microbiol. 76:6829–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nieto NC, Leonhard S, Foley JE, Lane RS. 2010. Coinfection of western gray squirrel (Sciurus griseus) and other sciurid rodents with Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in California. J. Wildl. Dis. 46:291–296 [DOI] [PubMed] [Google Scholar]
- 21. Nieto NC, Foley JE. 2008. Evaluation of squirrels (Rodentia: Sciuridae) as ecologically significant hosts for Anaplasma phagocytophilum in California. J. Med. Entomol. 45:763–769 [DOI] [PubMed] [Google Scholar]
- 22. Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, Runge M, Ganter M, Kehl A, Dumler JS, Garcia-Perez AL, Jensen J, Fingerle V, Meli ML, Ensser A, Stuen S, von Loewenich FD. 2011. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J. Clin. Microbiol. 49:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stuen S, Van De Pol I, Bergström K, Schouls LM. 2002. Identification of Anaplasma phagocytophila (formerly Ehrlichia phagocytophila) variants in blood from sheep in Norway. J. Clin. Microbiol. 40:3192–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Overzier E, Pfister K, Herb I, Mahling M, Böck G, Jr, Silaghi C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), questing ticks (Ixodes ricinus) and ticks infesting roe deer in southern Germany. Ticks Tick-Borne Dis., in press [DOI] [PubMed] [Google Scholar]