Abstract
Synechococcus elongatus strain PCC 7942 strictly depends upon the generation of photosynthetically derived energy for growth and is incapable of biomass increase in the absence of light energy. Obligate phototrophs' core metabolism is very similar to that of heterotrophic counterparts exhibiting diverse trophic behavior. Most characterized cyanobacterial species are obligate photoautotrophs under examined conditions. Here we determine that sugar transporter systems are the necessary genetic factors in order for a model cyanobacterium, Synechococcus elongatus PCC 7942, to grow continuously under diurnal (light/dark) conditions using saccharides such as glucose, xylose, and sucrose. While the universal causes of obligate photoautotrophy may be diverse, installing sugar transporters provides new insight into the mode of obligate photoautotrophy for cyanobacteria. Moreover, cyanobacterial chemical production has gained increased attention. However, this obligate phototroph is incapable of product formation in the absence of light. Thus, converting an obligate photoautotroph to a heterotroph is desirable for more efficient, economical, and controllable production systems.
INTRODUCTION
All cyanobacteria are photosynthetic organisms that utilize light energy for the reduction of carbon dioxide. Certain characterized cyanobacteria have been shown to consume organic molecules for increased biomass in the presence of light, allowing photoorganoheterotrophy, while some cyanobacterial species have been shown to consume organic molecules regardless of light conditions. Other cultured cyanobacterial species are obligate photoautotrophs and as such lack the ability to consume fixed carbon compounds for increased biomass or energy. The precise causes for this inflexible trophic behavior are currently unknown; however, the matter is being studied (1, 2).
Two principal hypotheses have been proposed for obligate photoautotrophy among microorganisms. In some photoautotrophs, the lack of efficient uptake of essential substrates, especially sugars, into the cells may be the major reason for obligate photoautotrophy (3). However, impermeability of membranes to essential nutrients may not be the only factor in obligate photoautotrophy, considering that some photoautotrophs incorporate organic compounds into cellular carbon incapable of sustaining growth (4). It was hypothesized that a biosynthetic or degradative lesion in central metabolism might cause obligate photoautotrophy (5). An incomplete tricarboxylic acid (TCA) cycle may be a vital reason in some phototrophic microorganisms. Wood et al. found that most photoautotrophic organisms lack at least one of the three components of 2-ketoglutarate dehydrogenase complex causing an incomplete TCA cycle (2). However, more recently, it has been shown that two alternative enzymes, 2-oxoglutarate decarboxylase and succinic semialdehyde dehydrogenase, convert 2-oxoglutarate to succinate and functionally complete the TCA cycle in cyanobacteria (6). Thus, we hypothesize that efficient uptake of sugars is the major missing factor inhibiting heterotrophy in obligate photoautotrophic cyanobacteria.
Sugar transporters have been exploited to expand trophic behavior and to export valuable biosynthetic products in a variety of organisms (3, 4, 7–11). Zaslavskaia et al. engineered a eukaryotic alga, Phaeodactylum tricornutum, to thrive on exogenous glucose in the absence of light by introducing a gene encoding a glucose transporter from human erythrocytes (Glut1 or Hup1) (3). The P. tricornutum strain expressing the Glut1 gene exhibited high growth rates in the presence of glucose. The cell density attained by the mutants exceeded that of the wild type by 15-fold (3). This result indicates that in some eukaryotic microalgae, the lack of efficient sugar uptake into the cells is a major reason for their obligate photoautotrophy.
Several investigations to examine obligate photoautotrophy in cyanobacteria with recombinant sugar transporter strategies have been attempted, yet a conclusive determination of the causes of photoautotrophy has proven to be elusive. The glcP gene, encoding a glucose transporter from the heterotrophic cyanobacterium, Synechocystis sp. strain PCC 6803, has been expressed from a plasmid-based expression system in Synechococcus elongatus strain PCC 7942 but could not be maintained, while genomic integration led to glucose sensitivity (4). Additionally, the fructose transport and regulation system of filamentous Anabaena variabilis was introduced into the closely related photoautotrophic cyanobacterium Anabaena sp. strain PCC 7120, allowing limited fructose consumption and utilization only in the dark; however, it was later discovered that Anabaena sp. strain PCC 7120 can consume fructose without the introduction of heterologous genes (9, 10). Furthermore, S. elongatus has been engineered to successfully express a sugar transport system to export photosynthetic end products, such as glucose, fructose, and sucrose (7, 8). This work established the availability of all required enzymes for the synthesis of sugars from the metabolic products of the Calvin Benson Bassham cycle. A reasonable assumption is that the reactions may be utilized in reverse for biomass increase from consumption of the corresponding sugar gradient.
Cyanobacteria are being considered as a platform for the conversion of renewable solar energy to biofuels (12–15). Current research supports the idea that cyanobacterial biosynthesis of fuels and industrial chemicals may be one of the most efficient approaches to reduce the need for imported petroleum and to minimize CO2 emissions (16). Cyanobacteria are also a largely untapped source of natural products for drug discovery (17). However, many cyanobacteria are obligate photoautotrophs and thus incapable of increasing biomass in the absence of light energy. Here we focus on the consumption of sugars for the production of cyanobacterial biomass. Glucose is a central metabolite universally used for the storage of energy and is the most plentiful biomolecule on earth in the form of cellulose. Research is investigating efficient harvesting of glucose from cellulose as a feedstock for biofuel production. Sucrose is another important feedstock that is produced on the economic scale needed for common human consumption, as well as being utilized for biologically derived fuels and chemicals. Xylose is the final feedstock of interest addressed herein, plentiful as a major portion of hemicellulosic materials, and is a sugar not metabolized by humans. Probing the limitations of S. elongatus regarding the consumption of each of these common feedstocks gives a greater understanding of the more complex and general obligate photoautotrophic behavior, as well as aiding in the development of industrially relevant and versatile chemical-producing photosynthetic organisms.
MATERIALS AND METHODS
Reagents.
The chemicals glucose, fructose, sucrose, and xylose were obtained from Sigma-Aldrich (St. Louis, MO). Isopropyl-β-d-thiogalactopyranoside (IPTG) was obtained from Fisher Scientific (Hanover Park, IL). Phusion polymerase was purchased from NEB (Ipswich, MA). KOD polymerase was purchased from EMD4Biosciences (San Diego, CA). Spectinomycin was purchased from MP Biomedicals (Santa Ana, CA). Oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (San Diego, CA).
Culture conditions.
All cyanobacterial strains were grown in BG-11 medium (18) at 30°C with shaking at 100 rpm. All medium modifications are described below. Cultures were maintained in a custom cabinet with the dimensions 56 cm by 36 cm by 76 cm. This cabinet was outfitted with 2 compact fluorescent lamp (CFL) natural-spectrum bulbs from Verilux, rated at 26 W. Light fluence rates were 25 μE s−1 m−2. Precultures for diurnal experiments were maintained for at least 72 h under diurnal lighting conditions to ensure proper circadian rhythm. All preculture was grown without the addition of sodium bicarbonate or any saccharide. Growth assays used a total volume of 10 ml of culture in 30-ml test tubes. All assays were conducted using biological triplicates. Cell growth was monitored by measuring the optical density at 730 nm (OD730) for each biological triplicate one time.
For growth assays using the S. elongatus strains, cells in exponential phase were diluted to an OD730 of 0.2 in 10 ml BG-11 medium including 20 μg/ml spectinomycin and 0.1 mM IPTG, with all other modifications described below. Wild-type assays omitted spectinomycin.
Assays were examined for contamination by plating 50 μl of the culture from the final time point of every sample on Luria Bertani (LB) plates. In a few of the samples, slight contamination was detected (<20 colonies out of at least 104 cyanobacterial cells). To further quantify the contamination, bright-field microscopy was utilized. Cells were counted within a Petroff-Hausser counting chamber slide to ensure a constant volume throughout. Counting chambers were chosen randomly, and green cells versus colorless cells were tallied. For all reported results where contamination was detected, green cells were greater than 99% of the culture (n ≥ 500).
Plasmid construction.
All plasmids used in this work are described in Table 1. All primers used are listed in Table 2. All installed genes in this study are listed in Table 3.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| AL257 | Synechococcus elongatus PCC 7942 (wild type) | S. S. Golden |
| AL358 | Ptrc: glcP integrated at NSI | This work |
| AL360 | Ptrc: GLUT1 integrated at NSI | This work |
| AL361 | Ptrc: galP integrated at NSI | This work |
| AL434 | Ptrc: xylE-xylA-xylB integrated at NSI | This work |
| AL504 | Ptrc: gfp integrated at NSI | This work |
| AL505 | Ptrc: galP-gfp integrated at NSI | This work |
| AL535 | Same as AL361 but ΔglgC | This work |
| AL536 | Same as wild type but ΔglgC | This work |
| AL1030 | Ptrc: cscK-cscB integrated at NSI | This work |
| AL1067 | Ptrc: xylE integrated at NSI | This work |
| Plasmids | ||
| pAM2991 | NSI targeting vector; Ptrc | 20 |
| pBBR1MCS-5 | Gmr; broad-host-range vector | 21 |
| pSA69 | P15A ori; Ampr; PLlacO1::alsS-ilvC-ilvD | 19 |
| pAL18 | Same as pAM2991 but Ptrc: GLUT1 lacIq | This work |
| pAL40 | Same as pAM2991 but Ptrc: galP lacIq | This work |
| pAL46 | Same as pAM2991 but Ptrc: glcP lacIq | This work |
| pAL61 | Same as pAM2991 but Ptrc: gfp lacIq | This work |
| pAL63 | Same as pAM2991 but Ptrc: gfp-galP lacIq | This work |
| pAL65 | Same as pAM2991 but Ptrc: xylE lacIq | This work |
| pAL70 | Same as pAM2991 but Ptrc: xylE-xylA-xylB lacIq | This work |
| pAL82 | glgC knockout vector; Gmr | This work |
| pAL288 | Same as pAM2991 but Ptrc: cscK-cscB lacIq | This work |
Table 2.
Oligonucleotides used in this study
| Primer name | Sequence (5′ → 3′) |
|---|---|
| JM5 | CCGGAATTCAATACCCAGTATAATTCCAGTTATATATTTTCGA |
| JM6 | CGGGATCCATCCTAGGTTACAGCGTAGCAGTTTGTTGT |
| JM7 | CGCCTAGGAACTTTAAGAAGGAGATATACCATGCAAGCCTATTTTGACCAGCTCG |
| JM8 | CGGGATCCTTACGCCATTAATGGCAGAAGTTGC |
| JM55 | ATGGAATTCATGTCAGCCAAAGTATGGGT |
| JM56 | GGATCCATTGGGACGTCACCTCCTATATTGCTGAAGGTACAGG |
| JM57 | GAGGTGACGTCATGACGCAATCTCGATTGC |
| JM58 | TAGAGGATCCTTAACCCAGTTGCCAGAGTG |
| JM66 | ATGGAATTCATGGCACTGAATATTCCATT |
| MC127 | CTAACAATTGATGCCTGACGCTAAAAAACAGGGGCG |
| MC128 | CTATAGATCTTTAATCGTGAGCGCCTATTTCGCGCAGTT |
| MC186 | CTATCTCGAGTTAATCGTGAGCGCCTATTTCGCGCAGTT |
| MC187 | CATGCCTGACGCTAAAAAACAGGGGCGGTCA |
| MC188 | TTACGGCCGCTGCCACCGCCGCTACCGCCATCGTGAGCGCCTATTTCGCGCAGTTTACG |
| MC189 | ACGATGGCGGTAGCGGCGGTGGCAGCGGCCGTAAAGGAGAAGAACTTTTCACTGGAGTT |
| MC190 | CTTAGCATGCTTTGTATAGTTCATCCATGCCATG |
| MC191 | CTATGAATTCCGTAAAGGAGAAGAACTTTTCACTGGAGTT |
| MC192 | CTAAGGATCCTTAGCATGCTTTGTATAGTTCATCCATGCCATG |
| IM171 | GCTAGCAGCTCAGATTACGC |
| IM573 | GGTGCTAGCCACCGTGGAAACGGATGAAGG |
| IM574 | CATTTTTGTCGACGCCGGGAAGCCGATCTCG |
| IM581 | GAGTAGGTGGCTACGTCTCC |
| GR005 | CCGCTCGAGTACCAGCGATCCGTGTCCCTACTCG |
| GR006 | CGAGCACGCGTCAATTGCCCTAAGACAGTTGTCGTC |
| GR015 | CGCCGAACTGTTTGAACAGC |
| GR050 | TAGTAACCTCCAGCCTTTTTTGCC |
Table 3.
Installed genes in this study
| Organism | Gene | Function |
|---|---|---|
| E. coli BW25113 | galP | MFS-type d-galactose/H+ transporter |
| xylE | MFS-type d-xylose/H+ transporter | |
| xylA | d-Xylose isomerase | |
| xylB | Xylulokinase | |
| Homo sapiens | GLUT1 | MFS-type d-glucose/H+ transporter |
| Synechocystis sp. PCC 6803 | glcP | MFS-type d-glucose/H+ transporter |
| E. coli ATCC 700927 | cscB | MFS-type d-sucrose/H+ transporter |
| cscK | Fructokinase |
The galP, xylE, xylA, and xylB genes were amplified from Escherichia coli BW25113 genomic DNA. The glcP gene was amplified from Synechocystis sp. PCC 6803 genomic DNA (ATCC). cscB and cscK were amplified from genomic DNA of E. coli ATCC 700927 (ATCC). galP was amplified using the primers MC127 and MC128, digested with MfeI and BgIII, and then ligated with pAM 2991 digested with EcoRI and BamHI to create pAL40. xylE was amplified using the primers JM05 and JM06, digested with EcoRI and BamHI, and ligated with pAM2991 digested with the same enzymes, creating pAL65. xylAB was amplified using the primers JM07 and JM08 and digested with AvrII and BamHI, followed by ligation to similarly digested pAL65 to create pAL70. glcP was amplified using the primers MC170 and MC171, digested with BamHI and EcoRI, and ligated to similarly digested pAM 2991 to create pAL46. cscB and cscK were amplified using the primers JM55 and JM56, digested with EcoRI and BamHI, and ligated to similarly digested pAM2991 to create pAL289.
pAL82 was constructed in order to delete the glgC gene from the S. elongatus chromosome (see Fig. S1 in the supplemental material). The region for homologous recombination (590,459 to 593,751) was amplified from S. elongatus genomic DNA using the primers GR005 and GR006. The product was digested with XhoI and MluI and ligated with similarly digested pSA69 (19), creating pGR01. To clone the gentamicin resistance gene, pBBR1MCS-5 (21) was used as the PCR template with the primers IM573 and IM574. The PCR product was digested with SalI and NheI and ligated with pGR01 cut with the same enzyme, creating pAL82. The correct deletion of glgC was confirmed by PCR with the primers GR050 and IM581 and IM573 and GR015 (see Fig. S1B). The complete segregation of ΔglgC was confirmed by PCR using the primers IM171 and GR015 (see Fig. S1C).
Transformation of S. elongatus.
Transformation of S. elongatus was performed as previously described (22). Strains were segregated several times by transferring colonies to fresh selective plates. Correct recombinants were confirmed by PCR and DNA sequencing to verify integration of targeting genes into the chromosome at NSI (see Fig. S2 in the supplemental material) (23). The strains used and constructed are listed in Table 1.
Confocal microscopy.
All confocal microscope images were taken using the Olympus_America FV1000 system located within the UCD NEAT Spectral Imaging Facility. A 488-nm laser was used for excitation of all mutants. The emission filter was set 500 nm to 600 nm. The pinhole aperture was set to 100 μm. The laser percentage was set to 11.5%. Cells were placed in glass-bottom dishes for imaging.
GFP assay on plate reader.
All green fluorescent protein (GFP) assays were done using a Microtek Synergy H1 plate reader (BioTek). BG-11 medium only was used as a blank, and excitation and emission wavelengths were set to 485 and 528 nm, respectively.
Glucose and xylose consumption assays.
Glucose and xylose concentrations in the culture medium were measured using a high-performance liquid chromatograph (Shimadzu) equipped with an Aminex HPX-87 column (Bio-Rad) and a refractive index detector. Samples were centrifuged and filtered using FiltrEX filter 96-well plates (Corning).
RESULTS AND DISCUSSION
Growth with glucose.
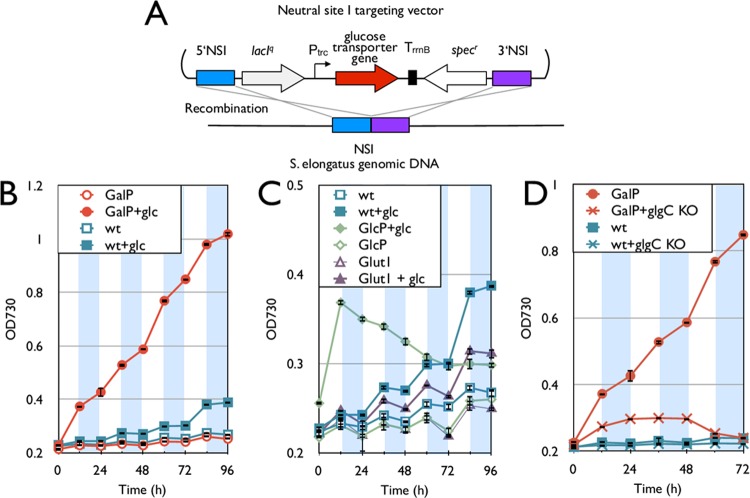
We tested a possible means to complement the inability of S. elongatus to grow in the dark based on our hypothesis that efficient uptake of sugars is a missing factor for heterotrophy. Glucose is a common energy storage molecule in S. elongatus in the form of glycogen (24–27). Glycogen is built up within the cell throughout the light phase of metabolism and then used as an energy source to maintain essential chemical processes throughout the dark phase (26). Therefore, all the required genes for the breakdown of endogenous glucose ought to be present in S. elongatus. We installed three glucose transporter genes from a variety of organisms into the S. elongatus chromosome in an attempt to confer heterotrophic behavior under diurnal conditions (Fig. 1). These transporters include GlcP from Synechocystis sp. PCC 6803 (28), GalP from Escherichia coli (29), and Glut1 from human erythrocytes (30). Only the eukaryotic gene for Glut1 was codon optimized for S. elongatus. Each gene was integrated into the S. elongatus chromosome at neutral site I (NSI) under the control of an isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible promoter, Ptrc (Fig. 1A) (22). Growth of the resulting three strains and the wild type was measured under diurnal illumination conditions in the presence and absence of glucose (Fig. 1B and C). To amplify the growth difference between strains that could and could not grow on extracellular glucose, conditions were set so as to limit the amount of CO2 (no bubbling) and light intensity (25 μE/m2/s).
Fig 1.
Installation of glucose transporter to S. elongatus. (A) Schematic representation of glucose transporter gene integration into the S. elongatus genome. (B) Growth curve of the galP strain (red) and wild type (blue) with and without 5 g/liter glucose. (C) Growth curve of the Glut1 (purple) and glcP (green) strains and the wild type. (D) Growth curve of the galP strain and the wild type with and without the glgC deletion (glgC KO). For panels B, C, and D, empty symbols are samples in BG-11 medium without glucose, while solid symbols indicate results with BG-11 containing 5 g/liter glucose. White and shaded areas indicate the light and dark cycles, respectively. All y axes denote OD730,though the scales differ for visibility. Error bars represent standard deviations (in triplicate). NSI, neutral site 1.
Throughout Results/Discussion, growth rate values reported from the light and dark cycles are derived from the 48-to-60-h and 60-to-72-h periods, respectively (growth rates for all periods, 0 to 96 h, are listed in Table 4). The baseline growth rate of wild-type S. elongatus based on the OD730 was 0.161 day−1 during the light cycle (48 h to 60 h) with no growth during the dark cycle (Fig. 1B and Table 4). The wild type grew at a higher rate (0.204 day−1) when in the presence of glucose and light, yet exhibited no growth in the dark cycle (Fig. 1B). No changes in cellular morphology, such as size and shape of the cells grown with or without glucose, were detected by microscopic observation (magnification, 1,000×). The only engineered strain to show a consistent increase in the growth rate in the presence of glucose contained galP (denoted as the galP strain) (Fig. 1B). In addition to increased growth compared to that of the wild type, the galP strain showed growth even during dark periods, whereas the wild type showed no growth (Fig. 1B and C). The growth rates of the galP strain under light and dark conditions in the presence of glucose were 0.540 day−1 and 0.199 day−1, respectively. However, the glucose consumption of the galP strain after 96 h was not detectable with HPLC analysis. Calculations using OD730 and cell counts predict the total change in dry biomass to be around 0.1 g/liter. Thus, using HPLC analysis, there was a high level of difficulty in detecting such a small change in the glucose concentration over a 96-h period while taking into account evaporation of water. The strain containing glcP showed increased growth (0.730 day−1) during the first light phase, but the growth stopped after that, which is consistent with the previous results (Fig. 1C) (4, 9). The strain containing GLUT1 exhibited growth similar to that of the wild type (Fig. 1C). All growth assays were confirmed to be free of any significant contamination through microscopy and plating analyses upon completion (see Materials and Methods). These results demonstrate that S. elongatus can metabolize glucose for growth once it is transported into the cells. While each of these three heterologously expressed transporters is part of the major facilitator superfamily (MFS), each had a varied effect upon cell growth. We can assume these variations are related to the ability of these membrane proteins in S. elongatus to be adequately expressed, folded, and localized, as well as differences in enzymatic activity. Thus, the difficulty of predicting heterologous enzymatic activity, especially for membrane proteins, remains high.
Table 4.
Growth rates under diurnal conditionsa
| Strain | OE | KO | Sugar | Growth rate (day−1) for time period (h), condition |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–12, L | 12–24, D | 24–36, L | 36–48, D | 48–60, L | 60–72, D | 72–84, L | 84–96, D | ||||
| AL257 | 0.082 ± 0.064 | ND | 0.110 ± 0.025 | ND | 0.161 ± 0.030 | ND | 0.163 ± 0.026 | ND | |||
| AL257 | Glc | 0.128 ± 0.008 | ND | 0.238 ± 0.017 | ND | 0.204 ± 0.018 | ND | 0.469 ± 0.012 | ND | ||
| AL536 | glgC | Glc | ND | ND | ND | ND | ND | ND | NA | NA | |
| AL257 | Suc | 0.298 ± 0.010 | 0.081 ± 0.007 | 0.319 ± 0.015 | ND | 0.310 ± 0.017 | 0.087 ± 0.027 | 0.298 ± 0.025 | 0.071 ± 0.019 | ||
| AL257 | Xyl | 0.149 ± 0.021 | 0.104 ± 0.017 | 0.285 ± 0.012 | ND | 0.350 ± 0.017 | 0.062 ± 0.012 | 0.446 ± 0.016 | 0.098 ± 0.016 | ||
| AL361 | galP | 0.128 ± 0.017 | ND | 0.067 ± 0.010 | ND | 0.126 ± 0.018 | ND | 0.158 ± 0.004 | ND | ||
| AL361 | galP | Glc | 0.992 ± 0.014 | 0.269 ± 0.083 | 0.428 ± 0.030 | 0.211 ± 0.015 | 0.540 ± 0.017 | 0.199 ± 0.012 | 0.285 ± 0.012 | 0.079 ± 0.021 | |
| AL535 | galP | glgC | Glc | 0.438 ± 0.016 | 0.159 ± 0.013 | ND | ND | ND | ND | NA | NA |
| AL358 | glcP | 0.122 ± 0.025 | ND | 0.118 ± 0.036 | ND | 0.112 ± 0.040 | ND | 0.279 ± 0.049 | ND | ||
| AL358 | glcP | Glc | 0.730 ± 0.008 | ND | ND | ND | ND | ND | ND | ND | |
| AL360 | GLUT1 | 0.140 ± 0.018 | ND | 0.112 ± 0.014 | ND | 0.115 ± 0.029 | ND | 0.292 ± 0.045 | ND | ||
| AL360 | GLUT1 | Glc | 0.219 ± 0.029 | ND | 0.212 ± 0.027 | ND | 0.201 ± 0.051 | ND | 0.352 ± 0.055 | ND | |
| AL1030 | cscKB | 0.134 ± 0.023 | ND | 0.106 ± 0.032 | ND | 0.141 ± 0.038 | ND | 0.148 ± 0.026 | ND | ||
| AL1030 | cscKB | Suc | 0.327 ± 0.029 | 0.157 ± 0.021 | 0.401 ± 0.026 | 0.122 ± 0.034 | 0.376 ± 0.031 | 0.128 ± 0.011 | 0.412 ± 0.008 | 0.126 ± 0.009 | |
| AL1067 | xylE | 0.102 ± 0.004 | ND | 0.141 ± 0.010 | ND | 0.161 ± 0.020 | ND | 0.172 ± 0.024 | ND | ||
| AL1067 | xylE | Xyl | 0.140 ± 0.021 | ND | 0.179 ± 0.007 | ND | 0.154 ± 0.015 | ND | 0.192 ± 0.011 | ND | |
| AL434 | xylEAB | ND | ND | 0.050 ± 0.011 | ND | 0.121 ± 0.011 | ND | 0.106 ± 0.014 | ND | ||
| AL434 | xylEAB | Xyl | 0.395 ± 0.026 | 0.351 ± 0.014 | 0.572 ± 0.025 | 0.336 ± 0.012 | 0.471 ± 0.012 | 0.291 ± 0.020 | 0.313 ± 0.015 | 0.082 ± 0.020 | |
These growth rates were calculated from the OD730 throughout diurnal conditions. All growth rates are the average rates for biological triplicates. The “±” signs indicate standard deviations of rates, where n = 3. Any growth rate calculated to be less than 0.050 day−1 is considered insignificant and is shown here as not detectable (ND). AL257 is the wild type. L, light condition; D, dark condition; OE, overexpression; KO, deletion; Xyl, xylose; Glc, glucose; Suc, sucrose; NA, not analyzed.
Since S. elongatus naturally stores fixed carbon in the form of glycogen (31), it was hypothesized that some portion of the transported glucose was captured and stored instead of contributing to cell growth. To explore this possibility, a necessary gene for the formation of glycogen, glgC, was deleted from both the galP strain (denoted as the galP-ΔglgC strain) and the wild type (see Fig. S1 in the supplemental material). These strains were assayed to determine any change in growth behavior. The galP-ΔglgC strain failed to show any growth in the presence of glucose beyond the first light cycle (Fig. 1D). It has been shown that the deletion of glgC in the wild type also impaired growth (27), although the effects of ΔglgC in this study were larger than those reported previously (27). Further analysis will be required to elucidate the mechanism of the growth defect.
To characterize the expression of galP in S. elongatus, gfp was fused to the 3′ end of galP (denoted as galP-gfp). The strains containing galP-gfp or gfp only were examined with fluorescent confocal microscopy (Fig. 2A and B). The strain containing galP-gfp showed a fluorescent signal only in the cellular membrane (Fig. 2B, right), while the gfp-containing control strain (AL504) (Table 1) showed a fluorescent signal throughout the cytoplasm (Fig. 2B, left), and the wild type did not show a fluorescent signal under these conditions (Fig. 2A). These results indicate that GalP from E. coli is successfully localized to the membrane of S. elongatus and may allow efficient transport of glucose into the cell.
Fig 2.
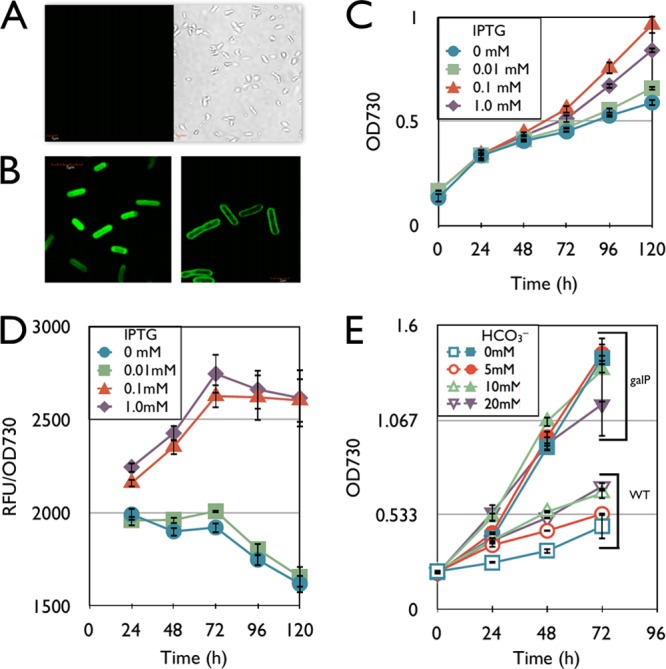
Characterization of the galP strain. All samples are grown in BG-11 medium with 5 g/liter glucose in continuous light. (A) Transmitted light (right) and confocal (left) images of the wild type. (B) Confocal microscope images of the gfp strain (left) and the galP-gfp strain (right). (C) Growth curve of the galP-gfp strain in the presence of various amounts of IPTG. (D) Fluorescence analysis of the galP-gfp strain during the course of the growth curve. The y axis indicates GFP fluorescence intensity divided by OD730. (E) Growth curve of the galP strain (without gfp) (filled symbols) and the wild type (open symbols) in the presence of various concentrations of sodium bicarbonate (NaHCO3). Error bars represent standard deviations (in triplicate).
Next, various concentrations of IPTG were added to the cultures of the galP-gfp strain, and the growth was measured under continuous-light conditions (Fig. 2C). The variation in IPTG concentrations led to a variation in growth rates of the cultures. This was also the case with the galP-containing strain, while the growth of the wild type was not altered with the addition of any amount of IPTG up to 1 mM. Optimum growth resulted from induction using 0.1 mM IPTG, while induction with a 1 mM concentration led to a slightly lower cell growth rate (Fig. 2C).
Fluorescence intensity of the cultures of the galP-gfp strain was measured throughout the growth assay (Fig. 2D). Time courses of GFP fluorescence intensity and the OD730 of the galP-gfp strain were measured with various concentrations of IPTG (Fig. 2D). The standardized fluorescent intensity (relative fluorescence units [RFU]/OD730) indicated that 1 mM IPTG and 0.1 mM IPTG in the medium caused similar levels of expression of GalP-GFP, and expression from Ptrc was saturated with 0.1 mM IPTG for this construct.
The effects of bicarbonate on heterotrophic growth were characterized (Fig. 2E). This experiment was used to determine if heterotrophic growth of the galP-containing strain was measurable only due to the carbon limitations introduced by the assay conditions. The galP strain showed similar growth with or without various concentrations of bicarbonate- and glucose-containing medium in continuous light, while a higher concentration of bicarbonate slightly enhanced the growth of the wild type (Fig. 2E). The growth rate of the wild type increased to 0.398 day−1 with 20 mM bicarbonate from 0.259 day−1 without bicarbonate, while the growth rate of the galP strain did not change in the presence of bicarbonate (∼0.636 day−1). These results suggest that carbon fixation is not the rate-limiting step for the growth of the galP strain in the presence of 5 g/liter glucose, while carbon fixation is growth limiting for the wild type under the same conditions. With the addition of bicarbonate in this experiment, the pH of the culture may increase as the bicarbonate is consumed. This basic pH may decrease sugar consumption by the H+ symporter (7). Thus, it is important to maintain the pH of the culture around 7.0, at which the transport of sugars would be in the importing direction for efficient growth.
Growth with sucrose.
Sucrose is a natural metabolite in S. elongatus, and it has been shown to be synthesized in response to osmotic pressure (7, 27). The wild type with 5 g/liter sucrose had an increased growth rate, 0.310 day−1 and 0.087 day−1, respectively, in the light and dark compared to the wild type without sucrose, at a rate of 0.161 day−1 and no growth in the light and dark, respectively (Fig. 3). This result shows that S. elongatus is weakly permeable to sucrose and can use sucrose as a carbon source. Seeking to improve these growth rates, a sucrose transporter gene and a fructokinase gene, cscB and cscK, respectively (E. coli ATCC 700927), were integrated into the S. elongatus genome (Fig. 3A). These genes were previously shown to be properly expressed in this organism (7). This strain was constructed to more fully allow sucrose into the cell through the CscB transporter and to be hydrolyzed to glucose and fructose by the endogenous sucrose invertase (putatively encoded by SYNPCC7942_0397). The fructokinase gene, cscK, was overexpressed to maximize the carbon flux to fructose-6-phosphate, a central metabolite in the oxidative pentose phosphate pathway (32). It was found that the cscK-cscB strain had increased growth rates, 0.376 day−1 (light) and 0.128 day−1 (dark), in the presences of 5 g/liter sucrose (Fig. 3C). Although sucrose enhanced the growth of the wild type, the effects of sucrose were larger in the cscB-cscK strain than in the wild type.
Fig 3.
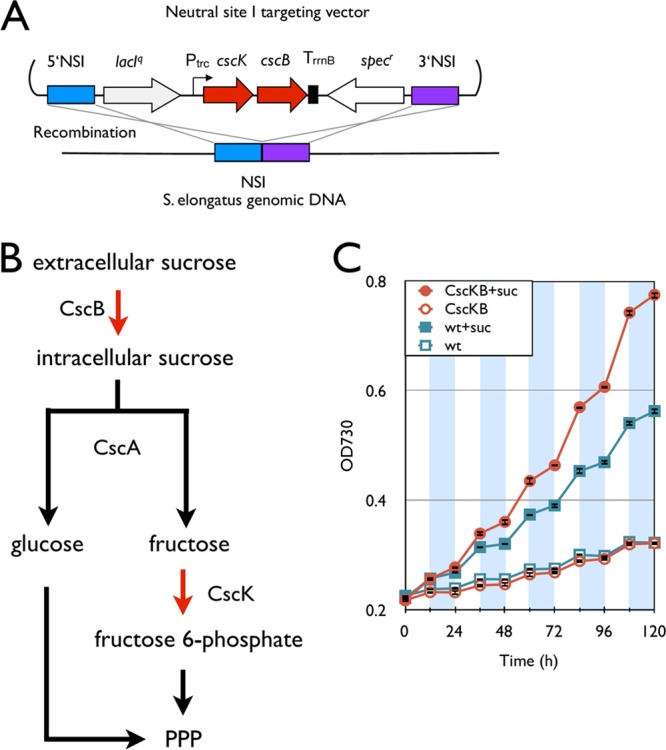
Installation of the sucrose degradation pathway. (A) Schematic representation of integration of the the sucrose degradation pathway into the S. elongatus chromosome. (B) Synthetic sucrose degradation pathway in S. elongatus. Red arrows indicate steps catalyzed by heterologous enzymes. PPP, pentose phosphate pathway. (C) Growth curves of the cscB-cscK strain (red) and the wild type (blue). Empty and filled symbols indicate growth without and with 5 g/liter sucrose, respectively. White and shaded areas indicate light and dark cycles, respectively. Error bars represent standard deviations (in triplicate).
Growth with xylose.
Xylose is the major part of abundantly available hemicellulosic materials and may be a promising inexpensive renewable feedstock for microbial production of biofuels and chemicals (33). Importantly, xylose is not a known metabolite of S. elongatus. To engineer S. elongatus so that it can utilize xylose, xylE, encoding a xylose transporter, from E. coli was integrated into S. elongatus (Fig. 4A). However, the xylose transporter not only failed to improve the growth of the strain in the presence of xylose under diurnal conditions, but this mutant had a significantly reduced growth rate (0.154 ± 0.015) compared to that of the wild type (0.350 ± 0.017) (Fig. 4C). Since xylose slightly improved the growth of the wild type (Fig. 4C), S. elongatus seems to be able to consume xylose slowly. However, the expression of xylE may greatly increase the amount of xylose accumulated within the cell owing to its slow metabolism, causing a metabolic imbalance. Thus, we hypothesized that some downstream enzymes for converting xylose to central metabolites efficiently are missing. We searched the S. elongatus genome sequence and found that the S. elongatus genome is not known to contain homologs of xylA and xylB, encoding xylose isomerase and xylulokinase, respectively. Xylose isomerase and xylulokinase are responsible for the first two steps of xylose degradation (Fig. 4B). To introduce xylose isomerase and xylulokinase into S. elongatus, an operon including xylE, xylA, and xylB from E. coli was integrated into the S. elongatus genome (denoted as the xylEAB strain) (Fig. 4A). This strain was shown to have a much higher heterotrophic growth rate under diurnal conditions than the wild type (Fig. 4C). This operon also allowed heterotrophic growth in the dark part of diurnal conditions, while the wild-type growth rate was marginal (Fig. 4C). The xylEAB strain had growth rates of 0.471 day−1 and 0.291 day−1 in the light and dark, respectively, in the presence of xylose. This is in contrast to the wild-type growth rates of 0.350 day−1 and 0.062 day−1 under the same conditions. The xylose consumption rate was measured by HPLC to average about 10 mg h−1 over the 96-h growth period. Interestingly, the maximum growth of the galP strain (0.992 ± 0.014) was faster than that of the xylEAB strain (0.572 ± 0.025) under the light condition in the presence of their respective sugars owing to rapid growth in the galP strain in the first 12 h (Fig. 1B and 4C). After the initial 12 h, the growth rates of the two strains were comparable in the light. However, the maximum growth in the dark of the xylEAB strain (0.351 ± 0.014) was faster than that of the galP strain (0.269 ± 0.083) in the presence of their respective sugars (Fig. 1B and 4C).
Fig 4.
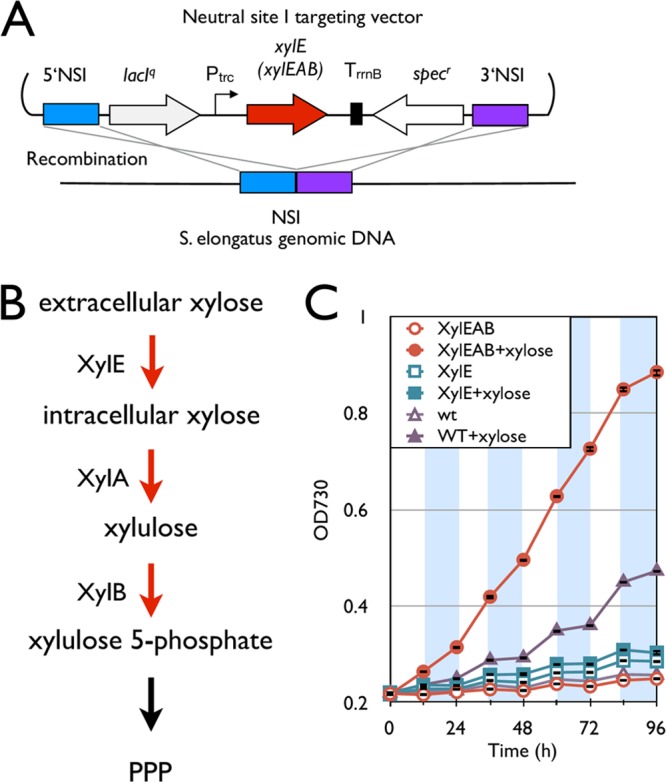
Installation of the xylose degradation pathway. (A) Schematic representation of integration of the xylose degradation pathway into the S. elongatus chromosome. (B) Synthetic xylose degradation pathway in S. elongatus. Red arrows indicate steps catalyzed by heterologous enzymes. (C) Growth curves of the xylE and xylEAB strains and the wild type. Empty and filled symbols indicate growth without and with 5 g/liter xylose, respectively. White and shaded areas indicate light and dark cycles. Error bars represent standard deviations (in triplicate).
We have successfully engineered S. elongatus so that it can grow continuously under diurnal conditions by using common fixed carbon sources for growth. In general, heterotrophic cyanobacteria utilize relatively few substrates for the buildup of biomass (18). The well-known photoheterotrophic cyanobacterium Synechocystis sp. 6803 can utilize glucose under photomixotrophic conditions of growth, with a reported growth rate of 1.38 (day−1) (34). This is slightly faster than the fastest growth shown here for the galP strain under photomixotrophic conditions, with a growth rate of about 0.99 (day−1). However, Synechocystis sp. 6803 has not been shown to consume xylose, demonstrating the versatile nature of this synthetic sugar transporter strategy. Sugar transporters, as well as other peripheral genes, were integrated for the increased growth rate of a model photoautotrophic cyanobacterium under both light and dark conditions. While the particular genes necessary for each specific feedstock may differ, these results suggest that the cause of autotrophy in this organism lies within the peripheral metabolic processes. Core catabolic pathways for common sugar utilization seem to be functional in S. elongatus.
Cyanobacteria are increasingly being studied for the production of valuable natural products, as well as biofuels and chemicals (35). Optimal industrial conditions must be explored in order to maximize productivity and lead to efficient utilization of resources to remain competitive. In order for any cyanobacterial chemical production to be economically competitive, the light energy must be supplied from the sun, a natural diurnal condition, with production possible for only a portion of the day. To improve this production scenario, the development of heterotrophic and/or mixotrophic growth conditions that utilize diverse feedstocks and production processes applicable to a major category of cyanobacteria is desirable. Additionally, even under light conditions, photoautotrophic growth is often limited by light deficiency due to mutual shading of cells. As a result, the overall productivity of a photoautotrophic system is low. In this work, we developed a strategy to allow growth of an obligate photoautotroph on diverse feedstocks. As such, this example of modification of an obligate photoautotroph for continuous growth under diurnal conditions demonstrates key advantages of using a synthetic biological approach to expand the natural feedstocks of a particular class of organisms.
In this work, we examined the growth behavior of engineered S. elongatus under diurnal conditions. We have determined one underlying cause of natural photoautotrophy in this bacterium by engineering the organism to allow diurnal growth. For complete organoheterotrophic conversion of S. elongatus, the strain must be able to grow in the complete absence of light. In future work, we will seek to engineer the strains from this work to allow growth under continuously dark conditions. The activities of all photosynthetic organisms are energy intensive, interconnected with central metabolism, and are strictly regulated according to light intensity. The activity of these reactions will sharply decrease once exposure to light ceases, although the fixation of CO2 has been observed up to 8 h into the dark cycle during diurnal conditions (36). In S. elongatus, light regulation takes place through two major routes: transcriptional and posttranslational. Each of these regulatory pathways uses the redox state of the electron transport chain as a way of sensing the light conditions (37, 38), transducing this signal through various mediator systems, such as KaiABC and the ferrodoxin/thioredoxin system (37, 39–41). We anticipate the need to decouple this regulation through continued metabolic engineering. Another possibility is exploring a light pulsing stratagem similar to the light-activated heterotrophic growth seen in Synechocystis sp. strain PCC 6803 (42). While diurnal conditions are of keen interest for cost-effective, industrial pursuits, further work with continuously dark conditions will more fully illuminate the causes of phototrophy seen in this model cyanobacterium.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a National Science Foundation grant (1132442).
We thank Gang-Yu Liu and Jie-Ren Li for assistance with confocal microscopy in the UCD NEAT Spectral Imaging Facility, John C. Meeks, J. Clark Lagarias, and Christine A. Rabinovitch-Deere for helpful comments on the manuscript, and Noeli Acoba for experimental assistance.
Footnotes
Published ahead of print 28 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03326-12.
REFERENCES
- 1. Chen GQ, Chen F. 2006. Growing phototrophic cells without light. Biotechnol. Lett. 28:607–616 [DOI] [PubMed] [Google Scholar]
- 2. Wood AP, Aurikko JP, Kelly DP. 2004. A challenge for 21st century molecular biology and biochemistry: what are the causes of obligate autotrophy and methanotrophy? FEMS Microbiol. Rev. 28:335–352 [DOI] [PubMed] [Google Scholar]
- 3. Zaslavskaia LA, Lippmeier JC, Shih C, Ehrhardt D, Grossman AR, Apt KE. 2001. Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 292:2073–2075 [DOI] [PubMed] [Google Scholar]
- 4. Zhang C-C, Jeanjean R, Joset F. 1998. Obligate phototrophy in cyanobacteria: more than a lack of sugar transport. FEMS Microbiol. Lett. 161:285–292 [DOI] [PubMed] [Google Scholar]
- 5. Cogne G, Gros JB, Dussap CG. 2003. Identification of a metabolic network structure representative of Arthrospira (Spirulina) platensis metabolism. Biotechnol. Bioeng. 84:667–676 [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Bryant DA. 2011. The tricarboxylic acid cycle in cyanobacteria. Science 334:1551–1553 [DOI] [PubMed] [Google Scholar]
- 7. Ducat DC, Avelar-Rivas JA, Way JC, Silver PA. 2012. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microbiol. 78:2660–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niederholtmeyer H, Wolfstadter BT, Savage DF, Silver PA, Way JC. 2010. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl. Environ. Microbiol. 76:3462–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stebegg R, Wurzinger B, Mikulic M, Schmetterer G. 2012. Chemoheterotrophic growth of the cyanobacterium Anabaena sp. strain PCC 7120 dependent on a functional cytochrome c oxidase. J. Bacteriol. 194:4601–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ungerer JL, Pratte BS, Thiel T. 2008. Regulation of fructose transport and its effect on fructose toxicity in Anabaena spp. J. Bacteriol. 190:8115–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CNS, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A, Kashiyama Y, Baker D, Yoshikuni Y. 2012. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308–313 [DOI] [PubMed] [Google Scholar]
- 12. Atsumi S, Higashide W, Liao JC. 2009. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 27:1177–1180 [DOI] [PubMed] [Google Scholar]
- 13. Liu X, Fallon S, Sheng J, Curtiss R., III 2011. CO2-limitation-inducible green recovery of fatty acids from cyanobacterial biomass. Proc. Natl. Acad. Sci. U. S. A. 108:6905–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu X, Sheng J, Curtiss R., III 2011. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 108:6899–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machado IMP, Atsumi S. 2012. Cyanobacterial biofuel production. J. Biotechnol. 162:50–56 [DOI] [PubMed] [Google Scholar]
- 16. Robertson D, Jacobson S, Morgan F, Berry D, Church G, Afeyan N. 2011. A new dawn for industrial photosynthesis. Photosynth. Res. 107:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan LT. 2007. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 68:954–979 [DOI] [PubMed] [Google Scholar]
- 18. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 19. Atsumi S, Hanai T, Liao JC. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 20. Ivleva NB, Bramlett MR, Lindahl PA, Golden SS. 2005. LdpA: a component of the circadian clock senses redox state of the cell. EMBO J. 24:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovach ME, Phillips RW, Elzer PH, Roop RM, II, Peterson KM. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800–802 [PubMed] [Google Scholar]
- 22. Golden S, Brusslan J, Haselkorn R. 1987. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 153:215–246 [DOI] [PubMed] [Google Scholar]
- 23. Bustos SA, Golden SS. 1991. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J. Bacteriol. 173:7525–7533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ball SG, Morell MK. 2003. From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 54:207–233 [DOI] [PubMed] [Google Scholar]
- 25. Preiss J. 1984. Bacterial glycogen synthesis and its regulation. Annu. Rev. Microbiol. 38:419–458 [DOI] [PubMed] [Google Scholar]
- 26. Smith AJ. 1983. Modes of cyanobacterial carbon metabolism. Ann. Microbiol. (Paris) 134B:93–113 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki E, Ohkawa H, Moriya K, Matsubara T, Nagaike Y, Iwasaki I, Fujiwara S, Tsuzuki M, Nakamura Y. 2010. Carbohydrate metabolism in mutants of the cyanobacterium Synechococcus elongatus PCC 7942 defective in glycogen synthesis. Appl. Environ. Microbiol. 76:3153–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang CC, Durand MC, Jeanjean R, Joset F. 1989. Molecular and genetical analysis of the fructose-glucose transport system in the cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 3:1221–1229 [DOI] [PubMed] [Google Scholar]
- 29. Hernandez-Montalvo V, Martinez A, Hernandez-Chavez G, Bolivar F, Valle F, Gosset G. 2003. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 83:687–694 [DOI] [PubMed] [Google Scholar]
- 30. Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. 1985. Sequence and structure of a human glucose transporter. Science 229:941–945 [DOI] [PubMed] [Google Scholar]
- 31. Stanier RY. 1975. The utilization of organic substrates by cyanobacteria. Biochem. Soc. Trans. 3:352–359 [DOI] [PubMed] [Google Scholar]
- 32. Bassham JA, Benson AA, Calvin M. 1950. The path of carbon in photosynthesis. J. Biol. Chem. 185:781–787 [PubMed] [Google Scholar]
- 33. Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD. 2010. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562 [DOI] [PubMed] [Google Scholar]
- 34. Vermaas W. 1996. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: principles and possible biotechnology applications. J. Appl. Phycol. 8:263–273 [Google Scholar]
- 35. McEwen JT, Atsumi S. 2012. Alternative biofuel production in non-natural hosts. Curr. Opin. Biotechnol. 23:744–750 [DOI] [PubMed] [Google Scholar]
- 36. Paul JH, Kang JB, Tabita FR. 2000. Diel patterns of regulation of rbcL transcription in a cyanobacterium and a prymnesiophyte. Mar. Biotechnol. (NY) 2:429–436 [DOI] [PubMed] [Google Scholar]
- 37. Buchanan BB, Balmer Y. 2005. Redox regulation: a broadening horizon. Annu. Rev. Plant Biol. 56:187–220 [DOI] [PubMed] [Google Scholar]
- 38. Golden SS. 1995. Light-responsive gene expression in cyanobacteria. J. Bacteriol. 177:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ivleva NB, Gao T, LiWang AC, Golden SS. 2006. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc. Natl. Acad. Sci. U. S. A. 103:17468–17473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lindahl M, Florencio FJ. 2003. Thioredoxin-linked processes in cyanobacteria are as numerous as in chloroplasts, but targets are different. Proc. Natl. Acad. Sci. U. S. A. 100:16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS. 2000. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289:765–768 [DOI] [PubMed] [Google Scholar]
- 42. Anderson SL, McIntosh L. 1991. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J. Bacteriol. 173:2761–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.