Abstract
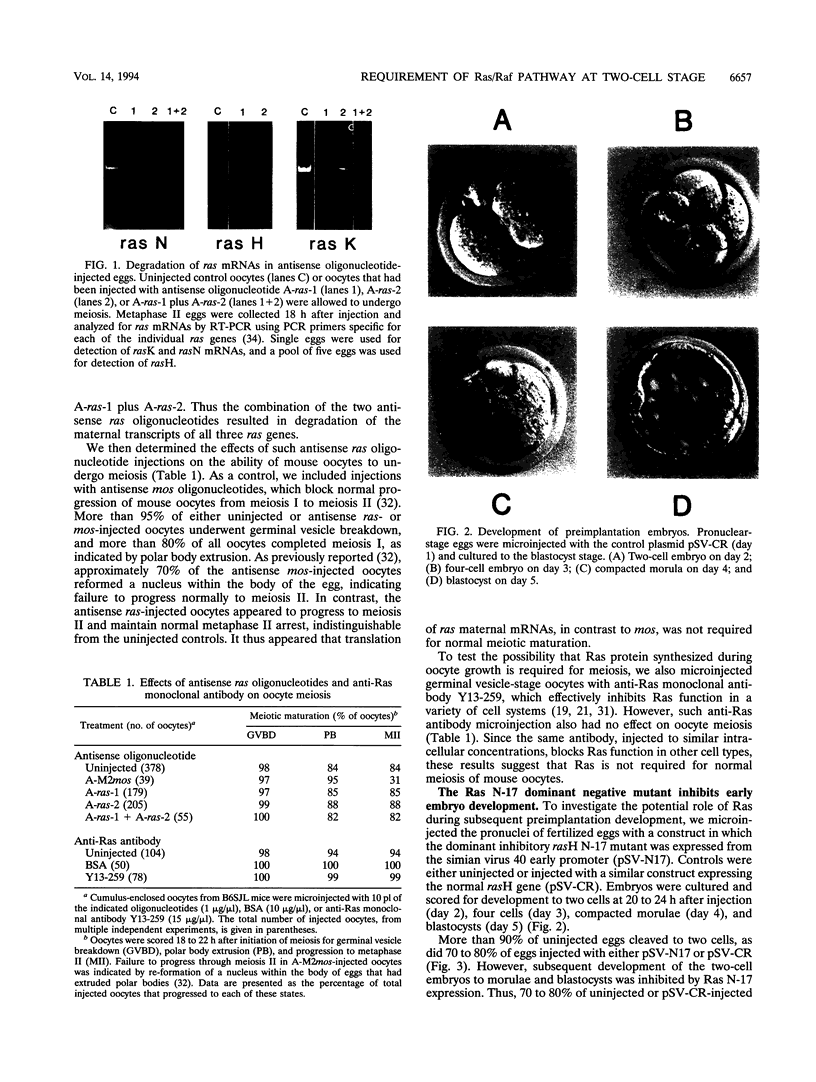
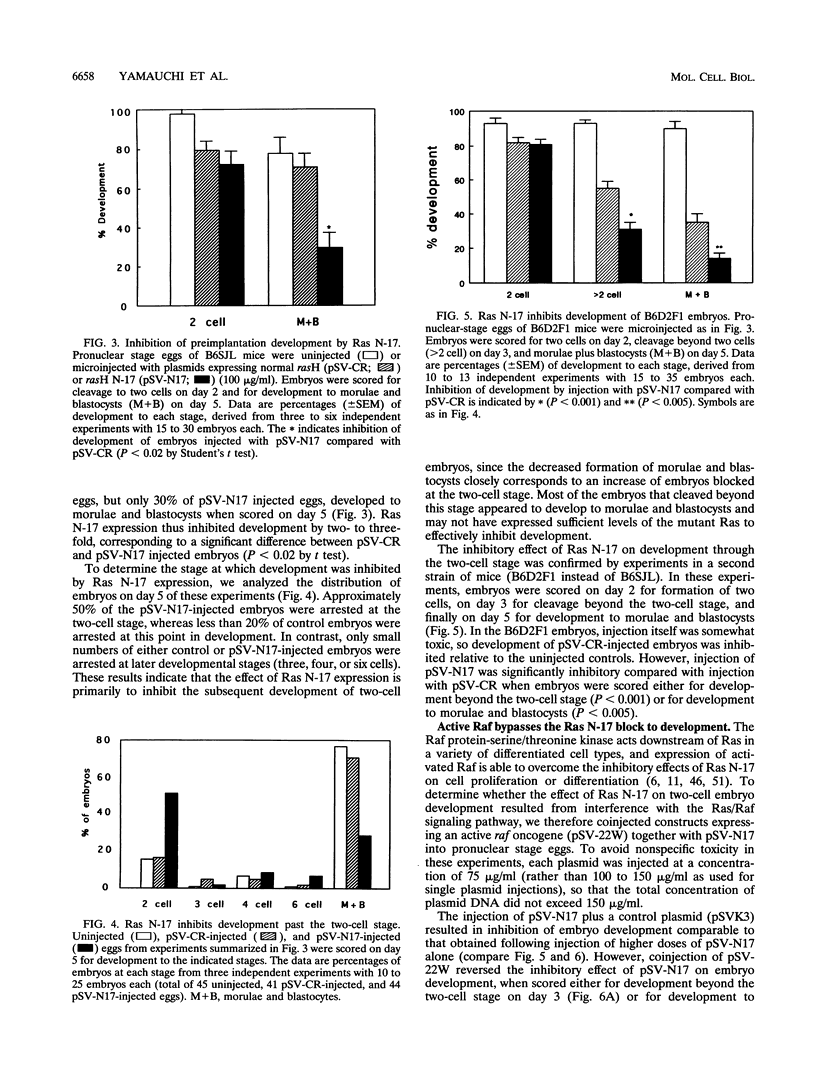
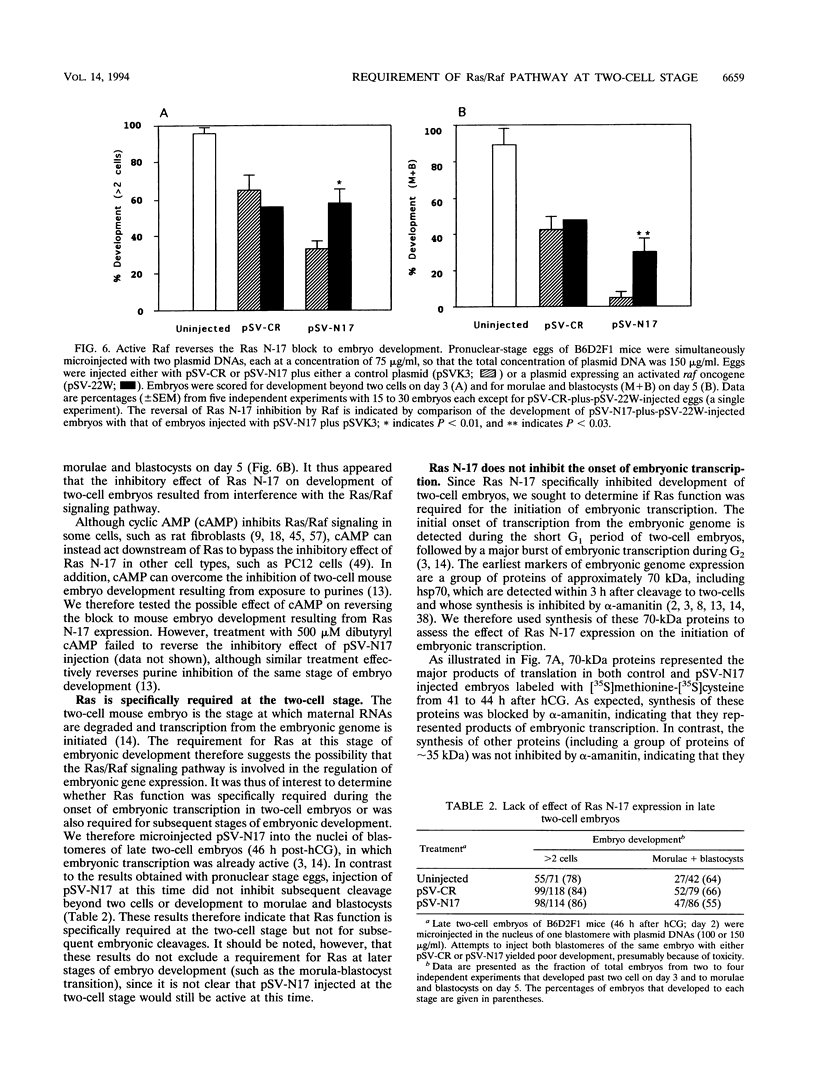
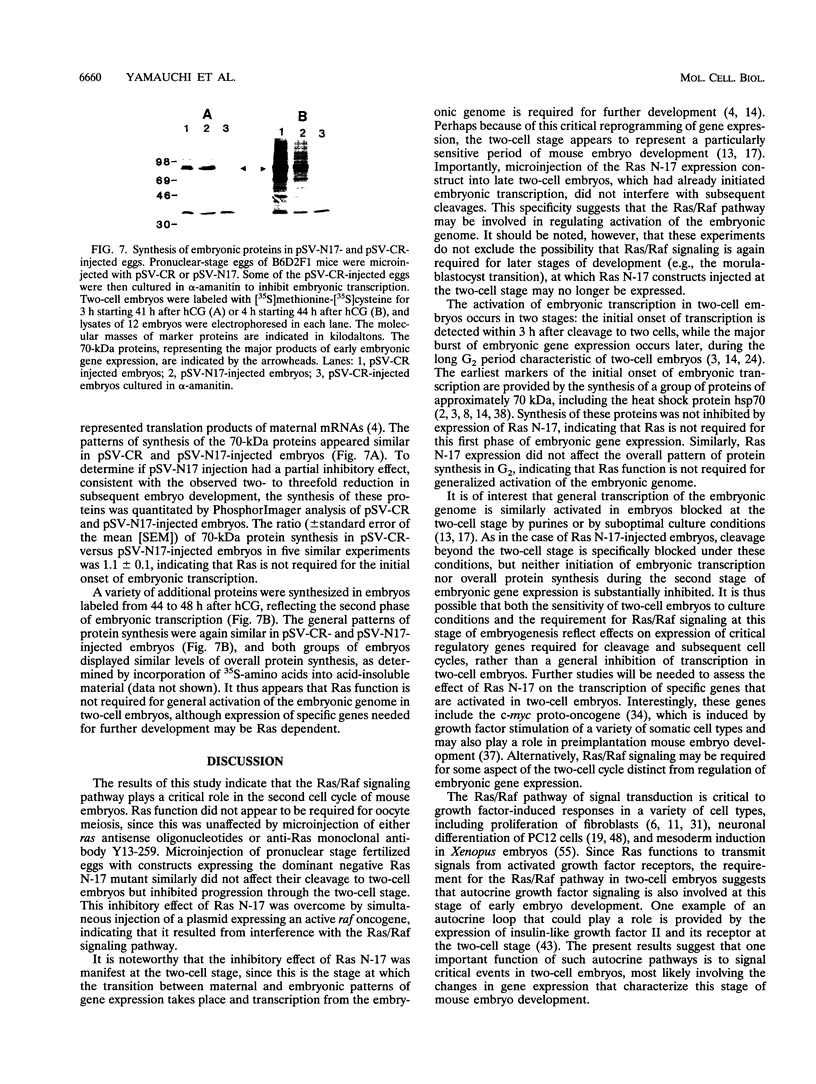
We have used microinjection of antisense oligonucleotides, monoclonal antibody, and the dominant negative Ras N-17 mutant to interfere with Ras expression and function in mouse oocytes and early embryos. Microinjection of either ras antisense oligonucleotides or anti-Ras monoclonal antibody Y13-259 did not affect normal progression of oocytes through meiosis and arrest at metaphase II. However, microinjection of fertilized eggs with constructs expressing Ras N-17 inhibited subsequent development through the two-cell stage. The inhibitory effect of Ras N-17 was overcome by simultaneous injection of a plasmid expressing an active raf oncogene, indicating that it resulted from interference with the Ras/Raf signaling pathway. In contrast to the inhibition of two-cell embryo development resulting from microinjection of pronuclear stage eggs, microinjection of late two-cell embryos with Ras N-17 expression constructs did not affect subsequent cleavages and development to morulae and blastocysts. It thus appears that the Ras/Raf signaling pathway, presumably activated by autocrine growth factor stimulation, is specifically required at the two-cell stage, which is the time of transition between maternal and embryonic gene expression in mouse embryos.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D. EGF receptor activities in mammalian development. Mol Reprod Dev. 1990 Sep;27(1):16–22. doi: 10.1002/mrd.1080270106. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Bolton V. N., Oades P. J., Johnson M. H. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984 Feb;79:139–163. [PubMed] [Google Scholar]
- Braude P., Pelham H., Flach G., Lobatto R. Post-transcriptional control in the early mouse embryo. Nature. 1979 Nov 1;282(5734):102–105. doi: 10.1038/282102a0. [DOI] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Troppmair J., Diaz-Meco M. T., Sithanandam G., Rapp U. R., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine couples Ras to activation of Raf protein kinase during mitogenic signal transduction. Mol Cell Biol. 1993 Dec;13(12):7645–7651. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Szeberényi J., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990 Oct;10(10):5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro C. M., Trounson A. The effect of protein on preimplantation mouse embryo development in vitro. J In Vitro Fert Embryo Transf. 1984 Sep;1(3):183–187. doi: 10.1007/BF01139212. [DOI] [PubMed] [Google Scholar]
- Conover J. C., Temeles G. L., Zimmermann J. W., Burke B., Schultz R. M. Stage-specific expression of a family of proteins that are major products of zygotic gene activation in the mouse embryo. Dev Biol. 1991 Apr;144(2):392–404. doi: 10.1016/0012-1606(91)90431-2. [DOI] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissore R. A., Jackson K. V., Kiessling A. A. Mouse zygote development in culture medium without protein in the presence of ethylenediaminetetraacetic acid. Biol Reprod. 1989 Nov;41(5):835–841. doi: 10.1095/biolreprod41.5.835. [DOI] [PubMed] [Google Scholar]
- Fissore R., O'Keefe S., Kiessling A. A. Purine-induced block to mouse embryo cleavage is reversed by compounds that elevate cyclic adenosine monophosphate. Biol Reprod. 1992 Dec;47(6):1105–1112. doi: 10.1095/biolreprod47.6.1105. [DOI] [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Goddard M. J., Pratt H. P. Control of events during early cleavage of the mouse embryo: an analysis of the '2-cell block'. J Embryol Exp Morphol. 1983 Feb;73:111–133. [PubMed] [Google Scholar]
- Graves L. M., Bornfeldt K. E., Raines E. W., Potts B. C., Macdonald S. G., Ross R., Krebs E. G. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Kremer N. E., D'Arcangelo G., Thomas S. M., DeMarco M., Brugge J. S., Halegoua S. Signal transduction by nerve growth factor and fibroblast growth factor in PC12 cells requires a sequence of src and ras actions. J Cell Biol. 1991 Nov;115(3):809–819. doi: 10.1083/jcb.115.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham K. E., Garrels J. I., Chang C., Solter D. Quantitative analysis of protein synthesis in mouse embryos. I. Extensive reprogramming at the one- and two-cell stages. Development. 1991 Aug;112(4):921–932. doi: 10.1242/dev.112.4.921. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- Manova K., Nocka K., Besmer P., Bachvarova R. F. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990 Dec;110(4):1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Mehta T. S., Kiessling A. A. Development potential of mouse embryos conceived in vitro and cultured in ethylenediaminetetraacetic acid with or without amino acids or serum. Biol Reprod. 1990 Oct;43(4):600–606. doi: 10.1095/biolreprod43.4.600. [DOI] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Wolfes H., Kiessling A. A., Cooper G. M. Microinjection of antisense c-mos oligonucleotides prevents meiosis II in the maturing mouse egg. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7038–7042. doi: 10.1073/pnas.86.18.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A., Avivi A., Zimmer Y., Givol D., Yarden Y., Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990 Aug;109(4):911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Pal S. K., Crowell R., Kiessling A. A., Cooper G. M. Expression of proto-oncogenes in mouse eggs and preimplantation embryos. Mol Reprod Dev. 1993 May;35(1):8–15. doi: 10.1002/mrd.1080350103. [DOI] [PubMed] [Google Scholar]
- Pal S. K., Zinkel S. S., Kiessling A. A., Cooper G. M. c-mos expression in mouse oocytes is controlled by initiator-related sequences immediately downstream of the transcription initiation site. Mol Cell Biol. 1991 Oct;11(10):5190–5196. doi: 10.1128/mcb.11.10.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Das S. K., Andrews G. K., Dey S. K. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Dey S. K., Andrews G. K. Antisense c-myc effects on preimplantation mouse embryo development. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10051–10055. doi: 10.1073/pnas.89.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poueymirou W. T., Schultz R. M. Differential effects of activators of cAMP-dependent protein kinase and protein kinase C on cleavage of one-cell mouse embryos and protein synthesis and phosphorylation in one- and two-cell embryos. Dev Biol. 1987 Jun;121(2):489–498. doi: 10.1016/0012-1606(87)90185-0. [DOI] [PubMed] [Google Scholar]
- Poueymirou W. T., Schultz R. M. Regulation of mouse preimplantation development: inhibition of synthesis of proteins in the two-cell embryo that require transcription by inhibitors of cAMP-dependent protein kinase. Dev Biol. 1989 Jun;133(2):588–599. doi: 10.1016/0012-1606(89)90061-4. [DOI] [PubMed] [Google Scholar]
- Pratt H. P., Bolton V. N., Gudgeon K. A. The legacy from the oocyte and its role in controlling early development of the mouse embryo. Ciba Found Symp. 1983;98:197–227. doi: 10.1002/9780470720790.ch12. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Sturm K. S., Behrendtsen O., Schultz G. A., Pedersen R. A., Werb Z. Insulin-like growth factor II acts through an endogenous growth pathway regulated by imprinting in early mouse embryos. Genes Dev. 1992 Jun;6(6):939–952. doi: 10.1101/gad.6.6.939. [DOI] [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberényi J., Erhardt P., Cai H., Cooper G. M. Role of Ras in signal transduction from the nerve growth factor receptor: relationship to protein kinase C, calcium and cyclic AMP. Oncogene. 1992 Nov;7(11):2105–2113. [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Warne P. H., Viciana P. R., Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993 Jul 22;364(6435):352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Involvement of p21ras in Xenopus mesoderm induction. Nature. 1992 May 21;357(6375):252–254. doi: 10.1038/357252a0. [DOI] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]