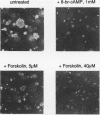
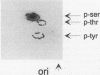
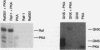Abstract
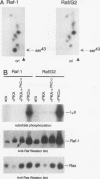
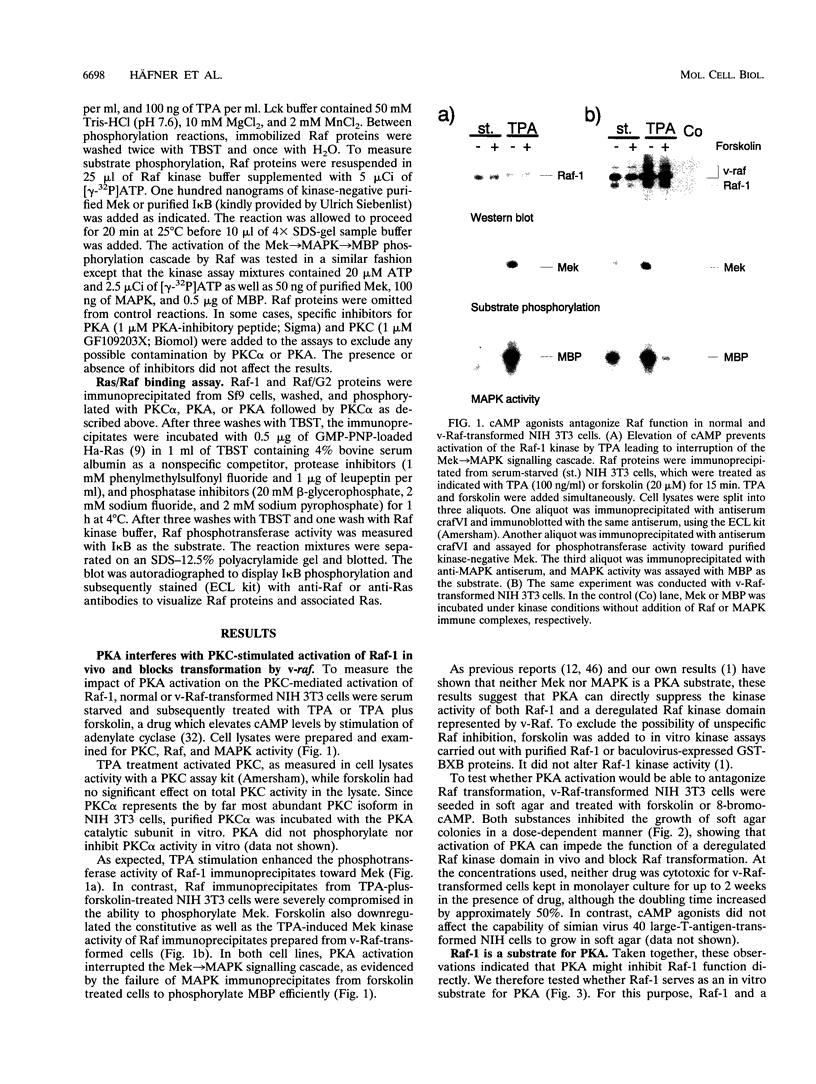
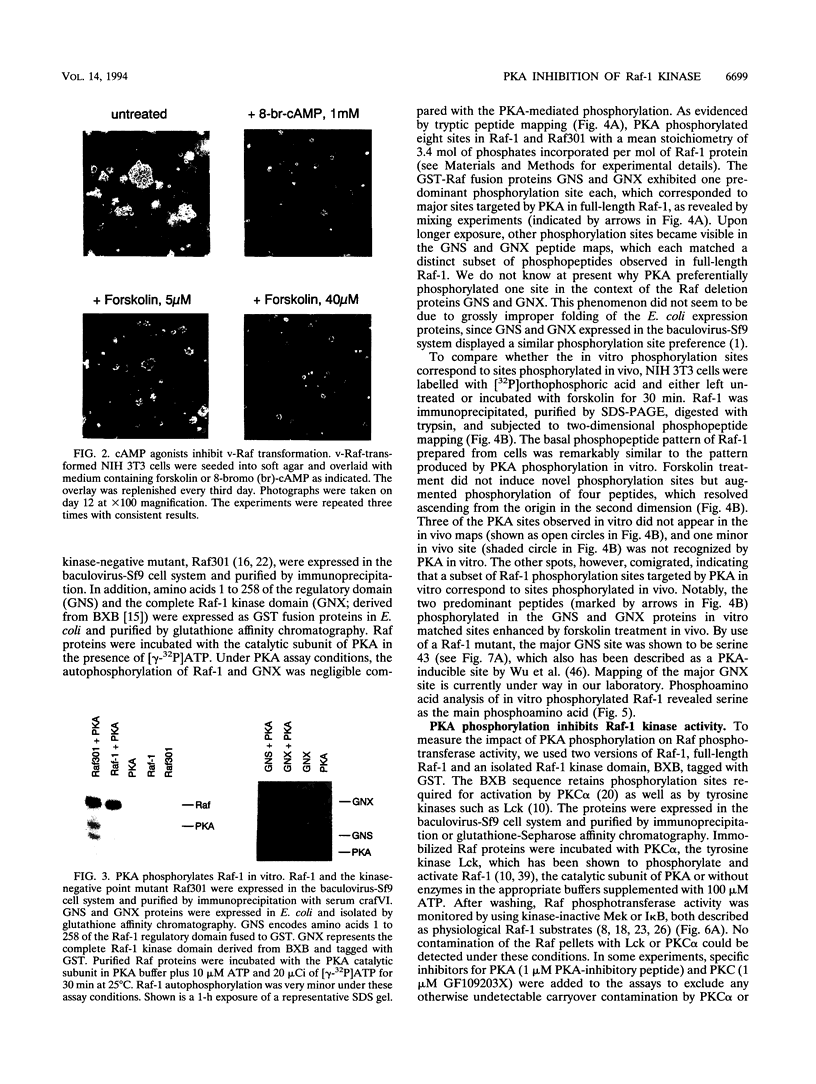
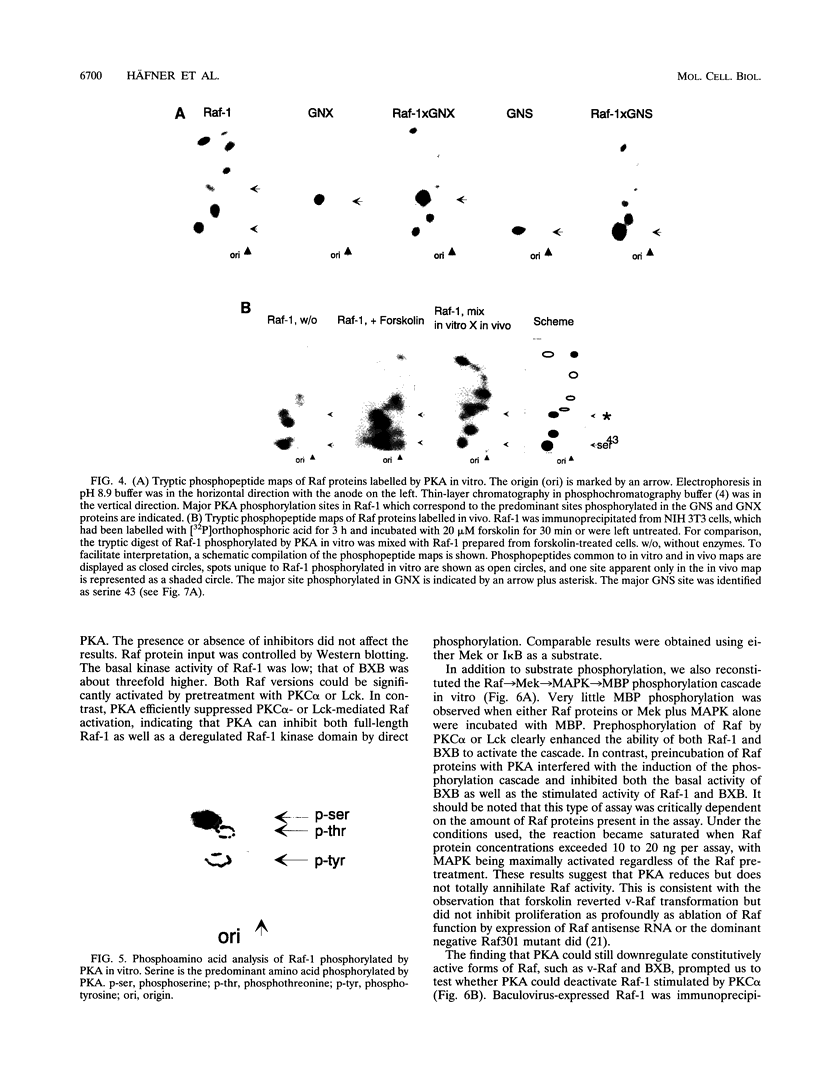
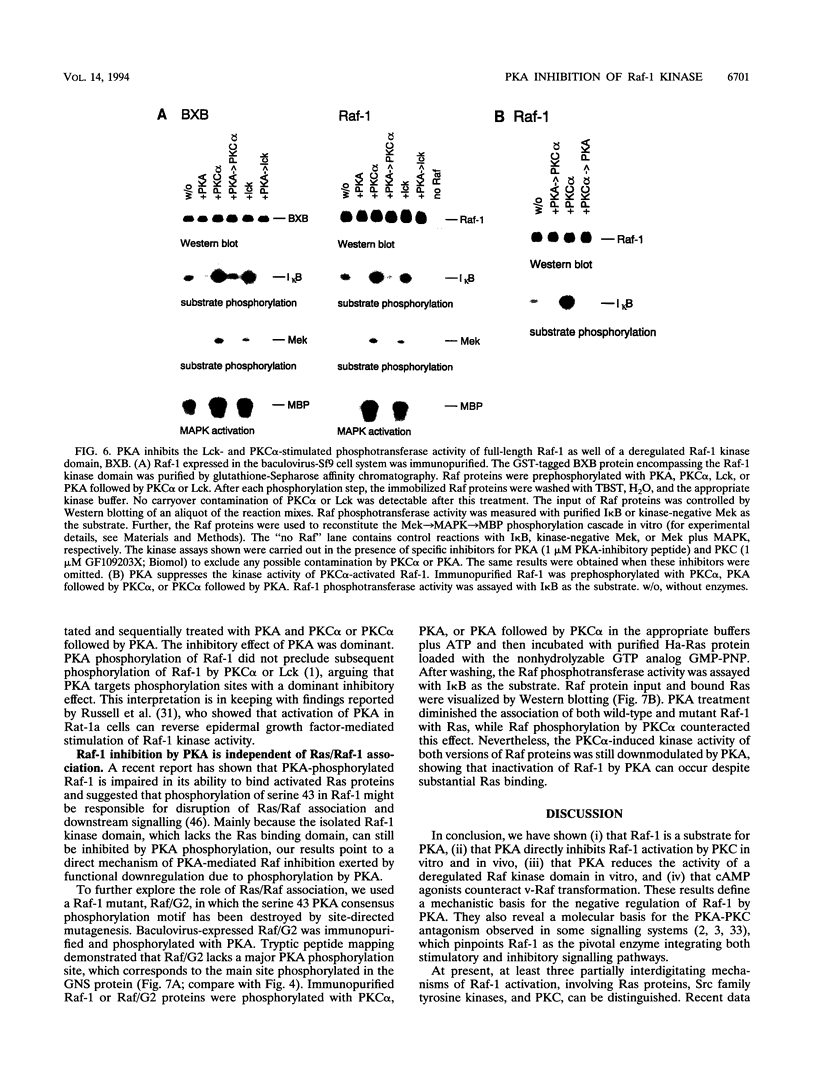
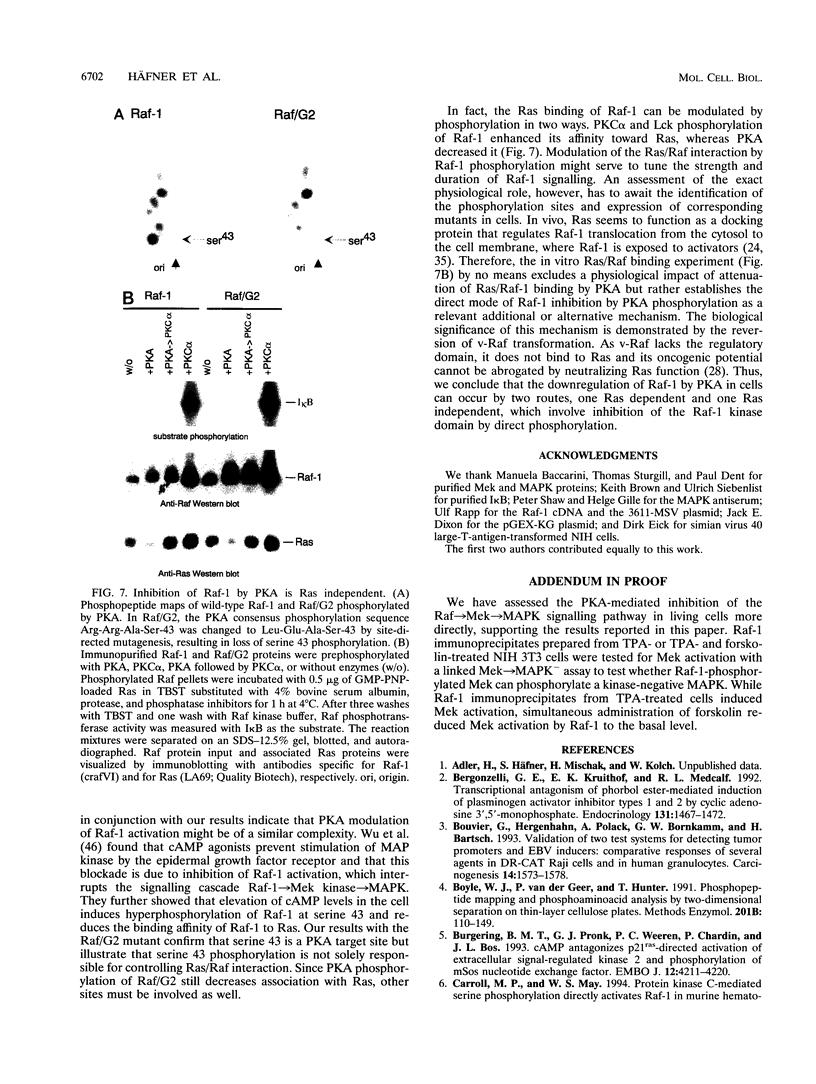
The cytoplasmic Raf-1 kinase is essential for mitogenic signalling by growth factors, which couple to tyrosine kinases, and by tumor-promoting phorbol esters such as 12-O-tetradecanoylphorbol-13-acetate, which activate protein kinase C (PKC). Signalling by the Raf-1 kinase can be blocked by activation of the cyclic AMP (cAMP)-dependent protein kinase A (PKA). The molecular mechanism of this inhibition is not precisely known but has been suggested to involve attenuation of Raf-1 binding to Ras. Using purified proteins, we show that in addition to weakening the interaction of Raf-1 with Ras, PKA can inhibit Raf-1 function directly via phosphorylation of the Raf-1 kinase domain. Phosphorylation by PKA interferes with the activation of Raf-1 by either PKC alpha or the tyrosine kinase Lck and even can downregulate the kinase activity of Raf-1 previously activated by PKC alpha or amino-terminal truncation. This type of inhibition can be dissociated from the ability of Raf-1 to associate with Ras, since (i) the isolated Raf-1 kinase domain, which lacks the Ras binding domain, is still susceptible to inhibition by PKA, (ii) phosphorylation of Raf-1 by PKC alpha alleviates the PKA-induced reduction of Ras binding but does not prevent the downregulation of Raf-1 kinase activity by PKA and (iii) cAMP agonists antagonize transformation by v-Raf, which is Ras independent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergonzelli G. E., Kruithof E. K., Medcalf R. L. Transcriptional antagonism of phorbol ester-mediated induction of plasminogen activator inhibitor types 1 and 2 by cyclic adenosine 3',5'-monophosphate. Endocrinology. 1992 Sep;131(3):1467–1472. doi: 10.1210/endo.131.3.1354603. [DOI] [PubMed] [Google Scholar]
- Bouvier G., Hergenhahn M., Polack A., Bornkamm G. W., Bartsch H. Validation of two test systems for detecting tumor promoters and EBV inducers: comparative responses of several agents in DR-CAT Raji cells and in human granulocytes. Carcinogenesis. 1993 Aug;14(8):1573–1578. doi: 10.1093/carcin/14.8.1573. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Pronk G. J., van Weeren P. C., Chardin P., Bos J. L. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 1993 Nov;12(11):4211–4220. doi: 10.1002/j.1460-2075.1993.tb06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. P., May W. S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994 Jan 14;269(2):1249–1256. [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- DiBattiste D., Golubic M., Stacey D., Wolfman A. Differences in the interaction of p21c-Ha-ras-GMP-PNP with full-length neurofibromin and GTPase-activating protein. Oncogene. 1993 Mar;8(3):637–643. [PubMed] [Google Scholar]
- Fabian J. R., Daar I. O., Morrison D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993 Nov;13(11):7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A. M., Vaillancourt R. R., Johnson G. L. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993 Aug 25;268(24):17896–17901. [PubMed] [Google Scholar]
- Graves L. M., Bornfeldt K. E., Raines E. W., Potts B. C., Macdonald S. G., Ross R., Krebs E. G. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hallberg B., Rayter S. I., Downward J. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J Biol Chem. 1994 Feb 11;269(6):3913–3916. [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Kölch W., Morrison D. K., Rapp U. R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- Hordijk P. L., Verlaan I., Jalink K., van Corven E. J., Moolenaar W. H. cAMP abrogates the p21ras-mitogen-activated protein kinase pathway in fibroblasts. J Biol Chem. 1994 Feb 4;269(5):3534–3538. [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Koide H., Satoh T., Nakafuku M., Kaziro Y. GTP-dependent association of Raf-1 with Ha-Ras: identification of Raf as a target downstream of Ras in mammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8683–8686. doi: 10.1073/pnas.90.18.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Weissinger E., Mischak H., Troppmair J., Showalter S. D., Lloyd P., Heidecker G., Rapp U. R. Probing structure and function of the raf protein kinase domain with monoclonal antibodies. Oncogene. 1990 May;5(5):713–720. [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Li S., Sedivy J. M. Raf-1 protein kinase activates the NF-kappa B transcription factor by dissociating the cytoplasmic NF-kappa B-I kappa B complex. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9247–9251. doi: 10.1073/pnas.90.20.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Russell M., Winitz S., Johnson G. L. Acetylcholine muscarinic m1 receptor regulation of cyclic AMP synthesis controls growth factor stimulation of Raf activity. Mol Cell Biol. 1994 Apr;14(4):2343–2351. doi: 10.1128/mcb.14.4.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Leli U., Nixon R. A. Opposing influences of protein kinase activities on neurite outgrowth in human neuroblastoma cells: initiation by kinase A and restriction by kinase C. J Neurosci Res. 1992 Nov;33(3):398–407. doi: 10.1002/jnr.490330306. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Thompson P. A., Ledbetter J. A., Rapp U. R., Bolen J. B. The Raf-1 serine-threonine kinase is a substrate for the p56lck protein tyrosine kinase in human T-cells. Cell Growth Differ. 1991 Dec;2(12):609–617. [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Warne P. H., Viciana P. R., Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993 Jul 22;364(6435):352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]