Abstract
Staphylococcal contamination of food products and staphylococcal food-borne illnesses continue to be a problem worldwide. Screening of food for the presence of Staphylococcus aureus and/or enterotoxins using traditional methods is laborious. Reliable and rapid multiplex detection methods from a single food extract or culture supernatant would simplify testing. A fluorescence-based cytometric bead array was developed for the detection of staphylococcal enterotoxin B (SEB), using magnetic microspheres coupled with either an engineered, enterotoxin-specific Vβ domain of the T-cell receptor (Vβ-TCR) or polyclonal antibodies. The binding affinity of the Vβ-TCR for SEB has been shown to be in the picomolar range, comparable to the best monoclonal antibodies. The coupled beads were validated with purified enterotoxins and tested in a variety of food matrices spiked with enterotoxins. The Vβ-TCR or antibody was shown to specifically bind SEB in four different food matrices, including milk, mashed potatoes, vanilla pudding, and cooked chicken. The use of traditional polyclonal antibodies and Vβ-TCR provides a redundant system that ensures accurate identification of the enterotoxin, and the use of labeled microspheres permits simultaneous testing of multiple enterotoxins from a single sample.
INTRODUCTION
Staphylococcus aureus is among the top five common pathogens associated with food-borne illness nationwide. The Centers for Disease Control and Prevention (CDC) estimates that there are about 240,000 illnesses with 1,000 hospitalizations and 6 deaths associated with staphylococcal food poisoning (SFP) annually (1). SFP is directly linked to small (25- to 30-kDa) exotoxins, known as staphylococcal enterotoxins (SEs), that are heat resistant, tolerate low pH, and often persist long after the microbe has been rendered nonviable. SEs are superantigens that induce nonspecific T-cell activation, which can rapidly cascade to a massive release of inflammatory mediators and may lead to toxic shock (2, 3). SFP occurs when improperly handled food contaminated with as little as 100 ng of SE is consumed. SFP is marked by severe gastrointestinal symptoms such as emesis, diarrhea, and/or abdominal pain after a 4-h incubation period (4).
Suspect food products are typically analyzed by using traditional culture techniques followed by identification of enterotoxigenic staphylococcal strains. Food extracts positive for bacteria may then be evaluated for the presence of preformed SEs (5). A recent outbreak in Japan from milk contaminated with staphylococcal enterotoxin A (SEA) illustrated the challenges of conventional methods, since viable bacteria were not detected (6, 7). PCR methods would be a useful screening tool for samples that are culture negative and enterotoxin positive (8). PCR methods are sensitive and can detect low levels of gene targets; however, this does not verify the presence of expressed proteins, and it is unacceptable as a definitive method for SE detection in the regulatory arena. Therefore, immunological techniques using either monoclonal or polyclonal SE antibodies are commonly employed to assess samples for the presence of SEs. The limit of detection for commercially prepared sandwich assays ranges from 0.25 to 1.0 ng/ml (14); however, two limitations of the commercial assays are an inability to analyze a single sample for multiple SEs and the confirmatory testing requirements for some assays. Lower SE levels can be detected using any method by concentrating samples, as demonstrated during the Japanese outbreak attributed to milk contaminated with SEA (6, 7), but one of our goals was to develop sensitive and specific assays that minimize sample processing.
In this work, we explored the use of engineered soluble receptor proteins that were developed as a way to neutralize SEs during the disease process (9–12). The SE binds to the T-cell receptor (TCR) variable region of the β chain (Vβ) (TCR-Vβ) on a T cell and to the class II major histocompatibility complex (MHC) molecule on an antigen-presenting cell. The formation of this “bridge” stimulates T cells that express a Vβ region for which the SE has specificity (12). While the normal affinity of the SE-Vβ interaction is quite low (in the micromolar range), the binding affinities of several different Vβs have been engineered to over 1-million-fold-higher affinities by yeast display (9–12). These Vβ regions have been expressed in Escherichia coli, and the soluble protein receptors have demonstrated high affinity, sensitivity, and specificity and exhibited dissociation constants in the picomolar range, as strong as, or stronger than, monoclonal antibodies (MAbs) (9–12).
In the present study, polyclonal antibodies or a superantigen-specific Vβ-TCR was coupled to uniquely labeled paramagnetic microspheres serving as the capture entity for the superantigen, to create a sandwich capture assay. Our objectives and initial studies focused on the sensitivity and specificity of assays for the detection of SEB in cell-free culture supernatants and minimally processed spiked food matrices. The specificity of the assay was verified by mass spectral analysis. Sensitivity and affinities for other SEs and Vβ-TCRs were also evaluated.
MATERIALS AND METHODS
Bacterial cultures.
Bacterial strains were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA, Chantilly, VA) and the American Type Culture Collection (ATCC, Manassas, VA). The strains were frozen at −80°C in nutrient broth with glycerol (15%, vol/vol).
Antibodies and Vβ-TCR.
Affinity-purified polyclonal staphylococcal antibodies targeting either enterotoxins (SEB and SEC) or toxic shock syndrome toxin (TSST) were purchased from Toxin Technology (Sarasota, FL). The Vβ-TCRs for SEB (G5-8), SEC (L3), and TSST (D10V) were developed and purified in-house (9–12). Polyclonal antibodies and Vβ-TCRs were used as capture proteins for the SEs, providing the assay with redundancy.
Microsphere coupling and validation.
Each antibody and Vβ-TCR (5 μg/ml) was covalently coupled to activated unique paramagnetic color-coded microspheres (1.25 × 106 beads/ml) by using an amine coupling kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Reagents required, but not included in the kit, were 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (50 mg/ml) and N-hydroxysulfosuccinimide (50 mg/ml) (Pierce ThermoFisher, Rockford, IL). Following coupling, the microspheres were washed with phosphate-buffered saline (PBS), blocked using the blocking buffer provided in the kit, washed, and finally resuspended in storage buffer, also provided in the kit. The final bead concentration was determined by using a TC10 automated cell counter (Bio-Rad, Hercules, CA).
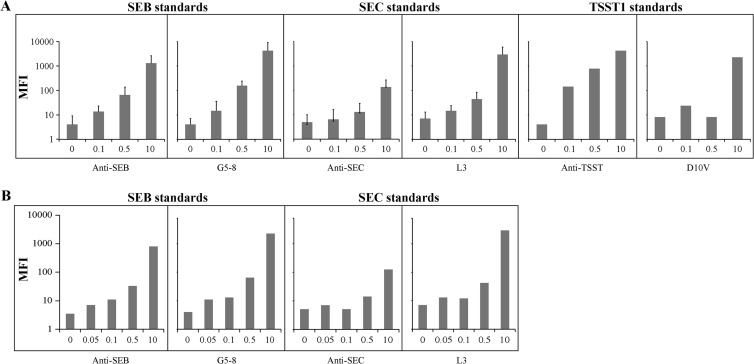
Validation of ligand attachment to the beads was performed in duplicate by adding biotinylated toxins SEB, SEC, and TSST (2 μg/ml) (Toxin Technology, Sarasota FL) to separate wells containing approximately 10,000 beads/ml. Following a 30-min incubation on an orbital shaker in the dark, the enterotoxin was removed, and the beads were resuspended in 50 μl of staining buffer with the reporter dye streptavidin R-phycoerythrin conjugate (SA-PE) (2 μg/ml) (Qiagen, Valencia, CA). SA-PE was removed after 10 min of incubation in the dark without shaking, and the beads were resuspended in storage buffer and then analyzed by using the BioPlex 200 system (Bio-Rad, Hercules, CA) (Fig. 1). Once coupling was validated for specific pairs, i.e., anti-SEB antibody and G5-8 with SEB, multiplex assays using combinations of antibodies, SEs, and Vβ-TCRs were evaluated by pooling the coupled microspheres into one well and testing as described above by using biotinylated enterotoxins. Each sample was run in duplicate (Fig. 2).
Fig 1.
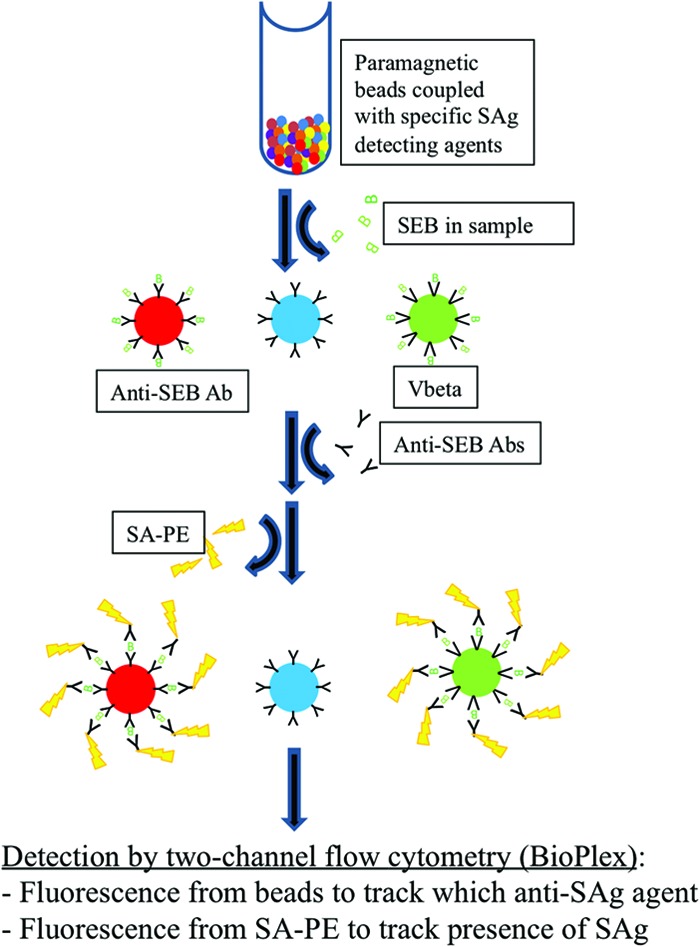
Concept diagram for the BioPlex assay. Paramagnetic beads were coupled with specific SE antibodies or Vβ-TCRs. SEs were incubated with approximately 10,000 beads/ml, followed by an incubation with detection antibodies and the reporter agent SA-PE. The analysis was performed by using two-channel flow cytometry (BioPlex). SAg, superantigen.
Fig 2.
Pooled bead sets tested with polyclonal detection agents or biotinylated enterotoxins. (A) Various concentrations of the indicated superantigens were added in a singleplex format to beads coupled with one of the six reagents (anti-SEB antibody [Ab], G5-8, anti-SEC antibody, L3, anti-TSST-1 antibody, or D10V). Biotinylated polyclonal antisuperantigen detection reagents were added, followed by SA-PE, and beads were assayed by flow cytometry (MFI values are shown). Reactions were run in duplicate, except for TSST. (B) In a multiplex format, biotinylated SEB and SEC were added directly to bead sets coupled with one of four capture agents, anti-SEB antibody, G5-8, anti-SEC antibody, or L3, followed by SA-PE. Beads were assayed by flow cytometry (MFI values are shown). Bead assays were evaluated in a single reaction.
Flow cytometry.
The BioPlex 200 flow cytometer is intended to read assays performed utilizing multiplexing suspension array technology. The assay components include paramagnetic fluorescently labeled microspheres, a flow cytometer, and a digital signal processor equipped with two lasers. One laser identifies the bead, and the second laser measures the fluorescence intensity of the reporter dye that distinguishes a positive or negative result. Readings were conducted and expressed as the median fluorescence intensity (MFI) of 100 beads, as suggested by the manufacturer.
Cell-free culture supernatant preparation.
Stock strains of S. aureus were transferred onto nutrient agar, and one colony was then inoculated into brain heart infusion (BHI) broth (pH 5.5) and incubated overnight at 35°C. Broth culture aliquots were centrifuged at 5,300 × g for 30 min to remove cellular debris. The supernatant was diluted 1:5 in PBS with 0.1% (wt/vol) bovine serum albumin (BSA) (PBS-BSA), and 100 μl of sample was added to 50 μl of the microspheres, with 4.5 × 104 beads/ml per well. The beads were incubated in the dark for 30 min on an orbital shaker and then washed twice with PBS-BSA. Biotinylated detection antibody was added (0.5 μg/ml), and the beads were incubated in the dark on an orbital shaker for 20 min and then washed twice with PBS-BSA. SA-PE (2 μg/ml) was added to each well, and the plate was incubated in the dark for 10 min without shaking. Unbound SA-PE was removed, and beads were resuspended in storage buffer and then analyzed by using the BioPlex 200 system.
Evaluation of receptor and antibody specificity by LC-MS.
Twenty micrograms of either anti-SEB antibody or G5-8 Vβ was covalently coupled to 2 mg of Dynabeads M-270 epoxy using the Dynabeads coimmunoprecipitation kit (final concentration, 10 μg/μl; Life Technologies, Grand Island, NY). Two hundred micrograms of coupled Dynabeads was washed three times with 500 μl phosphate-buffered saline with Tween (PBS-T) and then incubated with 1 ml of cell-free culture supernatant for 30 min at room temperature. After the supernatant was removed, the beads were washed three times with 500 μl PBS-T and once with 500 μl of 50 mM ammonium bicarbonate (Pierce, Rockford, IL). The beads were finally resuspended in 50 μl of 50 mM ammonium bicarbonate in 9% acetonitrile. The proteins were enzymatically digested by the addition of 1 μg of the serine protease trypsin. Digestion was allowed to proceed at 60°C for 4 h before being quenched by the addition of 1% acetic acid (final volume). The peptide samples were removed from the beads and analyzed via liquid chromatography (LC) (nanoAcquity; Waters, Milford, MA) followed by mass spectrometry (MS) (LTQ; Thermo Scientific, Waltham, MA). Details of the LC-MS analysis were described previously (13).
Food extract testing.
Aliquots of milk (6 ml) purchased from a local grocery store were spiked with SEB to a final concentration ranging from 0.1 to 7.0 ng/ml. A duplicate experiment that tested assay reproducibility was performed using SEB purchased from a different supplier (Sigma, St. Louis, MO). The pH of each spiked milk aliquot was adjusted to 4, the sample was centrifuged at 5,300 × g and filtered, and the pH was adjusted to 7. The extracted milk samples were diluted 1:5 in PBS-BSA, and 100 μl of sample was added to a flat-bottomed 96-well plate containing 50 μl of the microspheres, with 4.5 × 104 beads/ml per well. The plate was incubated in the dark for 30 min on an orbital shaker and then washed twice with PBS-BSA. Biotinylated detection antibody was added (0.5 μg/ml), and the plate was incubated in the dark on an orbital shaker for 20 min and then washed twice with PBS-BSA. SA-PE (2 μg/ml) was added to each well, and the plate was incubated in the dark for 10 min without shaking. SA-PE was removed, and the beads were resuspended in storage buffer and then analyzed by using the BioPlex 200 system (Fig. 1).
Canned chicken, prepared mashed potatoes, and prepared vanilla pudding were purchased from a local grocery store. Each food product was diluted 1:1 with PBS, homogenized, and split into two portions. One portion was spiked with SEB to a final concentration of 8 ng/ml, and the second portion was used as the negative control. Both portions were centrifuged and filtered, and the pH was adjusted to 7, as required. Twofold dilutions were prepared prior to mixing with the bead sets and analyzed by using the BioPlex 200 system, as described above.
Data analysis.
The BioPlex system results were calculated with MFI software. Since each matrix may be variable, negative samples (matrix plus beads) without the enterotoxin were used as the negative controls. A sample was considered positive if the value was ≥3 times the negative value, as described previously for a cytometric assay (16).
RESULTS AND DISCUSSION
The major challenge with immunoaffinity assays for enterotoxin detection is the availability of good-quality monoclonal antibodies. Therefore, the use of an engineered protein with high specificity and affinity was appealing, since cross-reactivity associated with polyclonal antibody use can be minimized and the production process for the engineered proteins can yield high-quality, standardized preparations. To examine the use of the Vβ-TCR reagents in a multiplex assay, polyclonal antibodies or a Vβ-TCR was coupled to uniquely labeled paramagnetic microspheres serving as the capture entity for the superantigen, to create a sandwich capture assay (Fig. 1).
Our data from the singleplex validation assays detected SEB, SEC, and TSST at approximately 0.1-ng/ml levels (Fig. 2A). Beads coupled with anti-SEB antibody, G5-8 (a Vβ against SEB but not SEC), anti-SEC antibody, and L3 (a Vβ against SEC but with a lower affinity for SEB) were pooled and tested with biotinylated SEB or SEC. The reactions with the Vβ-TCRs against SEB and SEC showed higher MFI than the antibody-coupled counterparts, a possible indicator of greater sensitivity (Fig. 2B). The Vβ reagents showed minimal cross-reactivity with other reagents (Fig. 3 and data not shown).
Fig 3.
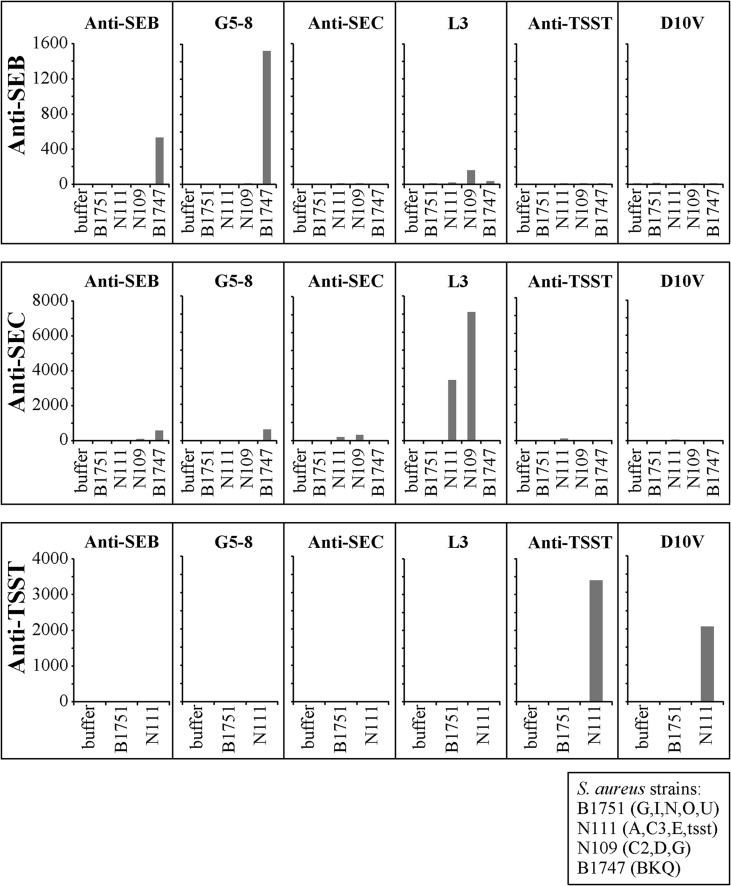
S. aureus culture supernatant assays. Cell-free culture supernatants of characterized Staphylococcus aureus strains were tested in multiplex assays for SEB, SEC, and TSST to determine assay sensitivity and specificity. The top panels show a multiplex reaction for SEB using the indicated immobilized reagents and an anti-SEB polyclonal detection agent. The middle panels show a multiplex reaction for SEC using the indicated immobilized reagents and an anti-SEC polyclonal detection agent. The bottom panels show a multiplex reaction for TSST-1 using the indicated immobilized reagents and an anti-TSST-1 polyclonal detection agent. The strains tested are indicated at the bottom right. The first panel shows a multiplex reaction for SEB. The bead set coupled with G5-8 shows a higher level of detection than the polyclonal anti-SEB antibody, with low-level detection with L3. This affinity can be attributed to homology between L3 and SEB. The second panel testing anti-SEC antibody shows a high level of detection using L3 but a very low level of detection with the bead set coupled with anti-SEC antibody. The bead sets coupled with anti-TSST antibody and D10V were highly specific and showed high levels of sensitivity.
To begin to assess the potential use of the Vβ reagents in a detection format with samples that contain unknown superantigens, we assayed the culture supernatants from four different strains with different genotypes for the superantigens (Fig. 3). Strain B1751 served as a negative control, as it expresses some superantigen genes but not SEB, SEC, or TSST-1. Strain B1747 (SEB positive [SEB+]) was positive for SEB protein with both the polyclonal detection agent and the Vβ-TCR G5-8. Strains N111 (SEC3+ TSST-1+) and N109 (SEC2+) were strongly positive with the Vβ-TCR L3 but only weakly positive with the polyclonal anti-SEC reagent. Strain N111 (SEC3+ TSST-1+) was strongly positive with the anti-TSST-1 polyclonal reagent and the TSST-1-specific Vβ-TCR D10V. Polyclonal detection reagents often yield cross-reactivity with SEB and SEC due to their structural similarities, but the G5-8 reagent's specificity for SEB can mitigate this cross-reactivity when used as the immobilized capture reagent.
One of the tools that we used to determine binding specificity was mass spectral analysis. Mass spectral evaluations were performed on cell-free staphylococcal culture supernatants. Dynabeads were coated with either anti-SEB or G5-8 capture agents, followed by incubation with culture supernatants of nine staphylococcal strains. Mass spectral analysis identified only SEB from the culture supernatants of the strains that were positive, as detected by the multiplex system (data not shown).
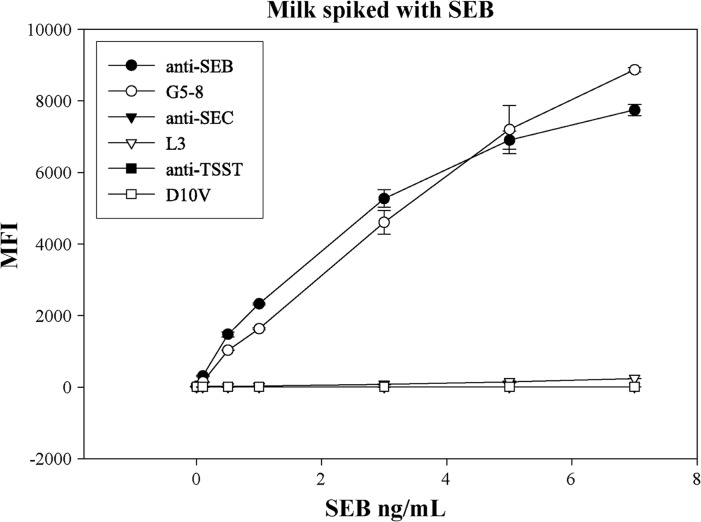
To apply the multiplex approach to food samples, various food matrices were spiked with different concentrations of SEB and assayed with various reagents. The milk spiked with SEB was evaluated with beads coupled with anti-SEB antibody, G5-8, anti-SEC antibody, L3, anti-TSST antibody, and D10V. The Vβ-TCR G5-8 and polyclonal antibodies exhibited similar levels of sensitivity and specificity (0.1 to 7.0 ng/ml) (Fig. 4). These experimental results were reproducible with SEB obtained from a different manufacturer (data not shown).
Fig 4.
Milk spiked with SEB. Milk was spiked with SEB and tested by using a multiplex assay for SEB, SEC, and TSST. The beads coupled with anti-SEB antibody and G5-8 showed a limit of detection of SEB of 0.1 ng/ml, while all other beads showed negative results.
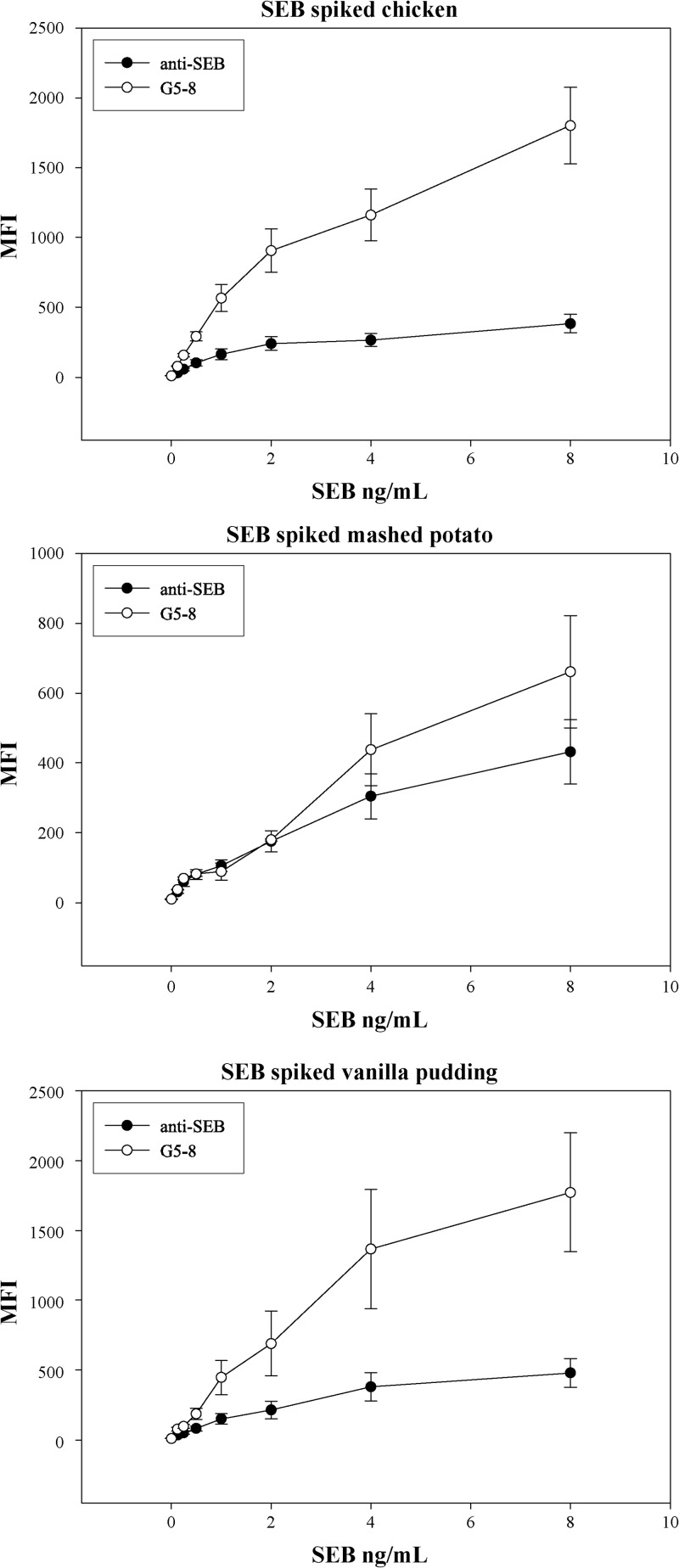
In contrast to the results with milk, the Vβ-TCR showed less interference than the polyclonal antibodies for the other food matrices, cooked chicken, prepared mashed potatoes, and prepared vanilla pudding (Fig. 5). The assays detected SEB at 0.125 ng/ml, lower than levels detected by commercial assays and with minimal sample processing. For example, the SEA outbreak in Japan required extensive sample concentration steps to identify the enterotoxin in milk powder (6, 7).
Fig 5.
Three different food matrices spiked with SEB. Canned chicken, mashed potatoes, and vanilla pudding were spiked and tested for SEB. Low levels were detected in all food matrices, but G5-8 showed a significantly higher level of detection. This demonstrates that the food matrices interfere less with the Vβ-TCR than the polyclonal antibody.
While our results from the chicken, mashed potatoes, and vanilla pudding suggested that the maximum signal of detection at the higher SEB concentrations was higher for the G5-8 Vβ, it is unclear why this was not observed with the standard SEB assays (Fig. 2A). This could be related to other components of these food matrices that inhibit the antibody-based assay but not the Vβ-based assay (Fig. 5).
Identification of contaminated food is essential for food safety and key for public health. A critical element toward this goal is the availability of reliable and sensitive assays that can rapidly detect bacterial toxins such as SEs. The use of monoclonal antibodies and now the engineered Vβ-TCRs allows for low-level detection in a multiplex fluorescent bead-based assay to enable accurate identification and quantification of staphylococcal enterotoxins from a single minimally processed sample.
The aim of our study was to design a robust, rapid immunoaffinity cytometric bead array for the detection of several staphylococcal enterotoxins in a single sample from either food matrices or cell-free culture supernatants. To this end, we have developed a redundant system using polyclonal antibodies and Vβ-TCRs that specifically bind SEB, SEC, or TSST. The lower limit of detection of our assay was at least as sensitive as or more sensitive than commercial assays, including the VidasSET2 assay (14, 17), and similar assays developed by other researchers (15). Further assay optimization may allow even more sensitive detection, and it will be useful to test spiked food in dual-multiplex assays using the Vβ-TCRs and antibodies that target other enterotoxins, such as SEA and SEC. For example, the ability to use smaller proteins such as the Vβ-TCRs (10 times smaller than intact antibodies) should enable the coating of beads at higher probe densities for possible improvements in sensitivity. This approach may also benefit from the controlled immobilization of the Vβ-TCRs using specific tags (e.g., C-terminal biotin), rather than the random linkage of free amines, which could lead to less optimal orientations. Finally, the use of engineered and bacterially expressed proteins such as Vβ-TCRs is advantageous since the use of traditional animal-expressed antibodies could be reduced and perhaps eliminated.
ACKNOWLEDGMENTS
The following isolates were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) Program, supported under NIAID/NIH contract no. HHSN272200700055C: NRS100, NRS109, NRS111, NRS113, NRS114, NRS120, NRS121, NRS166, NRS185, NRS193, NRS194, NRS387, and NRS483. This work was partially supported by a grant from the National Institutes of Health-supported Great Lakes Regional Center for Excellence in Biodefense and Emerging Diseases (grant U54 AI57153 to D.M.K.).
Footnotes
Published ahead of print 14 December 2012
REFERENCES
- 1. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77–104 [DOI] [PubMed] [Google Scholar]
- 4. Doyle MP, Beuchat LR, Montville TJ. (ed). 2007. Food microbiology: fundamentals and frontiers, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 5. Bennett RW, Lancette GA. 2001. Chapter 12. Staphylococcus aureus. In Hammack TS, Feng P, Jinneman K, Regan PM, Kase J, Orlandi P, Burkhardt W. (ed), FDA bacteriological analytical manual, 8th ed, revision A US Food and Drug Administration Center for Food Safety and Applied Nutrition, College Park, MD: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm [Google Scholar]
- 6. Anonymous 2001. Staphylococcus food poisoning in Japan. Infect. Agents Surveill. Rep. 22:185–186 http://idsc.nih.go.jp/iasr/22/258/tpc258.html [Google Scholar]
- 7. Asao T, Kumeda Y, Kawai T, Shibata T, Oda H, Haruki K, Nakazawa H, Kozaki S. 2003. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 130:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajkovic A, Benaissa EM, Uyttendaele M, Brolet P, Zorzi W, Heinen E, Foubert E, Debevere J. 2006. Immunoquantitative real-time PCR for detection and quantification of Staphylococcus aureus enterotoxin B in foods. Appl. Environ. Microbiol. 72:6593–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kieke MC, Sundberg E, Shusta EV, Mariuzza RA, Wittrup KD, Kranz DM. 2001. High affinity T cell receptors from yeast display libraries block T cell activation by superantigens. J. Mol. Biol. 307:1305–1316 [DOI] [PubMed] [Google Scholar]
- 10. Buonpane RA, Churchill HR, Moza B, Sundberg EJ, Peterson ML, Schlievert PM, Kranz DM. 2007. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat. Med. 13:725–729 [DOI] [PubMed] [Google Scholar]
- 11. Buonpane RA, Moza B, Sundberg EJ, Kranz DM. 2005. Characterization of T cell receptors engineered for high affinity against toxic shock syndrome toxin-1. J. Mol. Biol. 353:308–321 [DOI] [PubMed] [Google Scholar]
- 12. Mattis DM, Spaulding AR, Chuang-Smith ON, Sundberg EJ, Schlievert PM, Kranz DM. 15 November 2012. Engineering a soluble high-affinity receptor domain that neutralizes staphylococcal enterotoxin C in rabbit models of disease. Protein Eng. Des. Sel. [Epub ahead of print.] doi:10.1093/protein/gzs094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boehmer JL, DeGrasse JA, Lancaster VA, McFarland MA, Callahan JH, Ward JL. 2011. Evaluation of protein expression in bovine bronchoalveolar fluid following challenge with Mannheimia haemolytica. Proteomics 11:3685–3697 [DOI] [PubMed] [Google Scholar]
- 14. Vernozy-Rozand C, Mazuy-Cruchaudet C, Bavai C, Richard Y. 2004. Comparison of three immunological methods for detecting staphylococcal enterotoxins from food. Lett. Appl. Microbiol. 39:490–494 [DOI] [PubMed] [Google Scholar]
- 15. Miyamoto T, Kamikado H, Kobayashi H, Honjoh K, Iio M. 2003. Immunomagnetic flow cytometric detection of staphylococcal enterotoxin B in raw and dry milk. J. Food Prot. 66:1222–1226 [DOI] [PubMed] [Google Scholar]
- 16. Garber EAE, Venkateswaran KV, O'Brien TW. 2010. Simultaneous multiplex detection and confirmation of the proteinaceous toxins abrin, ricin, botulinum toxins, and Staphylococcus enterotoxins A, B, and C in food. J. Agric. Food Chem. 58:6600–6607 [DOI] [PubMed] [Google Scholar]
- 17. Pauly D, Kirchner S, Stoermann B, Schreiber T, Kaulfuss S, Schade R, Zbinden R, Avondet M, Dorner MB, Dorner BG. 2009. Simultaneous quantification of five bacterial and plant toxins from complex matrices using a multiplexed fluorescent magnetic suspension assay. Analyst 134:2028–2039 [DOI] [PubMed] [Google Scholar]