Abstract
Escherichia coli O157:H7 survived longer in soils from plastic-greenhouse cultivation than soils from the open field. Soil pH, organic carbon levels, and the ratio of bacterial phospholipid fatty acids (PLFAs) to fungal PLFAs played the significant roles in survival of O157:H7. Greater attention should be paid to the control of pathogen contamination under conditions of plastic-greenhouse cultivation.
TEXT
Escherichia coli O157:H7 is dangerous because of its resistance to low pH (2.5), its low infective dose (as few as 10 cells), and its high pathogenicity (1). Raw fruits and vegetables, especially leafy greens that are consumed raw, are well-known vehicles for transmitting O157:H7 and may be polluted via field application of raw manure or contaminated irrigation water (2, 3). O157:H7 survival in soils from vegetable field should be considered an important factor for the likelihood of vegetable contamination. In China, plastic-greenhouse cultivation of vegetables is becoming more popular because of the higher growth rate and yield. However, little is known about the effects of various soil cultivation practices on the survival of O157:H7. The aim of this study was therefore to assess its survival in soils from a vegetable field under conditions of different cultivation patterns.
Five soils (S0, S1, S4, S7, and S15) were collected from a depth of 0 to 20 cm in fields (sites) that were under conditions of various lengths of time (0, 1, 4, 7, and 15 years, respectively) of plastic-greenhouse cultivation in the same area. Three replicates were sampled for each site. The selected soil properties are listed in Table 1. O157:H7 cells were inoculated into the soil samples and gently mixed to establish a cell density of about 106 CFU per gram of dry weight (CFU · g−1). Forty grams (measured on the basis of analysis of oven-dried samples) of inoculated soil was placed in a 50-ml sterile plastic tube. All tubes (triplicates for each site, plus uninoculated controls) were incubated in the dark at 25 ± 1°C with the field capacity (soil water content) at −33 kPa. Sterile deionized water was added to the tubes every 2 days to maintain constant soil moisture status during the incubation. A soil subsample was taken from each tube at 0, 0.04, 1, 3, 5, 7, 10, 15, and 25 days after inoculation and subjected to 10-fold serial dilutions with 0.1% peptone buffer. The last three of the serial dilutions per sample were plated onto sorbitol SMAC-BCIG (sorbitol MacConkey–5-bromo-4-chloro-3-indoxyl-β-d-glucuronide) agar containing 100 μg · ml−1 of rifampin and 25 μg · ml−1 of nalidixic acid for enumeration (37°C, 16 h). The sampling was stopped after O157:H7 cells below the detection limit (approximately 100 CFU · g−1) appeared twice in succession during the incubation. Survival of O157:H7 was modeled by fitting the experimental data to the Weibull survival function proposed by Mafart et al. (4). The time needed to reach the detection limit (td) was calculated from the Weibull survival function.
Table 1.
Land use history and selected properties of the test soilsa
| Soil code | BP (nmol/g) | FP (nmol/g) | TP (nmol/g) | pH | WSOC (mg/kg) | MBC (mg/kg) | AP (mg/kg) | AK (mg/kg) | OC (g/kg) | % TN | % FC | % clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | 35.8 | 6.4 | 91.9 | 8.16 | 68.2 | 164.5 | 49.4 | 228.5 | 20.2 | 0.1 | 25.8 | 8.9 |
| S1 | 18.2 | 3.4 | 51.2 | 8.00 | 35.5 | 325.9 | 67.5 | 111.0 | 12.1 | 0.8 | 23.1 | 6.2 |
| S4 | 26.5 | 4.7 | 73.9 | 8.31 | 73.5 | 106.8 | 68.6 | 68.0 | 11.8 | 0.1 | 25.1 | 8.0 |
| S7 | 30.0 | 5.2 | 77.2 | 8.14 | 64.8 | 98.8 | 91.4 | 156.0 | 8.3 | 0.2 | 25.8 | 5.7 |
| S15 | 23.8 | 4.0 | 56.6 | 7.85 | 53.5 | 380.3 | 100.4 | 161.0 | 13.0 | 0.1 | 23.0 | 0.4 |
S0, S1, S4, S7, and S15 represent the soil samples from a vegetable field with various lengths of plastic-greenhouse cultivation (0, 1, 4, 7, and 15 years, respectively); BP, bacterial PLFAs; FP, fungal PLFAs; TP; total PLFAs; WSOC, water-soluble organic carbon; MBC, microbial-biomass carbon; AP, available phosphorus; AK, available potassium; OC, organic carbon; TN, total nitrogen; FC, field capacity (soil water content at −33 kPa).
Enumeration analysis indicated that there was no O157:H7 present in the noninoculated control samples during the entire incubation period. The Weibull model can fit the experimental data very well for the test soils with a mean R2 of 0.979, and the model parameters p and δ ranged from 0.88 to 1.36 and from 4.01 to 7.56, respectively. The average survival time (td) of O157:H7 for the test soils under conditions of plastic-greenhouse cultivation was 21.9 days, which is higher than that of the open-field soils (S0, 17.9 days).
Variations of survival dynamics were observed from different soils (Fig. 1). At the beginning of inoculation, the decline rates among the five soils were similar. After 7 days, O157:H7 declined the slowest in S15 (P < 1) and the fastest in S0 (P > 1). S0 with higher levels of phospholipid fatty acids (PLFAs) might contribute to the mortality of O157:H7. Our observation is in agreement with previous reports that ecosystems with lower microbial community complexity are more susceptible to bacterial invasion and more amenable to invaders than those with higher microbial community complexity (5).
Fig 1.
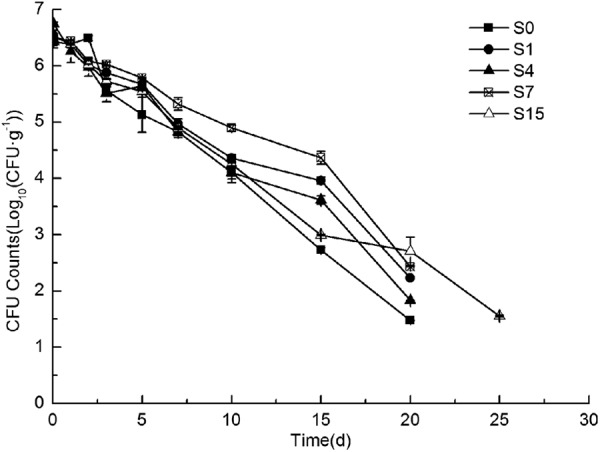
Survival of Escherichia coli O157:H7 in the test soils. The error bars are ± the standard errors. CFU · g−1, the CFU per gram of dry soil; d, days. The soil codes of S0, S1, S4, S7, and S15 in the figure are the same as shown in Table 1.
Simple correlation and stepwise multiple regression analysis further suggested that the abundance of PLFAs displayed a negative effect on O157:H7 survival in the test soils. Additionally, not only the microbial diversity but also certain groups of microorganisms might contribute to the suppression of invading pathogens. Results from the statistical analyses showed that 18:1ω7c, 16:1ω7c/15 iso 2OH, 18:0 iso, and 18:0 ante/18:2ω6,9c PLFAs were the most important ones that can significantly impact O157:H7 survival in the test soils. The results also suggest that soil microorganisms containing these PLFAs might play a more important role in O157:H7 survival.
Simple correlation analysis was performed to evaluate the effect of soil properties on O157:H7 survival in the test soils. Significantly negative correlations between td and organic carbon (OC, r = −0.856), clay (r = −0.727), pH (r = −0.526), bacterial PLFAs (r = −0.678), and total PLFAs (r = −0.698) and positive correlations between available phosphorus (r = 0.795) and sand contents (r = 0.797) were observed. Further studies of the relationships between td and soil properties were conducted using stepwise multiple regression analysis (Table 2). Coefficients of both multiple correlation and partial regression reached at least the 0.05 significance level. Stepwise regression analysis showed that soil pH and OC and the ratio of bacterial PLFAs to fungal PLFAs (B/F) were the most important factors impacting O157:H7 survival in the test soils. The td values were lower in soils with a higher OC because of a higher activity of indigenous microbial community and adsorption (6–9). Soil pH was positively correlated with clay contents (r = 0.836) and with the total PLFAs (r = 0.638). The combined effect of high clay content, the stronger indigenous microorganisms, and high pH value resulted in a reduced survival capacity of O157:H7 in the test soils. Path analysis results (Fig. 2) further imply that pH and OC (the abiotic factors) play a more important role in controlling O157:H7 survival in soils than the biotic factors (B/F) do.
Table 2.
Stepwise multiple-liner regression analysis of soil properties and the survival time of Escherichia coli O157:H7 in soilsa
| Regression equation | R2 | F value | Parameter or T value of the partial regression coefficient |
|
|---|---|---|---|---|
| Parameter | T value | |||
| td = 75.017 − 5.807 × pH − 0.386 × OC − 0.328 × B/F | 0.987 | 273.868c | Constant | 21.953c |
| pH | −14.538c | |||
| OC | −24.238c | |||
| B/F | −2.373b | |||
td (survival time), time needed to reach the detection limit, 100 CFU per gram (dry weight) of soil; OC, organic carbon; B/F, the ratio of bacterial PLFAs to fungal PLFAs.
Correlation is significant at the 0.05 probability level.
Correlation is significant at the 0.001 probability level.
Fig 2.
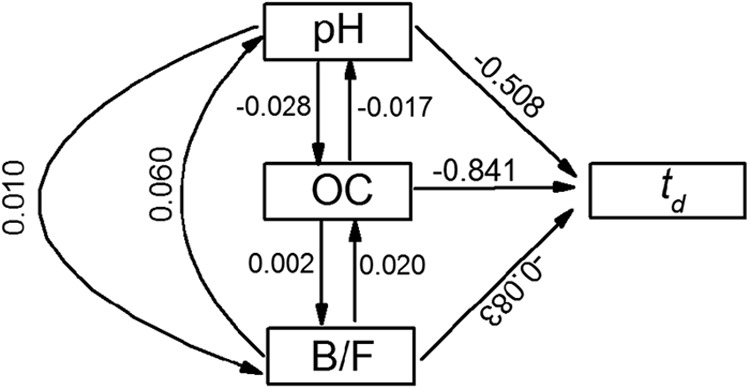
Path analysis for the effects of soil properties on the survival time (td) of Escherichia coli O157:H7 in soils. td, time needed to reach the detection limit, 100 CFU per gram of soil dry weight; OC, organic carbon; B/F, the ratio of bacterial PLFAs to fungal PLFAs.
Our results highlight a higher contamination risk of O157:H7 under conditions of plastic-greenhouse cultivation with a longer survival time. Therefore, it is necessary to properly manage farm wastes such as manure to control O157:H7 dissemination into open environments, especially under conditions of plastic-greenhouse cultivation.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (no. 40971255).
Footnotes
Published ahead of print 4 January 2013
REFERENCES
- 1. Tilden J, Jr, Young W, McNamara AM, Custer C, Boesel B, Lambert-Fair MA, Majkowski J, Vugia D, Werner SB, Hollingsworth J, Morris JG., Jr 1996. A new route of transmission for Escherichia coli: infection from dry fermented salami. Am. J. Public Health 86:1142–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fremaux B, Prigent-Combaret C, Vernozy-Rozand C. 2008. Long-term survival of Shiga toxin-producing Escherichia coli in cattle effluents and environment: an updated review. Vet. Microbiol. 132:1–18 [DOI] [PubMed] [Google Scholar]
- 3. Hilborn ED, Mermin JH, Mshar PA, Hadler JL, Voetsch A, Wojtkunski C, Swartz M, Mshar R, Lambert-Fair MA, Farrar JA, Glynn MK, Slutsker L. 1999. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 159:1758–1764 [DOI] [PubMed] [Google Scholar]
- 4. Mafart P, Couvert O, Gaillard S, Leguerinel I. 2002. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 72:107–113 [DOI] [PubMed] [Google Scholar]
- 5. Matos A, Kerkhof L, Garland JL. 2005. Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microb. Ecol. 49:257–264 [DOI] [PubMed] [Google Scholar]
- 6. Calvet R. 1989. Adsorption of organic chemicals in soils. Environ. Health Perspect. 83:145–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klánová K. 1994. Effect of chemical fertilizers on the transport of Escherichia coli, Pseudomonas aeruginosa and Salmonella infantis through sand columns. Folia Microbiol. (Praha) 39:283–286 [DOI] [PubMed] [Google Scholar]
- 8. Fisk AC, Murphy SL, Tate RL. 1999. Microscopic observations of bacterial sorption in soil cores. Biol. Fertil. Soils 28:111–116 [Google Scholar]
- 9. Liu B, Tu C, Hu S, Gumpertz M, Ristaino JB. 2007. Effect of organic, sustainable, and conventional management strategies in grower fields on soil physical, chemical, and biological factors and the incidence of Southern blight. Appl. Soil Ecol. 37:202–214 [Google Scholar]


