Abstract
We isolated a Bacillus sp. strain that could display broad-spectrum biofilm inhibition. The broad biofilm prevention could be achieved mainly by direct contact between inhibitor and target cells or was accompanied by an interaction with secreted inhibitory compounds. The repression of cell surface fimbria-like appendages of a biofilm producer was also observed; this was considered to contribute to the reduction in mixed biofilms.
TEXT
Bacterial biofilm can cause enhanced risks of health threats and a multitude of industrial problems (1). The traditional strategy for biofilm prevention is antimicrobial agent application. However, the performance of a biocidal approach is somewhat limited by biofilm hyperresistance phenotypes (1). As a result, the development of nonantimicrobial antibiofilm approaches, which focus on the direct limitation of bacterial surface adhesion and biofilm formation, is increasing (2). Recent studies have suggested that many microbes secrete nonantibiotic compounds within bacterial communities, including signaling antagonists (3, 4), active biosurfactants (5, 6), and enzymes (7–9), which may regulate biofilm architecture or modulate bacterial interaction. In mixed bacterial communities, bacteria communicate with one another in various ways. Besides signaling molecule secretion, cellular communication can also occur through contact during a negative competitive interaction. This phenomenon has been observed in Escherichia coli cells, which touch other bacteria and inhibit bacterial growth, and has been termed contact-dependent growth inhibition (10).
In this study, we demonstrated that a bacterium, Bacillus sp. strain SW9, exhibited broad-spectrum biofilm inhibition characteristics in mixed culture biofilms. Interestingly, the biofilm inhibition task was achieved mainly via direct cell-to-cell contact without affecting bacterial growth and was accompanied by interaction with secreted inhibitory molecules. The evidence for this inhibition was subsequently investigated.
Bacillus sp. SW9 displays broad-spectrum antibiofilm activity toward biofilm-forming bacteria.
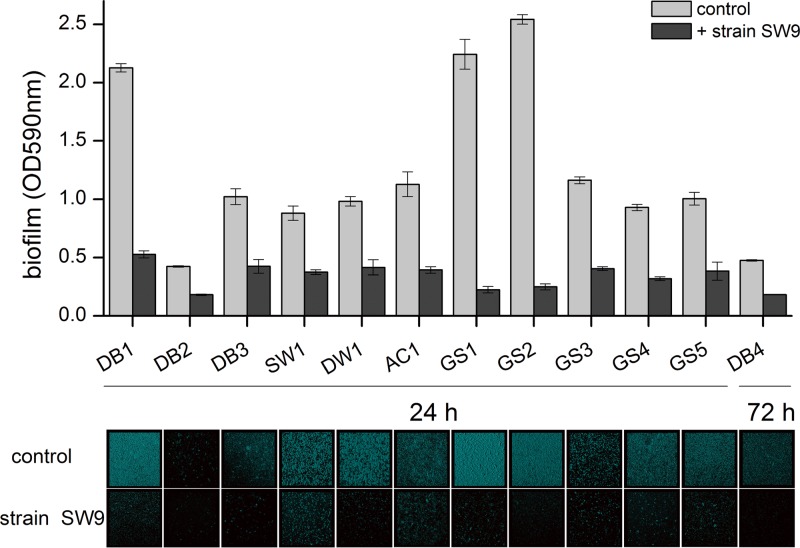
More than 70 bacterial strains were isolated from different water treatment environments: source water, tap water, biofilms attached to the granular activated carbon in a full-scale drinking-water biofilter (Pinghu, China), drinking-water biofilms attached to the pipeline in a drinking-water distribution system (Ningbo, China), and granule sludge in a simulated reactor treating artificial wastewater. Thirteen strains, including the inhibitory bacterial strain (Bacillus sp. SW9) that cannot form biofilm and 12 bacterial strains with strong biofilm-forming ability, were used in this study (Table 1). The monospecies and dual-species biofilms of these biofilm formers and their mixed counterparts with Bacillus sp. SW9 were assayed by the use of 96-well polyvinylchloride (PVC) microtiter plates and R2A medium at 28°C as described previously (11). After 24 h of incubation, the biofilms were stained with crystal violet, the dye was dissolved with ethanol, and the biofilm biomass was determined by measuring its absorbance at 590 nm. Results showed that in the presence of strain SW9, the biofilm formation capacities of all tested biofilm-forming bacteria were significantly reduced (Fig. 1). The biofilm biomasses were reduced 57.0% to 90.2% compared to their controls (P < 0.001). This suggests that Bacillus sp. SW9 exhibited strong biofilm inhibitory activity against various species of biofilm-forming bacteria.
Table 1.
Identities and sources of the bacterial strains
| No. | Strain | Identity | Source |
|---|---|---|---|
| 1 | DB1 | Pleomorphomonas oryzae | Drinking-water biofilm |
| 2 | DB2 | Acidovorax ebreus | Drinking-water biofilm |
| 3 | DB3 | Acidovorax sp. | Drinking-water biofilm |
| 4 | DB4 | Bradyrhizobium sp. | Drinking-water biofilm |
| 5 | SW1 | Brevundimonas sp. | Drinking-water source |
| 6 | DW1 | Brevundimonas sp. | Drinking water |
| 7 | AC1 | Brevibacillus sp. | Activated carbon granule |
| 8 | GS1 | Acinetobacter sp. | Granule sludge |
| 9 | GS2 | Thauera sp. | Granule sludge |
| 10 | GS3 | Flavobacterium sp. | Granule sludge |
| 11 | GS4 | Sphingopyxis sp. | Granule sludge |
| 12 | GS5 | Comamonas sp. | Granule sludge |
| 13 | SW9 | Bacillus sp. | Drinking-water source |
Fig 1.
Effect of Bacillus sp. SW9 on bacterial biofilm formation. Bacterial biofilms of various species were developed in the presence or absence of the bacterium Bacillus sp. SW9 in a 96-well microtiter plate. The plate was incubated at 28°C for a period of 24 or 72 h. Quantitative assays are shown at the top. Experiments were conducted in triplicate, and error bars represent standard deviations (SD). Confocal micrographs are shown at the bottom.
Biofilm inhibition happens by direct cell-to-cell contact with Bacillus sp. SW9, accompanied by interaction with diffusible inhibitory molecules.
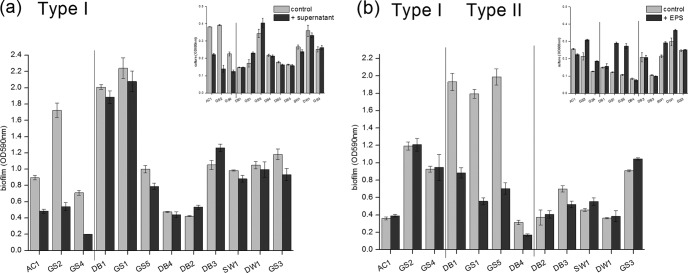
In mixed-species biofilms, biofilm prevention can be achieved either by responding to secreted inhibitory compounds or via direct contact with the inhibitor cells (10, 12). To determine whether Bacillus sp. SW9 secreted factors that inhibited biofilm formation, we tested the effects of filter-sterilized supernatant and capsular extracellular polymeric substances (EPS) from its stationary-phase culture on biofilm formation. The results showed that these two secreted bacterial compounds displayed completely different biofilm inhibition patterns (Fig. 2; see also Table S1 in the supplemental material). With the addition of bacterial supernatant, measurable inhibition of planktonic growth and biofilm formation was detected for three of the tested strains (type I strains), indicating that one group of inhibitory compounds were rich in the supernatant and had both bactericidal and biofilm-inhibiting effects on some bacteria (Fig. 2a). Meanwhile, in the presence of bacterial capsular EPS, biofilm inhibition was observed without the cell viability being affected for another four tested strains (type II strains), implying that another group of inhibitory compounds were rich in the capsular EPS and might behave like a biosurfactant by shielding bacterial surface characteristics and thus inhibit biofilm formation by some bacteria (13–15). Furthermore, the supernatant and capsular EPS of a coculture also exhibited an inhibition pattern similar to that of their single-culture counterpart. This ruled out the presence of inhibitory compounds with respect to the simultaneous appearance of both bacteria (data not shown). However, both secreted bacterial compounds failed to inhibit biofilm formation for all the tested strains, suggesting that other mechanisms might be more important for the broad-spectrum biofilm inhibition by Bacillus sp. SW9.
Fig 2.
Effect of extracellular substances of Bacillus sp. SW9 on bacterial biofilm formation. Supernatant (a) and capsular EPS (b). The types of biofilm-forming bacteria were cultured with different Bacillus sp. SW9 extracts and were grown for 24 h at 28°C. Experiments were conducted in triplicate, and error bars represent SD.
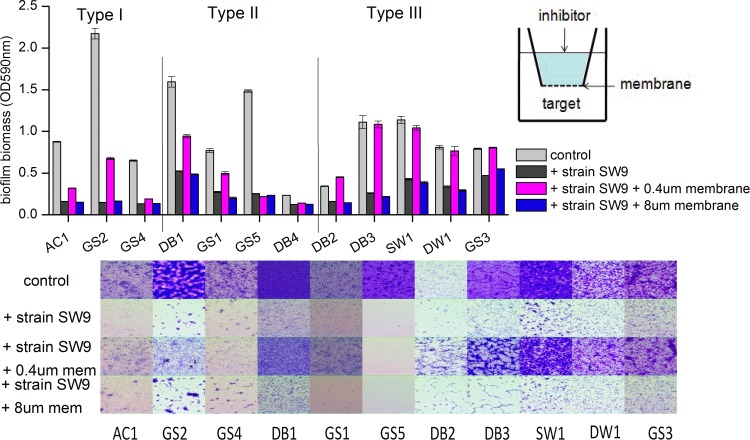
Next, we examined the possibility that contact between the inhibitor and the target bacterium might induce broad-spectrum biofilm prevention. The complexes were cultured in 24-well polystyrene plates and separated by porous polyethylene terephthalate (PET) membranes. The biofilm inhibition was partially or completely abolished when contact between inhibitor and target cells was blocked by 0.4-μm pores; however, complete inhibition of biofilms occurred when 8-μm pores were used, allowing inhibitor and target cells to mix (Fig. 3; see also Table S2 in the supplemental material). Interestingly, in the case of type I and II strains, coculture with 0.4-μm membrane separation did not significantly increase the biofilm-forming bacterial biofilms to the same extent as that of the monoculture, which indicated that Bacillus sp. SW9 might secrete some small diffusible molecules to inhibit biofilm formation by these strains. This result was consistent with our earlier conclusion that some active agents from the supernatant and capsular EPS of Bacillus sp. SW9 were responsible only for narrow-spectrum biofilm inhibition. Nevertheless, significant differences in biofilm biomass between cocultures with 0.4-μm and 8.0-μm filters were observed for most tested bacteria, with the exception of strain GS5. In addition, biofilm inhibition was not accompanied by growth defects through the spread-plate method (data not shown). These results support the hypothesis that the broad-spectrum biofilm prevention mediated by the bacterium Bacillus sp. SW9 required direct cell-to-cell contact between inhibitor and target cells. Here, strains relying solely on contact-dependent biofilm inhibition were defined as type III strains. For strain GS5, biofilm prevention is more likely due to the strong effect of diffusible inhibitory compounds. Competitive interaction through direct cell contact between bacteria is not without precedent. Aoki et al. (10) described a contact-dependent growth inhibition system in which certain pathogenic Escherichia coli strains bound to and inhibited the growth of susceptible target cells within a mixed bacterial population. Recently, such growth inhibition has been identified in some other strains (16–18). However, the present work is the first known report on the system of contact-dependent biofilm inhibition without growth defects. The presence of Bacillus sp. SW9 would lead to population shifts of biofilms in the environment because some dominant biofilm-forming bacteria might be expelled and others might refill their niches. However, the role of strain SW9 in environmental biofilms has yet to be investigated, since the microbial community structure of environmental biofilms is extremely complicated.
Fig 3.
Contact-dependent biofilm inhibition with Bacillus sp. SW9. Inhibitor strain SW9 was added to the top chamber of a 24-well plate containing either a 0.4-μm or an 8-μm PET membrane. Target biofilm-forming bacteria were added to the bottom well. The plate was incubated at 28°C for a period of 24 h. Quantitative assays are shown at the top. Experiments were conducted in triplicate, and error bars represent SD. Micrographs are shown at the bottom.
Bacillus sp. SW9 induces cell surface alteration.
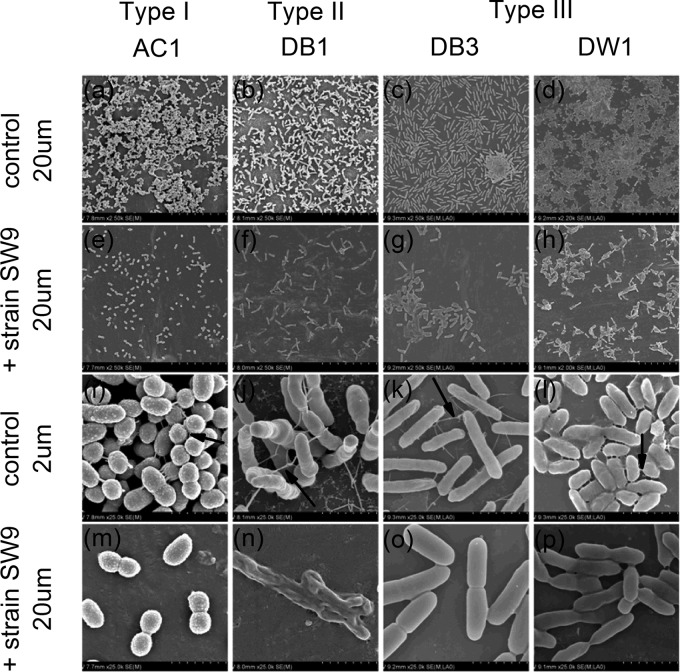
Cell surface extracellular appendages such as flagella, pili, and fimbriae are important to the biofilm formation process (19). Some antibiofilm molecules, which are secreted by bacteria and are devoid of antibacterial activity against free-living cells, were reported to repress the expression of fimbrial genes and further to prevent biofilm formation. For instance, the surface arginine deiminase of a Streptococcus sp. abolished Porphyromonas gingivalis biofilm by downregulating the expression of both short and long fimbriae (20–22). In another study, Lactobacillus acidophilus strains released exopolysaccharides, acting as signaling molecules to affect genes related to curli formation and further prevent biofilm formation by a wide range of Gram-negative and -positive bacteria (23). Our scanning electron microscopy (SEM) results revealed that the individual type III biofilm-forming strains formed much denser biofilms (Fig. 4a to d) than the biofilms that were developed by cocultures of the strains with Bacillus sp. SW9 (Fig. 4e to h). Examination of the monospecies biofilms at higher magnification showed that the cells involved were well connected by filaments (Fig. 4i to l). These filaments emanated from the cells and extended to the abiotic surfaces, as well as to other cells, thus promoting the cell-to-surface and cell-to-cell interactions. In contrast, such filamentous structures were occasionally detected in multispecies biofilms, and cells were tightly aggregated with the extracellular matrix (Fig. 4m to p). It is thus likely that the antibiofilm effect was induced by Bacillus sp. SW9-regulated fimbrial repression.
Fig 4.
SEM micrographs of biofilms developed by biofilm-forming strains with or without Bacillus sp. SW9 on PVC surfaces. (a to d) Dense biofilms developed by the biofilm-forming bacteria; scale bar, 20 μm. (e to h) Loose biofilms developed by the coculture of biofilm-forming bacteria and strain SW9; scale bar, 20 μm. (i to l) Cells were well connected with filaments (indicated by arrows); scale bar, 2 μm. (m to p) Cells were tightly bound with extracellular matrix or loosely connected with fewer filaments; scale bar, 2 μm.
In summary, we isolated a certain bacterium, Bacillus sp. SW9, that could inhibit the biofilm formation of a wide range of bacteria via direct cell-to-cell contact or in combination with secreted inhibitory compounds. Additionally, the fimbrial repression was considered to be responsible for the broad-spectrum biofilm inhibition. Further investigation should include the molecular basis underlying fimbrial repression and biofilm inhibition, which might involve the exploration of a novel strategy in controlling bacterial biofilm formation.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the National Hitech R&D Program (2012AA062607), the National Natural Science Foundation of China (51078343 and 51108440), Fujian Provincial Natural Science Foundation (2009J06028), and the Chinese Academy of Sciences (KZCX2-YW-T08 and KZCX2-YW-JC406 and the 100 Talents Program).
Footnotes
Published ahead of print 21 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02796-12.
REFERENCES
- 1. Nadell CD, Xavier JB, Foster KR. 2009. The sociobiology of biofilms. FEMS Microbiol. Rev. 33:206–224 [DOI] [PubMed] [Google Scholar]
- 2. Kaplan JB. 2010. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 89:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shepherd RW, Lindow SE. 2009. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Appl. Environ. Microbiol. 75:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bijtenhoorn P, Schipper C, Hornung C, Quitschau M, Grond S, Weiland N, Streit W. 2011. BpiB05, a novel metagenome-derived hydrolase acting on N-acylhomoserine lactones. J. Biotechnol. 155:86–94 [DOI] [PubMed] [Google Scholar]
- 5. Boles BR, Thoendel M, Singh PK. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 57:1210–1223 [DOI] [PubMed] [Google Scholar]
- 6. Irie Y, O'Toole GA, Yuk MH. 2005. Pseudomonas aeruginosa rhamnolipids disperse Bordetella bronchiseptica biofilms. FEMS Microbiol. Lett. 250:237–243 [DOI] [PubMed] [Google Scholar]
- 7. Boyd A, Chakrabarty AM. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:2355–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J. Bacteriol. 185:4693–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu TK, Collins JJ. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 104:11197–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248 [DOI] [PubMed] [Google Scholar]
- 11. Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175–179 [DOI] [PubMed] [Google Scholar]
- 12. Gillor O, Kirkup BC, Riley MA. 2004. Colicins and microcins: the next generation antimicrobials. Adv. Appl. Microbiol. 54:129–146 [DOI] [PubMed] [Google Scholar]
- 13. Schembri MA, Dalsgaard D, Klemm P. 2004. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 186:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valle J, Da Re S, Henry N, Fontaine T, Balestrino D, Latour-Lambert P, Ghigo JM. 2006. Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 103:12558–12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J. Bacteriol. 193:3879–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemonnier M, Levin BR, Romeo T, Garner K, Baquero MR, Mercante J, Lemichez E, Baquero F, Blázquez J. 2008. The evolution of contact-dependent inhibition in non-growing populations of Escherichia coli. Proc. Biol. Sci. 275:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dassanayake RP, Call DR, Sawant AA, Casavant NC, Weiser GC, Knowles DP, Srikumaran S. 2010. Bibersteinia trehalosi inhibits the growth of Mannheimia haemolytica by a proximity-dependent mechanism. Appl. Environ. Microbiol. 76:1008–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sawant AA, Casavant NC, Call DR, Besser TE. 2011. Proximity-dependent inhibition in Escherichia coli isolates from cattle. Appl. Environ. Microbiol. 77:2345–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie H, Cook GS, Costerton JW, Bruce G, Rose TM, Lamont RJ. 2000. Intergeneric communication in dental plaque biofilms. J. Bacteriol. 182:7067–7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie H, Lin X, Wang Wu B-YJ, Lamont RJ. 2007. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 153:3228–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christopher AB, Arndt A, Cugini C, Davey ME. 2010. A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development. Microbiology 156:3469–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y, Oh S, Kim SH. 2009. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157: H7. Biochem. Biophys. Res. Commun. 379:324–329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.