Abstract
In this study, we developed a large-scale screening of bacterial strains in order to identify novel candidate probiotics with immunomodulatory properties. For this, 158 strains, including a majority of lactic acid bacteria (LAB), were screened by two different cellular models: tumor necrosis factor alpha (TNF-α)-activated HT-29 cells and peripheral blood mononuclear cells (PBMCs). Different strains responsive to both models (pro- and anti-inflammatory strains) were selected, and their protective effects were tested in vivo in a murine model of influenza virus infection. Daily intragastric administrations during 10 days before and 10 days after viral challenge (100 PFU of influenza virus H1N1 strain A Puerto Rico/8/1934 [A/PR8/34]/mouse) of Lactobacillus plantarum CNRZ1997, one potentially proinflammatory probiotic strain, led to a significant improvement in mouse health by reducing weight loss, alleviating clinical symptoms, and inhibiting significantly virus proliferation in lungs. In conclusion, in this study, we have combined two cellular models to allow the screening of a large number of LAB for their immunomodulatory properties. Moreover, we identified a novel candidate probiotic strain, L. plantarum CNRZ1997, active against influenza virus infection in mice.
INTRODUCTION
Probiotics are defined by the Food and Agriculture Organization (FAO) of the United Nations World Health Organization (WHO) as “live microorganisms which, when administered in adequate amounts, confer health benefits on the host” (1). Their use is more and more popular for both prevention and treatment of a number of human diseases (2–5). Most probiotic microorganisms belong to lactic acid bacteria (LAB). However, not all probiotics display the same properties, and careful selection of specific strains based on their claimed beneficial effects is needed. Although several criteria have been proposed to identify novel probiotic strains, some studies have reported the selection of potential candidates at the preclinical level (i.e., animal trials), including evaluation of both safety and potential efficacy (6–8). The efficacy of probiotic bacteria is greatly influenced by their functional properties, such as antimicrobial activity, persistence in the gastrointestinal tract (GIT) for intestine-targeted probiotics, and immunomodulatory properties (9, 10).
Despite advances in medicine, common respiratory virus infections (RVI) such as the common cold or flu continue to cause a considerable economic burden; fortunately, some probiotic strains have been studied for their positive effects on these virus infections (11, 12). Indeed, products containing probiotics have been shown to have an immunomodulatory effect and a protective effect against RVI in both mice and humans (13–15). For example, oral daily administration of the LAB Lactobacillus plantarum L-137 (a strain selected for its proinflammatory properties in vitro) before and after influenza virus H1N1 challenge in mice enhanced survival and decreased virus titers in lungs of infected mice (15). Furthermore, several other LAB strains, such as Lactobacillus fermentum CECT5716 or Lactobacillus casei DN114-001, were described recently to enhance the effects of influenza virus vaccination and to improve antibody responses to influenza virus vaccination in humans, respectively (16, 17). Also, a mixture of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, and Bifidobacterium bifidum MF 20/5 reduced the severity of symptoms related to common cold episodes in humans (14). In another clinical trial, the most commonly used probiotic strain, Lactobacillus rhamnosus GG, tested alone or in association with B. animalis subsp. lactis BB-12, reduced the incidence of RVI (18, 19). A clinical trial using Lactobacillus acidophilus strain NCFM alone or in association with Bifidobacterium animalis subsp. lactis BI-07 showed that these probiotics reduce influenza-like symptoms (fever, rhinorrhea, cough incidence, and duration of antibiotic prescription) (20). In addition, in another interesting study, Boge et al. (16) demonstrated that daily consumption of a probiotic fermented dairy drink improves antibody responses to influenza virus vaccination in the elderly in two randomized controlled trials. Altogether, these preclinical and clinical trials suggest that probiotics can be successfully used as preventive and therapeutic agents in RVI.
Over the last 5 years, the interest in the immunomodulatory properties of LAB strains has significantly increased. In this context, a wide variety of strain-dependent properties have been reported (8, 10, 13), and several in vitro cellular models have been developed in order to analyze and classify the immunomodulatory properties of these strains. The aim of this study is thus to determine the immunomodulatory properties of a large set of LAB (158 strains) using two different cellular models, tumor necrosis factor alpha (TNF-α)-activated HT-29 cells and peripheral blood mononuclear cells (PBMCs), in order to identify the most efficient strain and develop a new probiotic supplement able to alleviate symptoms of RVI and, more particularly, the common cold. After establishing the immunomodulatory profile (based on their cytokine production profile) of each strain of the collection, different strains responsive to both models (pro- and anti-inflammatory strains) were selected and tested in vivo in a murine model of RVI, an influenza virus infection, to determine their protective effects.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The collection of this study comprised 158 strains isolated from different biotopes and including a majority of LAB (90 Lactobacillus spp., 31 Lactococcus spp., 31 Bifidobacterium spp., 3 Streptococcus spp., 2 Pediococcus spp., and 1 Bacillus sp.; INRA Collection CIRM, Rennes, France) (Table 1). Lactococcus spp. and Streptococcus spp. were grown in M17 medium (Difco, KS) supplemented with 5% lactose at 30°C without shaking, Lactobacillus and Pediococcus spp. were grown in MRS medium (Difco, KS) at 37°C without shaking, Bifidobacterium spp. were grown in MRS medium supplemented with 10% cysteine (Sigma-Aldrich, France) at 37°C under anaerobic conditions (GENbox Microaer; bioMérieux, Marcy l'Etoile, France) without shaking, and Bacillus spp. were grown in Luria-Bertani medium (Difco, KS) at 37°C with shaking.
Table 1.
Strains used in this study
| Organism | Collection no. | Origin |
|---|---|---|
| Lactobacillus delbrueckii | VEL12191 | Yogurt |
| Lactobacillus delbrueckii | VEL12192 | Yogurt |
| Lactobacillus helveticus | VEL12193 | Yogurt |
| Lactobacillus paracasei | VEL12194 | Human tooth decay |
| Lactobacillus paracasei | VEL12195 | Vegetable |
| Lactobacillus plantarum | VEL12196 | Unknown |
| Lactobacillus plantarum | VEL12197 | Fermented vegetable |
| Lactobacillus rhamnosus | VEL12198 | Unknown |
| Lactobacillus pentosus | VEL12199 | Corn silage |
| Lactobacillus pentosus | VEL12200 | Cheese |
| Lactobacillus johnsonii | VEL12201 | Unknown |
| Lactobacillus johnsonii | VEL12202 | Human blood |
| Lactobacillus johnsonii | VEL12203 | Unknown |
| Lactobacillus casei | VEL12204 | Cheese |
| Lactobacillus casei | VEL12205 | Cheese |
| Lactobacillus casei | VEL12206 | Unknown |
| Lactobacillus casei | VEL12207 | Cheese |
| Lactobacillus brevis | VEL12208 | Healthy donor |
| Lactobacillus fermentum | VEL12209 | Human saliva |
| Lactobacillus fermentum | VEL12210 | Fermented vegetable |
| Lactobacillus zeae | VEL12211 | Vegetable |
| Lactobacillus zeae | VEL12212 | Vegetable |
| Lactobacillus intestinalis | VEL12213 | Animal bowels |
| Lactobacillus crispatus | VEL12214 | Healthy donor |
| Lactobacillus crispatus | VEL12215 | Human eyes |
| Lactobacillus crispatus | VEL12216 | Chicken cecum |
| Lactobacillus gasseri | VEL12217 | Human saliva |
| Lactobacillus gasseri | VEL12218 | Human tooth decay |
| Lactobacillus salivarius | VEL12219 | Human saliva |
| Lactobacillus salivarius | VEL12220 | Human saliva |
| Lactobacillus sakei | VEL12221 | Fermented drink |
| Lactobacillus reuteri | VEL12222 | Healthy donor |
| Lactobacillus reuteri | VEL12223 | Healthy donor |
| Lactobacillus curvatus | VEL12224 | Meat |
| Lactobacillus curvatus | VEL12225 | Meat |
| Lactobacillus curvatus | VEL12226 | Unknown |
| Lactobacillus delbrueckii subsp. lactis | VEL12227 | Cheese |
| Lactobacillus delbrueckii subsp. lactis | VEL12228 | Cheese |
| Lactobacillus acidophilus | VEL12229 | Unknown |
| Lactobacillus helveticus | VEL12230 | Cheese |
| Lactobacillus rhamnosus | VEL12231 | Unknown |
| Lactobacillus brevis | VEL12232 | Unknown |
| Lactobacillus lactis | VEL12233 | Unknown |
| Lactobacillus johnsonii | VEL12234 | Unknown |
| Lactobacillus johnsonii | VEL12235 | Cheese |
| Lactobacillus delbrueckii subsp. bulgaricus | VEL12236 | Yogurt |
| Lactobacillus paracasei subsp. paracasei | VEL12237 | Fermented vegetable |
| Lactobacillus plantarum | VEL12238 | Yak milk |
| Lactobacillus plantarum | VEL12239 | Bakery starter |
| Lactobacillus paracasei subsp. paracasei | VEL12240 | Fermented vegetable |
| Lactobacillus plantarum | VEL12241 | Fermented vegetable |
| Lactobacillus casei BL23 MnKat− | VEL11757 | Recombinant strain |
| Lactobacillus casei BL23 MnKat+ | VEL11758 | Recombinant strain |
| Lactobacillus plantarum 8826 | VEL11685 | Human saliva |
| Lactobacillus acidophilus NCFM | VEL12085 | Human donor |
| Lactobacillus casei DN114-001 | VEL12079 | Commercial strain |
| Lactobacillus salivarius Ls33 | VEL12093 | |
| Lactobacillus casei BL23 | VEL12016 | Milk |
| Lactobacillus bulgaricus | VEL12374 | Unknown |
| Lactobacillus brevis | VEL12348 | Unknown |
| Lactobacillus bulgaricus | VEL12349 | Unknown |
| Lactobacillus bulgaricus | VEL12350 | Milk |
| Lactobacillus bulgaricus | VEL12351 | Unknown |
| Lactobacillus crispatus | VEL12352 | Unknown |
| Lactobacillus crispatus | VEL12353 | Unknown |
| Lactobacillus fermentum | VEL12354 | Unknown |
| Lactobacillus gasseri | VEL12355 | Unknown |
| Lactobacillus helveticus | VEL12356 | Unknown |
| Lactobacillus lactis | VEL12357 | Cheese |
| Lactobacillus lactis | VEL12358 | Yak milk |
| Lactobacillus plantarum | VEL12359 | Unknown |
| Lactobacillus paraplantarum | VEL12360 | Commercial starter |
| Lactobacillus paracasei | VEL12361 | Fermented milk |
| Lactobacillus paracasei | VEL12362 | Unknown |
| Lactobacillus rhamnosus | VEL12363 | Unknown |
| Lactobacillus salivarius | VEL12364 | Commercial starter |
| Lactobacillus salivarius | VEL12365 | Unknown |
| Lactobacillus sakei | VEL12366 | Unknown |
| Lactobacillus rhamnosus | VEL12386 | Unknown |
| Lactobacillus vaginalis | VEL12367 | Unknown |
| Lactobacillus delbrueckii | VEL12377 | Unknown |
| Lactobacillus delbrueckii | VEL12378 | Unknown |
| Lactobacillus delbrueckii | VEL12379 | Unknown |
| Lactobacillus delbrueckii | VEL12380 | Unknown |
| Lactobacillus delbrueckii | VEL12381 | Unknown |
| Lactobacillus delbrueckii | VEL12382 | Unknown |
| Lactobacillus delbrueckii | VEL12383 | Unknown |
| Lactobacillus delbrueckii | VEL12384 | Unknown |
| Lactobacillus fermentum | VEL12385 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12242 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12243 | Cheese |
| Lactococcus lactis subsp. lactis | VEL12244 | Cheese |
| Lactococcus lactis subsp. lactis | VEL12245 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12246 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12247 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12248 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12249 | Cheese |
| Lactococcus lactis subsp. lactis | VEL12250 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12251 | Fermented milk |
| Lactococcus raffinolactis | VEL12252 | Milk |
| Lactococcus lactis subsp. lactis | VEL12253 | Milk |
| Lactococcus lactis subsp. lactis | VEL12254 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12255 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12256 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12257 | Unknown |
| Lactococcus garvieae | VEL12258 | Unknown |
| Lactococcus plantarum | VEL12259 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12260 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12261 | Unknown |
| Lactococcus lactis subsp. lactis | VEL12262 | Yak milk |
| Lactococcus lactis subsp. hordniae | VEL12263 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12264 | Unknown |
| Lactococcus lactis subsp. cremoris | VEL12265 | Cheese |
| Lactococcus lactis subsp. lactis | VEL12266 | Cheese starter |
| Lactococcus lactis subsp. lactis | VEL12267 | Cheese |
| Lactococcus lactis subsp. cremoris NCDO712 | J60011 | Recombinant strain |
| Spontaneous mutant Lactococcus lactis subsp. cremoris NCDO712 | Spox K | Recombinant strain |
| Lactococcus lactis subsp. cremoris NCDO712 | MG1363 | Milk |
| Lactococcus lactis SL106 | VEL12347 | Unknown |
| Lactococcus lactis TIL46 | VEL12375 | Unknown |
| Bifidobacterium longum | VEL12268 | Unknown |
| Bifidobacterium thermophilum | VEL12269 | Rumen bovine |
| Bifidobacterium longum | VEL12270 | Fermented drink |
| Bifidobacterium adolescentis | VEL12271 | Healthy donor |
| Bifidobacterium thermophilum | VEL12272 | Rumen bovine |
| Bifidobacterium longum | VEL12273 | Fermented drink |
| Bifidobacterium longum | VEL12274 | Fermented drink |
| Bifidobacterium bifidum | VEL12275 | Healthy donor |
| Bifidobacterium breve | VEL12276 | Healthy donor |
| Bifidobacterium adolescentis | VEL12277 | Healthy donor |
| Bifidobacterium bifidum | VEL12328 | Healthy donor |
| Bifidobacterium bifidum | VEL12329 | Healthy donor |
| Bifidobacterium sp. | VEL12330 | Healthy donor |
| Bifidobacterium sp. | VEL12331 | Healthy donor |
| Bifidobacterium sp. | VEL12332 | Healthy donor |
| Bifidobacterium sp. | VEL12333 | Healthy donor |
| Bifidobacterium sp. | VEL12334 | Healthy donor |
| Bifidobacterium angulatum | VEL12335 | Unknown |
| Bifidobacterium adolescentis | VEL12336 | Unknown |
| Bifidobacterium pseudocatenulatum | VEL12337 | Unknown |
| Bifidobacterium longum | VEL13385 | Unknown |
| Bifidobacterium longum subsp. animalis | VEL12338 | Unknown |
| Bifidobacterium angulatum | VEL12339 | Unknown |
| Bifidobacterium infantis | VEL12340 | Unknown |
| Bifidobacterium animalis | VEL12341 | Unknown |
| Bifidobacterium sp. | VEL12342 | Healthy donor |
| Bifidobacterium sp. | VEL12343 | Healthy donor |
| Bifidobacterium sp. | VEL12344 | Healthy donor |
| Bifidobacterium sp. | VEL12345 | Healthy donor |
| Bifidobacterium sp. | VEL12346 | Healthy donor |
| Bifidobacterium bifidum CIP 56.7 (ATCC 29251) | VEL12376 | Unknown |
| Streptococcus thermophilus | VEL12368 | Unknown |
| Streptococcus thermophilus | VEL12369 | Unknown |
| Pediococcus pentosaceus | VEL12370 | Unknown |
| Pediococcus acidilactici | VEL12371 | Unknown |
| Streptococcus thermophilus | VEL12372 | Unknown |
| Bacillus subtilis | VEL12373 | Unknown |
| Lactobacillus delbrueckii | VEL12387 | Unknown |
Bile salts stress resistance assessment.
Resistance to bile salts was studied to mimic the passage of the strains in the GIT. A high-throughput method based on sterile microwell plates (384 wells; Greiner, Bio-one) was used. From a culture of each strain grown overnight, the optical density at 600 nm (OD600) was adjusted to 1 in peptone water. Five hundred microliters from this adjusted culture was added either to 500 μl of a cholic acid (Sigma-Aldrich, France) and deoxycholic acid (Sigma-Aldrich, France) solution in peptone water (0.05% [vol/wt] for each acid) or to 500 μl of peptone water. After 1 h at 37°C, stressed and nonstressed bacterial suspensions were centrifuged. Pellets were resuspended in 1 ml of appropriate culture medium and diluted 10 times. Sixty microliters from those suspensions was deposited into a well already containing 60 μl of medium. Four serial 2-fold dilutions were then performed. Growth of the stressed and nonstressed strains was monitored every 15 min for 19 h by measuring the OD600 using a thermoregulated plate reader (InfiniteM200 Pro; Tecan, France). The resistance of each strain to bile salts stress was determined by measuring the growth delay (i.e., delay for the time to reach mid-exponential phase) between stressed and nonstressed cultures. For each strain, 5 growth delays (in each experiment) corresponding to the five dilutions on the microplate were averaged. Experiments were performed in triplicates.
Experiments with the HT-29 cell line.
The human colon carcinoma cell line HT-29 was cultured in 24-well culture plates in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Switzerland) supplemented with 10% heat-inactivated fetal calf serum (FCS)–1% glutamine at 37°C in a 10% CO2–air atmosphere. Medium was changed every day. Experiments were initiated on day 7 after seeding, when cells were at confluence (∼1.83 × 106 cells/well). Twenty-four hours before bacterial coculture (day 6), the culture medium was changed for a medium with 5% heat-inactivated FCS and 1% glutamine. On the day of coculture, bacteria were added at a multiplicity of infection (MOI) of 1:40 in 50 μl DMEM in a total volume of 500 μl. Cells were stimulated simultaneously with recombinant human TNF-α (5 ng/ml; Peprotech, NJ) for 6 h at 37°C in 10% CO2. All samples were analyzed in triplicate. After coincubation, cell supernatants were collected and frozen at −80°C until further analysis of interleukin-8 (IL-8) concentrations by an enzyme-linked immunosorbent assay (ELISA) (Biolegend, San Diego, CA).
Experiments with PBMCs.
Commercial PBMCs (StemCell Technologies SARL, Grenoble, France) from healthy donors were used. For the first screening (i.e., 158 strains), we used PBMCs from one healthy donor (American man, Caucasian, aged <65 years, with a body mass index of <30), nonsmoking, with no drugs with anti-inflammatory effects taken during 15 days prior to sampling and negative for HIV and hepatitis A and B viruses. For the confirmation phase, we used PBMCs from 3 different healthy donors presenting similar health characteristics. After reception, cells were stored in liquid nitrogen until use. To prepare PBMCs for coculture experiments with bacteria, the vial cells were thawed at 37°C in a water bath and then transferred into a medium containing RPMI 1640 medium (Lonza, Switzerland) supplemented with 10% heat-inactivated FCS, 1% l-glutamine, and 0.1% penicillin-streptavidin. DNase (10 mg/ml) was added to this mix to avoid clumping. Cells were then centrifuged at 200 × g for 15 min, counted by using trypan blue, and spread onto 24-well plates at 1 × 106 cells/well. Bacteria were added in triplicate at an MOI of 1:10 in 20 μl of phosphate-buffered saline (PBS) in a total volume of 1 ml, and plates were incubated for 24 h at 37°C in 10% CO2. Samples were finally stored at −80°C until further analysis of IL-10 and IL-12p70 concentrations by ELISA (Mabtech, Sweden).
Mice.
Specific-pathogen-free BALB/c mice (females, 6 weeks of age; Janvier, France) were maintained under normal husbandry conditions in the animal facilities of the National Institute of Agricultural Research (UEAR, INRA, Jouy-en-Josas, France). All animal experiments were started after the animals were allowed 2 weeks of acclimation and were performed according to European Community rules of animal care and with authorization 78-149 of the French Veterinary Services.
Influenza virus infection of mice.
Influenza virus H1N1 strain A Puerto Rico/8/1934 (A/PR8/34; a mouse-adapted strain) was grown in allantoic cavities of 11-day-old fertile chicken eggs for 2 days at 35°C (21). The viral titer (i.e., PFU determination) was quantified by a standard plaque assay using Madin-Darby canine kidney (MDCK) cells, and the virus stock was stored at −80°C until use. For intranasal infection, mice were fully anesthetized by intraperitoneal injection of ketamine (Imalgene 1000, Merial, France) and xylazine (Rompun, Alcyon, France) (0.1% ketamine plus 0.06% xylazine; 150 μl for a mouse weighing 20 g) and then infected by intranasal application of 50 μl of virus suspension (25 μl into each nostril).
Preparation of live bacterial inocula and administration in mice.
Candidate probiotic bacteria were grown as described above. Pellets from cultures grown overnight were then harvested by centrifugation at 3,000 × g at 4°C and washed with sterile PBS. The pellet was suspended in PBS to a final concentration of 5 × 109 CFU/ml. Plate counts were performed with all inocula to corroborate the CFU administered. As a positive control for our in vivo experiments, we used L. casei DN114-001 and L. rhamnosus GG, two probiotic strains having well-documented in vitro and in vivo immunomodulatory properties and protective effects in different models of influenza virus infection, as positive controls (13, 16, 22, 23).
Groups of mice (n = 8) were daily administered intragastrically 1 × 109 CFU of each strain suspended in 200 μl of PBS (with a feeding needle) 10 days before and 10 or 14 days after virus challenge. PBS was used as a negative control. Mice were monitored daily for mortality, weight loss, and visual score by scientists blinded to the study. For visual score, we used the following five-point scale, as reported previously (24): 5, healthy (no clinical symptoms); 4, mild (fur slightly ruffled); 3, moderate (fur moderately ruffled and lethargy); 2, severe (fur severely ruffled and thin); 1, very severe (no reaction to stimulation).
Sample collection.
On days 10 and 14 postinfection, blood samples were obtained from the retro-orbital venous plexus and centrifuged, and sera were stored at −80°C until further analysis. Mice were then sacrificed by cervical dislocation, and bronchoalveolar lavage fluid (BALF) was collected. To recover BALF, a catheter was tied to the exposed trachea, and a hypodermic needle and syringe were attached and used to inject and withdraw the lungs with a total volume of 1 ml of PBS. BALF samples were stored at −80°C until further analysis. Furthermore, lungs were collected for virus titration and stored at −80°C until analysis.
Virus quantification in mouse lungs.
Total RNA was isolated by using a Qiagen RNeasy minikit (Qiagen, France). Reverse transcription was carried out with Superscript II reverse transcriptase (Invitrogen, France) and the specific influenza A virus (IAV) M1 primer (21), 5′-TCT AAC CGA GGT CGA AAC GTA-3′, according to the supplier's recommendations. Virus titers were quantified by quantitative PCR. We used the specific IAV M1 primer 5′-TCT AAC CGA GGT CGA AAC GTA-3′ and the Mastercycler Realplex system (Eppendorf, France). The PCR conditions and cycles were as follows: initial DNA denaturation for 10 min at 95°C followed by 40 cycles (15 s at 95°C, 20 s at 64°C, and 30 s at 72°C), followed by 15 s at 95°C, 15 s at 60°C, 20 min of melting curve (to assess the purity of the PCR product), and 15 s at 95°C. To normalize gene expression, β-actin levels were determined for all samples (sense primer 5′-AGA AAA TCT GGC ACC ACA CC-3′ and antisense primer 5′-CTC CTTAAT GTC ACG CAC GA-3′) (21).
Statistical analyses.
For animal experiments, the formula used to calculate sample size was n = 1 + 2 × C × (s/d)2 (where s is the standard deviation, d is the difference to be observed with a C value of 10.51 [for an alpha value of 0.05 and a beta value of 0.1], and s/d equals 0.5). With these values, n equals 6.255, which means a sample size of 7 animals (and we used an extra animal to take into account the fact that mice can sometimes escape virus infection). Data were analyzed by using Dunnett's test or t test to compare the differences between groups and controls using Prism software. A P value of <0.05 was considered significant.
RESULTS
In vitro screening of the strain collection.
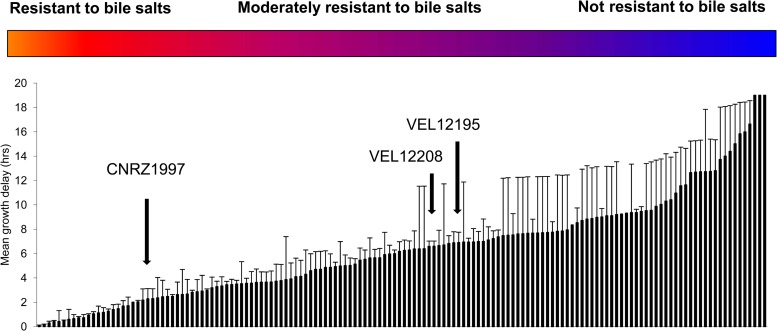
Our bacterial strain collection was first analyzed in a stress resistance experiment. As shown in Fig. 1, 44% of the strains (70 strains) had a growth delay of less than 5 h and were qualified as resistant to bile salts. We then determined the immunomodulatory properties of the 158 strains in the two cellular models: TNF-α-stimulated HT-29 cells and PBMCs.
Fig 1.
Classification of the bacterial strains according to their resistance to bile salts. Results are expressed as the mean delay in growth after exposure to the stress.
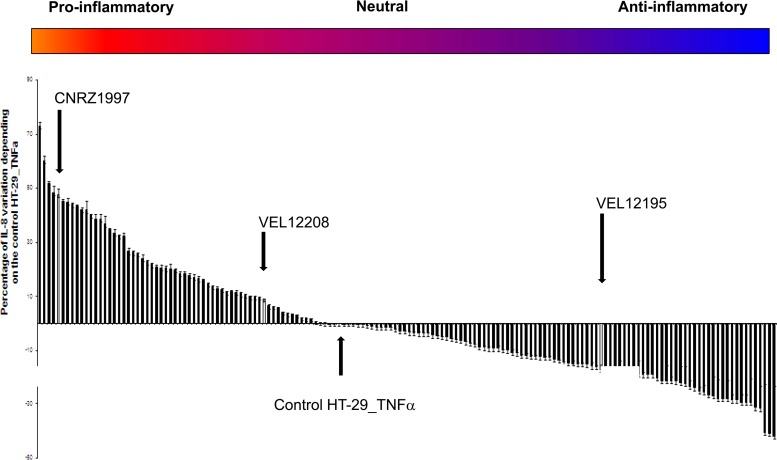
For the HT-29 model, we analyzed TNF-α-induced IL-8 secretion by HT-29 cells. Since IL-8 is considered a major inflammatory mediator, bacteria enhancing its secretion are considered to have proinflammatory properties, while those inhibiting its secretion are considered to have anti-inflammatory properties. As shown in Fig. 2, the 158 strains revealed a very distinct pattern of secretion of IL-8 in TNF-α-stimulated HT-29 cells and can be classified as highly to weakly proinflammatory, including some neutral strains. None of the bacteria tested alone (i.e., nonstimulated HT-29 cells) induced IL-8 secretion (data not shown).
Fig 2.
Classification of bacterial strains according to IL-8 production by HT-29 cells stimulated with TNF-α. Cytokine production after the coincubation of bacteria and HT-29 cells for 6 h was analyzed by ELISA. The results are expressed as a percentage of induction of the HT-29/TNF-α/PBS control ± standard error of the mean (n = 3).
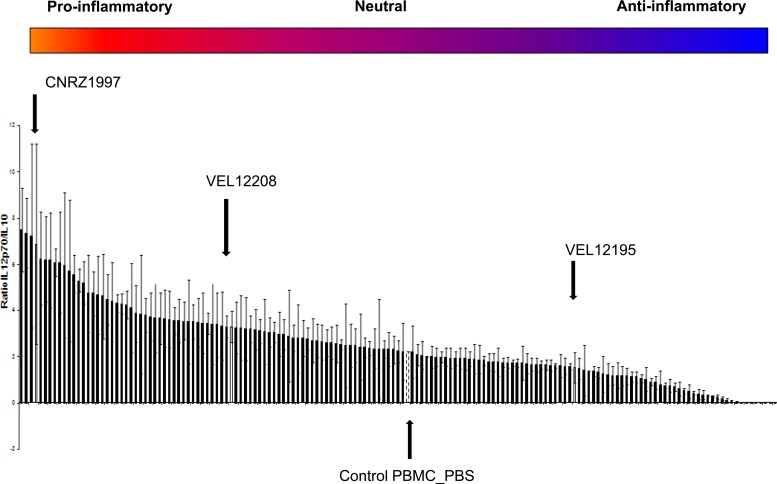
Alongside the establishment of either the anti- or proinflammatory profile of the 158 strains using the HT-29 model, we then determined their ability to modulate IL-12p70 and IL-10 secretion by PBMCs. The IL-12p70/IL-10 ratio allows us to classify strains with a strong or a weak proinflammatory profile, with a high versus low IL-12p70/IL-10 ratio, respectively. As shown in Fig. 3, the 158 tested strains again displayed different immunomodulatory profiles after coincubation with PBMCs, confirming partially the results obtained with the HT-29 model.
Fig 3.
IL-12p70/IL-10 ratio after incubation of bacteria with PBMCs from one donor. Cytokines were quantified by ELISA after coincubation of bacteria and PBMCs for 24 h. Ratios of IL-12p70/IL-10 ± standard errors of the means are shown (n = 3).
Selection of the most interesting candidate probiotic strains.
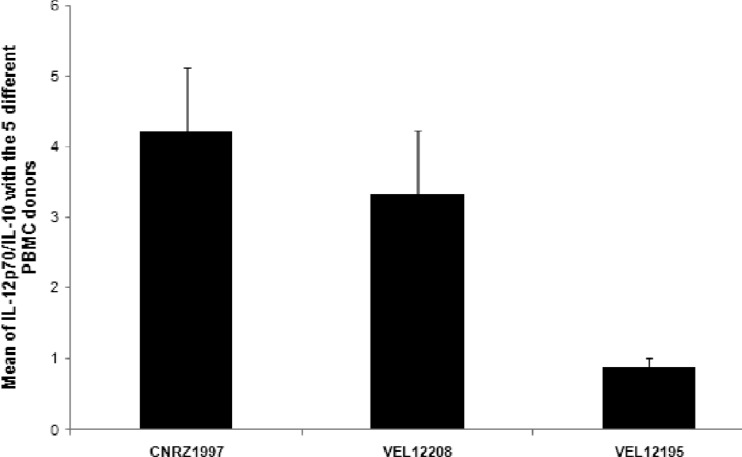
In order to determine the potential probiotic effect of the most interesting strains, 3 different responsive strains (Fig. 2 and 3, arrows) were chosen for further in vivo experiments: one with a highly proinflammatory profile (L. plantarum CNRZ1997), one with a weakly proinflammatory profile (L. brevis VEL12208), and a third with a markedly anti-inflammatory profile (L. paracasei VEL12195). We first confirmed the immunomodulatory profile of the 3 selected strains by calculating the IL-12p70/IL-10 ratio after coculture of the candidate strains with PBMCs from 5 different donors. As expected, the results obtained with the 5 different donors confirmed the profile of each strain and clearly showed a significant difference between anti- and proinflammatory strains (Fig. 4).
Fig 4.
IL-12p70/IL-10 ratios for the selected strains with 5 different PBMC donors. Cytokines were quantified by ELISA after coincubation of bacteria and PBMCs for 6 h. Ratios of IL-12p70 to IL-10 ± standard errors of the means are shown (n = 3).
In vivo effects of selected strains in a murine model of IAV infection.
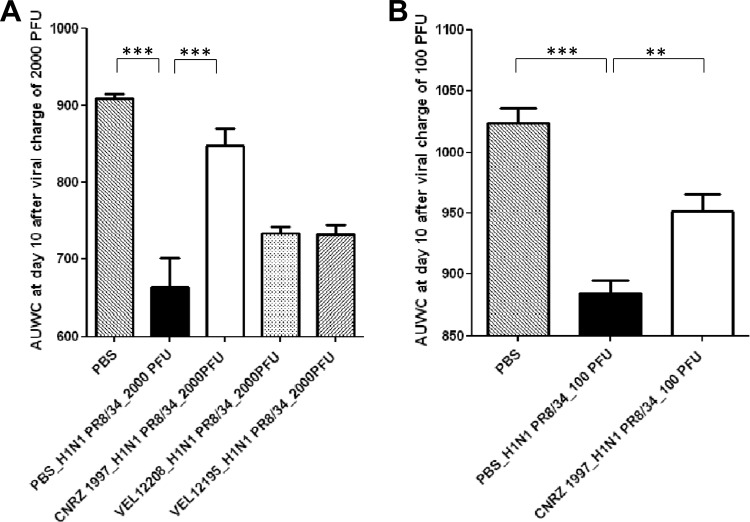
The effects of the 3 selected strains in a murine model of influenza virus infection were then investigated (Fig. 5). Ten days after virus challenge (2,000 PFU/mouse), the area under the weight curve (AUWC), representative of the cumulative weight loss of mice over the time course of the assay, revealed severe injury to PBS-treated mice (Fig. 6A). Mice treated with the proinflammatory strain CNRZ1997 showed the highest AUWC value compared to nontreated mice or mice treated with either a weakly proinflammatory (VEL12208) or anti-inflammatory (VEL12195) strain. Surprisingly, no significant differences were observed between nontreated mice and mice treated with either the L. casei DN114-001 or L. rhamnosus GG positive-control strain (data not shown).
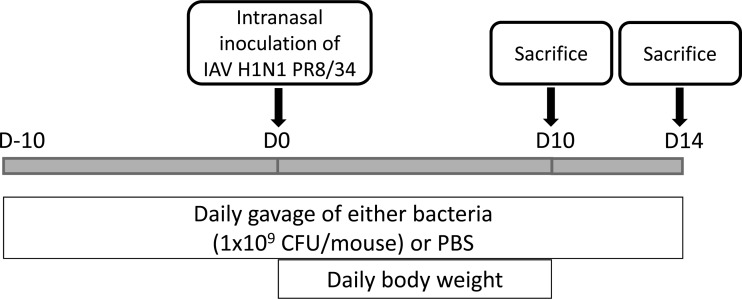
Fig 5.
Protocol used to study the immunomodulatory effect of selected bacteria on mice infected with the A/PR8/34 strain (a mouse-adapted strain).
Fig 6.
(A) Area under the weight curve (AUWC) for the different groups after a severe virus infection with 2,000 PFU of the A/PR8/34 strain. Statistically significant differences were determined by Dunnett's test (***, P < 0.001). (B) Area under the weight curve for the different groups after a moderate viral infection with 100 PFU of A/PR8/34 strain. Statistically significant differences were determined by Dunnett's test (***, P < 0.001; **, P < 0.01).
These promising results demonstrate a tendency of L. plantarum strain CNRZ1997 to decrease the body weight loss after infection with a high dose of IAV (i.e., 2,000 PFU/mouse). We then decided to test the probiotic effects of strain CNRZ1997 with a lower dose of IAV (100 PFU/mouse). This lower virus dose was used to induce moderate symptoms in mice, which can be significantly counterbalanced by our candidate probiotic bacterium.
PBS-treated and infected mice displayed a weight loss of ∼30% (at day 10) compared to healthy control mice, while mice treated with proinflammatory L. plantarum strain CNRZ1997 displayed a reduction in weight loss of only ∼10% of body weight (at day 10). In addition, weight loss was delayed in CNRZ1997-treated mice over the time course of the assay, and an earlier initiation of recovery was also observed than for PBS-treated mice (data not shown). These results were confirmed by AUWC analysis (Fig. 6B).
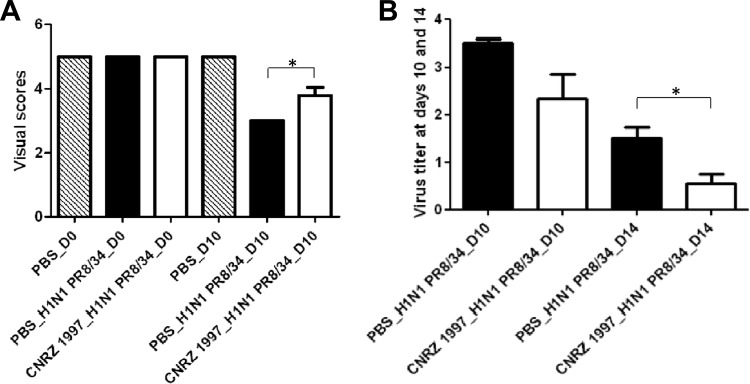
To better characterize the beneficial effects of strain CNRZ1997 in the murine model of IAV infection, we used a five-point scale of visual scores. At day 0, the 3 groups of mice showed a score of 5 (Fig. 7A). At day 10, PBS-treated and infected mice presented a score of 3 (moderate symptoms), while mice treated with strain CNRZ1997 showed a score of ∼4.
Fig 7.
(A) Visual score for mice after treatment with L. plantarum CNRZ1997 after virus infection with 100 PFU of the A/PR8/34 strain. Statistically significant differences were determined by Dunnett's test (*, P < 0.05). (B) Virus titer in lungs at day 10 after virus infection. Results are expressed as the log number of copies of the virus genome in 2.5 μg of total RNA. Statistically significant differences were determined by Student t test (*, P < 0.05).
Influenza virus titers in infected mice.
Virus titration in the lungs of infected mice revealed that CNRZ1997-treated mice presented a lower virus titer than infected control mice at days 10 and 14 postinfection (Fig. 7B). However, at day 10, no significant difference was observed in virus loads between control mice and mice receiving strain CNRZ1997; this difference became significant at day 14.
DISCUSSION
Despite advances in medicine, RVI (such as the common cold or flu) continue to cause a considerable economic burden; fortunately, some probiotic strains have been studied for their positive effects on certain infectious diseases, and in the last 5 years, their use to prevent and treat RVI has significantly increased (11, 12). There is thus a clear interest in the identification and characterization of new candidate strains with well-demonstrated probiotic properties against RVI.
In this study, we determined the immunomodulatory properties of 158 strains of LAB in order to identify the most interesting candidate probiotic strain able to alleviate RVI symptoms. For this, we first performed a large-scale screening of the 158 strains for their resistance to bile salts. Indeed, bile salts may constitute a deleterious factor preventing a given strain from exerting its beneficial properties in vivo. A high-throughput method was used to rapidly characterize strain resistance. Among the tested strains, L. plantarum CNRZ1997 had a delay of 2.9 h. Eighty strains (62.5% of the collection), including VEL12208 and VEL12195, showed moderate resistance, with a growth delay of between 5 and 13 h. Eight strains were not resistant to bile salts. This simple yet robust protocol could also be used to test other properties, such as other stresses or the ability to grow under specific conditions.
The immunomodulatory effects of the bacterial collection were then assessed by using two cellular models: TNF-α-activated HT-29 cells and peripheral blood mononuclear cells (PBMCs). These in vitro cellular models are commonly used to validate the immunomodulatory properties (anti- or proinflammatory cytokine profile) of a given candidate bacterium before validation in animal models. Indeed, these tests have a predictive value and allow a reduction of the number of bacterial strains to be tested in animal models, which are expensive and time-consuming. One of these predictive in vitro models is PBMCs, in which the immunomodulation potential of probiotic strains is usually assessed by measuring IL-10 and IL-12 cytokine levels after stimulation with live bacterial strains. In this manner, Foligne et al. (9) successfully established a correlation between this PBMC model, the in vivo immunomodulation potential of probiotic strains, and the ability to prevent experimental colitis in mice. Bacteria inducing higher levels of the anti-inflammatory cytokine IL-10 and lower levels of the proinflammatory cytokine IL-12 in vitro displayed the best protection in the murine colitis model (15). The human intestinal epithelial cell line HT-29 has been also used successfully to evaluate the immunomodulatory properties of bacteria (25, 26). Once coincubation with bacterial strains was achieved, IL-8 quantification led to the selection of anti-inflammatory bacteria (reducing IL-8 production) and proinflammatory bacteria (enhancing IL-8 production) (25, 27). After we tested our bacterial collection with these two cellular models, we selected 3 different responsive strains according to their immunomodulatory profile, one with a highly proinflammatory profile (L. plantarum CNRZ1997), one with a weakly proinflammatory profile (L. brevis VEL12208), and one with a markedly anti-inflammatory profile (L. paracasei VEL12195), for in vivo experiments using a model of A/PR8/34 strain infection in mice. Based on the preliminary results observed with a high dose of IAV (2,000 PFU), we found that L. plantarum strain CNRZ1997 (highly proinflammatory profile) was the most interesting candidate among the three candidate strains tested, with beneficial effects against IAV. The results obtained with a less challenging model (lower dose of IAV of 100 PFU) as well as with the five-point scale of visual scores confirmed these observations. Indeed, this strain was effective in preventing body weight loss (Fig. 6B) and clinical condition alterations by alleviating significantly the symptoms of the infected mice (P < 0.05) (Fig. 7A). Kawase et al. (28) previously found similar results (i.e., improved clinical symptoms) when studying the oral administration of two LAB strains, L. rhamnosus GG and L. gasseri TMC0356, in a murine model of IAV infection.
In addition, when we analyzed virus titration in lungs of infected mice at 10 and 14 days postinfection, we found that strain CNRZ1997 was able to decrease virus titers in these infected mice. This result indicates a positive effect of strain CNRZ1997 in mice by perhaps accelerating IAV elimination from the lungs. These results are similar to those obtained previously by Kawashima et al. (29), where L. plantarum strain LpYU (with a proinflammatory profile observed in vitro) was tested in a model of virus infection with the H1N1 virus (A/NWS/33).
In this work, we decided to use strains GG and DN114-001 as positive controls in our model of IAV, since they have already been successfully studied for their anti-IAV properties in preclinical trials (23) and in a human clinical trial against RVI (13). Interestingly, the AUWC results observed for mice treated with either GG or DN114-001 showed that there are no protective effects of these two well-known probiotic strains in our IAV model. These unexpected results can be explained by the fact that strain GG was reported previously to be protective against IAV in mice after “intranasal” administration, in contrast with our study, where we used an “intragastric” route. In order to confirm this, we performed experiments with L. rhamnosus GG by the intranasal route in our IAV model, and we confirmed a protective effect; however, we cannot favor this route, since the aim of this study was to develop a probiotic oral dietary supplement. Second, most trials using strain DN114-001 against IAV have been done with the fermented final product (which means that it contained L. casei strain DN114-001 combined with two cultures commonly used in yogurt, Streptococcus thermophilus and Lactobacillus bulgaricus), while in our study, we used DN114-001 as a single isolated strain, so we cannot discard a synergistic effect of strain DN114-001 and the fermented final product.
The effects of probiotic bacteria against infectious diseases, such as IAV, have been demonstrated, but the mechanisms of action are not yet fully understood; however, some hypotheses have been advanced. For example, oral administration of acidic exopolysaccharide extracts of L. delbrueckii OLL1073R-1 to mice infected with IAV provided protection (30). In another study, the protective properties of L. plantarum strain LpYU against IAV were dependent on Toll-like receptor 2 (TLR2), which recognizes the peptidoglycan (PG) and the lipoteichoic acids (LTA) of Gram-positive bacteria (29). These two results seem interesting to explore in order to identify the mechanisms of action of our proinflammatory strain CNRZ1997.
Most of the studies using LAB to prevent and treat IAV infection have been performed with intranasal administration of either live or heat-killed bacteria (15, 31, 32). In this study, we chose to test live bacteria administered orally, in order to develop a potential probiotic supplement for use in humans. Of note, parallel research demonstrated that the proinflammatory L. plantarum strain CNRZ1997 is compatible with all manufacturing and formulation technological processes (data not shown), and currently, a double-blind, randomized, controlled trial is in progress in order to investigate whether the consumption of this strain influences the severity of symptoms and the incidence and duration of common cold infections. In conclusion, our results suggest the feasibility of a large in vitro screening of bacterial strains using two cellular models (TNF-α-activated HT-29 cells and PBMCs), in order to determine their immunomodulatory properties based on a cytokine profile. Oral administration of the most proinflammatory strain (L. plantarum CNRZ1997) conferred protection against IAV infection. Further research is being conducted in our laboratory to identify the mechanism of action of this new candidate probiotic bacterium.
ACKNOWLEDGMENTS
Noura Kechaou is the recipient of a grant from the Fonds Unique Interministériel (FUI; France). We thank the Fonds de Compétitivité des Entreprises (FCE; France) from the Direction Générale des Entreprises (contract no. 08 2 90 6473) for financial support.
We thank all the UEAR staff (INRA, Jouy-en-Josas, France) who helped us during in vivo experiments, in particular Laetitia Guedeville and Alice Belleivaire, as well as Edwige Bouguyon for virus preparation and quantification.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. Food and Agriculture Organization 2002. FAO/WHO Working Group report on drafting guidelines for the evaluation of probiotics in food, p 11 Food and Agriculture Organization, London, United Kingdom [Google Scholar]
- 2. van der Aa LB, van Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, Ben Amor K, Sprikkelman AB. 2011. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 66:170–177 [DOI] [PubMed] [Google Scholar]
- 3. Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. 2006. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect. Dis. 6:374–382 [DOI] [PubMed] [Google Scholar]
- 4. Pham LC, Hoogenkamp MA, Exterkate RA, Terefework Z, de Soet JJ, ten Cate JM, Crielaard W, Zaura E. 2011. Effects of Lactobacillus rhamnosus GG on saliva-derived microcosms. Arch. Oral Biol. 56:136–147 [DOI] [PubMed] [Google Scholar]
- 5. Bermudez-Humaran LG, Kharrat P, Chatel JM, Langella P. 2011. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Fact. 10(Suppl 1):S4 doi:10.1186/1475-2859-10-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sorokulova I. 2008. Preclinical testing in the development of probiotics: a regulatory perspective with Bacillus strains as an example. Clin. Infect. Dis. 46(Suppl 2):S92–S95; discussion, S144–S151. doi:10.1086/523334 [DOI] [PubMed] [Google Scholar]
- 7. Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG. 2009. Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 47:1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Moller PL, Michaelsen KF, Paerregaard A, Sandstrom B, Tvede M, Jakobsen M. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annuk H, Shchepetova J, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M. 2003. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 94:403–412 [DOI] [PubMed] [Google Scholar]
- 11. Hori T, Kiyoshima J, Shida K, Yasui H. 2002. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin. Diagn. Lab. Immunol. 9:105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY, Lee HJ, Woo SH, Kim HM, Lee JB, Park SY, Choi IS, Song CS. 2012. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 93:138–143 [DOI] [PubMed] [Google Scholar]
- 13. Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. 2010. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 103:58–68 [DOI] [PubMed] [Google Scholar]
- 14. de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, Schrezenmeir J. 2006. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine 24:6670–6674 [DOI] [PubMed] [Google Scholar]
- 15. Maeda N, Nakamura R, Hirose Y, Murosaki S, Yamamoto Y, Kase T, Yoshikai Y. 2009. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 9:1122–1125 [DOI] [PubMed] [Google Scholar]
- 16. Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. 2009. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 27:5677–5684 [DOI] [PubMed] [Google Scholar]
- 17. Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J. 2007. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 23:254–260 [DOI] [PubMed] [Google Scholar]
- 18. Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. 2001. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322:1327 doi:10.1136/bmj.322.7298.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rautava S, Salminen S, Isolauri E. 2009. Specific probiotics in reducing the risk of acute infections in infancy—a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 101:1722–1726 [DOI] [PubMed] [Google Scholar]
- 20. Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. 2009. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 124:e172–e179 doi:10.1542/peds.2008-2666 [DOI] [PubMed] [Google Scholar]
- 21. Le Goffic R, Bouguyon E, Chevalier C, Vidic J, Da Costa B, Leymarie O, Bourdieu C, Decamps L, Dhorne-Pollet S, Delmas B. 2010. Influenza A virus protein PB1-F2 exacerbates IFN-beta expression of human respiratory epithelial cells. J. Immunol. 185:4812–4823 [DOI] [PubMed] [Google Scholar]
- 22. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, Radaelli G. 2007. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr. Res. 62:215–220 [DOI] [PubMed] [Google Scholar]
- 23. Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, Yausi H. 2010. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 50:597–602 [DOI] [PubMed] [Google Scholar]
- 24. Kawase M, He F, Kubota A, Harata G, Hiramatsu M. 2010. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 51:6–10 [DOI] [PubMed] [Google Scholar]
- 25. Grimoud J, Durand H, de Souza S, Monsan P, Ouarne F, Theodorou V, Roques C. 2010. In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int. J. Food Microbiol. 144:42–50 [DOI] [PubMed] [Google Scholar]
- 26. Preising J, Philippe D, Gleinser M, Wei H, Blum S, Eikmanns BJ, Niess JH, Riedel CU. 2010. Selection of bifidobacteria based on adhesion and anti-inflammatory capacity in vitro for amelioration of murine colitis. Appl. Environ. Microbiol. 76:3048–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanz Y, De Palma G. 2009. Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int. Rev. Immunol. 28:397–413 [DOI] [PubMed] [Google Scholar]
- 28. Kawase M, He F, Kubota A, Yoda K, Miyazawa K, Hiramatsu M. 2012. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol. Med. Microbiol. 64:280–288 [DOI] [PubMed] [Google Scholar]
- 29. Kawashima T, Hayashi K, Kosaka A, Kawashima M, Igarashi T, Tsutsui H, Tsuji NM, Nishimura I, Hayashi T, Obata A. 2011. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 11:2017–2024 [DOI] [PubMed] [Google Scholar]
- 30. Nagai T, Makino S, Ikegami S, Itoh H, Yamada H. 2011. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 11:2246–2250 [DOI] [PubMed] [Google Scholar]
- 31. Takeda S, Takeshita M, Kikuchi Y, Dashnyam B, Kawahara S, Yoshida H, Watanabe W, Muguruma M, Kurokawa M. 2011. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 11:1976–1983 [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi N, Saito T, Uematsu T, Kishi K, Toba M, Kohda N, Suzuki T. 2011. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 11:199–203 [DOI] [PubMed] [Google Scholar]