Abstract
The filamentous, heterocystous cyanobacterium Anabaena sp. strain PCC 7120 is one of the simplest multicellular organisms that show both morphological pattern formation with cell differentiation (heterocyst formation) and circadian rhythms. Therefore, it potentially provides an excellent model in which to analyze the relationship between circadian functions and multicellularity. However, detailed cyanobacterial circadian regulation has been intensively analyzed only in the unicellular species Synechococcus elongatus. In contrast to the highest-amplitude cycle in Synechococcus, we found that none of the kai genes in Anabaena showed high-amplitude expression rhythms. Nevertheless, ∼80 clock-controlled genes were identified. We constructed luciferase reporter strains to monitor the expression of some high-amplitude genes. The bioluminescence rhythms satisfied the three criteria for circadian oscillations and were nullified by genetic disruption of the kai gene cluster. In heterocysts, in which photosystem II is turned off, the metabolic and redox states are different from those in vegetative cells, although these conditions are thought to be important for circadian entrainment and timekeeping processes. Here, we demonstrate that circadian regulation is active in heterocysts, as shown by the finding that heterocyst-specific genes, such as all1427 and hesAB, are expressed in a robust circadian fashion exclusively without combined nitrogen.
INTRODUCTION
Circadian clocks are endogenous self-sustained oscillators with a period of ∼24 h. A variety of organisms, from bacteria to plants and mammals, use circadian clocks to adapt to daily environmental changes. Cyanobacteria are the simplest organisms known to exhibit circadian rhythms, although a recent analysis suggested that a prototype of circadian regulation may also be involved in the cellular metabolism of some halobacterial species (1). Most molecular, genetic, and physiological studies of cyanobacterial circadian systems have been performed with the unicellular strain Synechococcus elongatus sp. strain PCC 7942 (here called Synechococcus). In contrast, the circadian regulation in multicellular, filamentous cyanobacteria has not been investigated at the molecular level. In the filamentous species Anabaena flos-aquae, which produces neurotoxins and forms blue blooms, the rate of cellular autolysis in a population appears to be controlled by a circadian clock (2). However, its underlying mechanism for the population dynamics remains unknown, and no molecular genetic analysis has been established in this species. Among the many filamentous species, Anabaena sp. strain PCC 7120 is a well-established genetic model strain in terms of its multicellularity, developmental program, and nitrogen metabolism. In the absence of a combined nitrogen source, Anabaena differentiates cells called “heterocysts” at a rate of one every ∼10 to 15 cells; these are specialized for oxygen-sensitive nitrogen fixation. In these cells, photosystem II activity is turned off, respiration is enhanced, and a thick envelope consisting of layers of polysaccharide and glycolipid is developed to reduce the intracellular oxygen concentration (3–5). Therefore, Anabaena sp. PCC 7120 should be the simplest model organism in which to study circadian systems in relation to multicellularity and morphological development.
Here, we report the first molecular description of the circadian system in Anabaena sp. PCC 7120. For detailed analysis, we wanted to identify clock-controlled genes that exhibit robust cycling under continuous conditions with high amplitude. In Synechococcus, the rhythmic genes with the highest amplitude are kaiB and kaiC, which are organized into an operon that is essential for circadian timing, and their expression peaks at subjective dusk under continuous-light (LL) conditions (6, 7). The cycling of the kaiBC operon is driven by the transcription-translation feedback loop, in which the Kai clock proteins regulate their own expression. This was originally thought to be the core of the circadian oscillation in Synechococcus (6). Although we have demonstrated that the kaiBC expression rhythm is not an essential requirement for circadian regulation (8, 9), most previous molecular and physiological studies have successfully used a bioluminescent reporter (luciferase) to monitor the rhythm of the high-amplitude kaiBC promoter activity under LL (10, 11). In Synechococcus, KaiA activates the autophosphorylation of KaiC, and this activation is antagonized by KaiB. In the presence of ATP, we reconstituted the circadian KaiC phosphorylation cycle in vitro by simply incubating KaiC with KaiA and KaiB (12). KaiA also functions as a positive regulator of kaiBC transcription in the presence of KaiC protein in Synechococcus (13). Anabaena contains homologues of the kaiA, kaiB, and kaiC genes of Synechococcus. Interestingly, the N-terminal two-thirds of Synechococcus KaiA is missing in the KaiA homologue in most filamentous species (14). Uzumaki et al. (15) replaced the kaiA gene with its Anabaena homologue in Synechococcus, which exhibited a lower-amplitude kaiBC promoter rhythm with a longer period of ∼40 h, and reported that Anabaena KaiA was able to homodimerize and activate Synechococcus KaiC phosphorylation in vitro, as does Synechococcus KaiA. The crystal structures of the Anabaena KaiA and KaiB homologues have also been analyzed in an initial structural biological analysis of the cyanobacterial clock proteins (16). However, no in vivo analysis of the expression of the kai genes in Anabaena has been reported.
Here, we found that in Anabaena the kaiA, kaiB, and kaiC homologues show only very low-amplitude (or arrhythmic) expression under LL. Therefore, we performed a DNA microarray analysis and found that the expression of 78 genes was significantly rhythmic. Two bacterial luciferase reporter strains that exhibited robust bioluminescence rhythms with different phase relationships were constructed. The bioluminescence satisfied all three criteria for circadian rhythms: free running, with a period of ∼24 h under LL; temperature compensation of the period; and entrainment to a light-dark regimen. Genetic disruption of the kai gene cluster nullified all tested transcriptional rhythms.
The metabolic conditions are plausibly quite different in the autotrophic vegetative cells and the diazotrophic heterocysts. In Synechococcus, the robustness and photic input pathways of the core circadian timing mechanism have been proposed to be affected by metabolic states, such as the redox state and ATP concentration, via modifying the biochemical properties of the Kai protein complex (17–19). Thus, to understand a possible link among metabolism, differentiation, and circadian regulation in Anabaena, it is important to determine whether circadian regulation is active in heterocysts and, if so, the extent to which the circadian input and output are common to both the vegetative and differentiated cells. Therefore, we performed a microarray analysis without combined nitrogen. By combining a transcriptomic analysis of heterocyst-enriched samples and the expression profile of the hetR-null mutant strain (20), we identified a subset of genes that are expressed exclusively in heterocysts, with robust Kai-based circadian cycling under LL. Thus, circadian regulation functions within both the differentiated and vegetative cells of Anabaena sp. PCC 7120.
MATERIALS AND METHODS
Bacterial strains.
The wild-type Anabaena sp. strain PCC 7120 and its derivatives used in this study are listed in Table S1 in the supplemental material. For the bioluminescent reporter strains, the ∼500-bp 5′ upstream regions (5′-USRs) from the coding regions of pecB (alr0523) and all3173 were amplified by PCR, using Anabaena genomic DNA as the template, and were cloned into the SalI-KpnI sites of pRL488 (20), a shuttle vector that contains the Vibrio fischeri luciferase gene set, luxAB, to construct pIL392 and pIL393, respectively. The Vibrio harveyi luxCDE genes, which catalyze the biosynthesis of aldehyde substrates, from pAM1619 (10) were then amplified and cloned into the SalI-BamHI sites of pBluescript II KS(+) (Stratagene) to produce pIL373. For the induction of luxCDE in Anabaena, the ∼500-bp 5′-USR of the constitutively expressed gene all0011 was amplified using Anabaena genomic DNA as the template and then cloned into the SalI site of pIL373 to produce pIL374. The EcoRI segment containing the 5′-USR all0011::luxCDE cassette from pIL374 was then cloned into the EcoRI sites of pIL392 and pIL393 to produce the pIL407 (pecB::luxAB) and pIL411 (all3173::luxAB) reporter plasmids, respectively. pIL407 or pIL411 was transferred by conjugation into the wild-type Anabaena sp. PCC 7120 in the presence of neomycin (50 μg/ml) to segregate the bioluminescent reporter strain ILC292 or ILC379, respectively. For the kaiABC-null mutant strains, the 1,000-bp 5′-USR from the kaiA coding region, a streptomycin/spectinomycin resistance gene cassette (Ω fragment) (21), and the 1,000-bp 3′ downstream region from the kaiC coding region were amplified separately, tandemly connected by using the mutagenic PCR method (22), and then cloned into the BglII site of pRL271 (23), an integration plasmid for genomic DNA, to produce pIL204. pIL204 was transferred by conjugation into the wild-type Anabaena sp. PCC 7120, and double recombinants were selected with spectinomycin and sucrose (sacB selection) to obtain the kaiABC-null mutant strain, ILC352. For the bioluminescence assays with kaiABC-null mutant strains, pIL407 and pIL411 were transferred by conjugation into ILC352 to produce the kaiABC-nullified reporter strains ILC505 and ILC507, respectively.
Culture conditions.
For the microarray and Northern blotting analyses, Anabaena cells were grown in BG-11 medium containing 17.6 mM NaNO3 as a combined nitrogen source (24) buffered with 20 mM HEPES-NaOH (pH 7.5) or in BG-110 (without combined nitrogen) medium in a continuous-culture system in a 1.5-liter flat glass bottle for algal culture (Fujimoto Rika, Tokyo, Japan; width, 160 mm; diameter, 40 mm; height, 500 mm) at an optical density at 750 nm (OD750) of 0.2 or 0.25 at 30°C under white fluorescent light at 30 μmol m−2 s−1 (here called “standard conditions”), illuminating the lateral flat surface of the bottle. To synchronize the circadian clock, the culture was acclimated to two light-dark (LD) cycles (12 h-12 h) and transferred into the light for the indicated periods. For the bioluminescence assays, the cells were cultured in BG-11 or BG-110 liquid medium in the presence or absence of antibiotics (10 μg/ml neomycin or 20 μg/ml spectinomycin) under standard conditions for several days up to an OD750 of ∼0.2. A 10-μl aliquot of diluted culture with an OD750 of 2 × 10−3 was then inoculated onto BG-11 or BG-110 solid medium containing 1.5% agar in 35-mm plates, maintained under standard conditions for 6 to 10 days, acclimated to two LD cycles, transferred into the light, and subjected to the bioluminescence assay.
Heterocyst-enriched fraction analysis.
The heterocyst-enriched fraction was obtained from heterocyst-induced cell filaments 48 h after nitrate depletion, as described previously (25). Microscopic observation confirmed that 80% of the cells in the fraction were heterocysts. Total RNAs were extracted and subjected to microarray analysis as described previously, using the current versions of oligonucleotide arrays (25). As the control sample, the total RNA extracted from cells that had been cultured for 48 h after nitrate depletion without heterocyst enrichment was also subjected to microarray analysis. Based on the fluorescent signals in eight independent experiments, we calculated the enrichment ratio (Ri) as the value for each expression level of a gene i in the heterocyst-enriched fraction (target signal) divided by that in the control sample (control signal). We calculated the ratio for each amount of transcript in a heterocyst to that in a vegetative cell, based on the following two assumptions: (i) the total RNA contents purified from a heterocyst and a vegetative cell were estimated to be equivalent, and (ii) the genes with maximum enrichment ratios were expressed not in vegetative cells but only in heterocysts. When one heterocyst develops per n cells on average and the average expression levels of gene i in a single heterocyst and in a single vegetative cell are defined as Hi and Vi, respectively, the enrichment ratio Ri can be calculated as follows: Ri = n(0.8Hi + 0.2Vi)/[Hi + (n − 1)Vi].
Because the maximum ratio (Rasl3656) was 9.28 for the asl3656 gene, encoding a hypothetical protein (Hasl3656 = 1; Vasl3656 = 0), n was estimated to be 11.6, which is within the normal range for heterocyst differentiation. Note that the maximum ratio was slightly higher than the ratio of 8.98 for nifK, which encodes a nitrogenase subunit and is expressed exclusively in heterocysts, validating our second assumption. Therefore, the induction ratio Ii, defined as Hi/Vi for gene i, can be calculated as follows: Ii = (10.6Ri − 2.32)/(9.28 − Ri).
Northern blotting.
Northern blotting was performed by loading 1 or 2 μg of total RNA into each lane of an electrophoretic gel, which was blotted and then probed with digoxigenin-labeled DNA (PCR) probes, as described previously (8).
DNA microarray analysis.
A DNA microarray analysis was performed as described previously (25, 26), with some modifications. Briefly, for the circadian expression analysis, 5 to 7 μg of each total RNA from Anabaena cells collected at appropriate times and 4 μl of an Anabaena-specific primer mix (17.2 nM each) were used in a reverse transcription (RT) reaction to generate Cy3-labeled cDNAs (target samples). Totals of 5 to 7 μg of evenly mixed RNA samples (containing RNAs from hour 4 to 48 in LL) were used to produce Cy5-labeled cDNAs as a control sample. The total RNA and an Anabaena-specific primer mix were denatured at 90°C for 5 min and then gradually cooled to 42°C over 20 min. The RT reaction was performed in 20 μl containing the total RNA, Anabaena-specific primer mix, 10 mM dithiothreitol (DTT), 62.5 to 250 μM (each) dATP, dCTP, and dGTP, 25 to 100 μM dTTP, 100 μM Cy3-dUTP or Cy5-dUTP (GE Healthcare), 20 U of RNase inhibitor (TaKaRa Bio), and 200 U of SuperScript II or III reverse transcriptase (Invitrogen, CA) in 1× first-strand buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2). The mixtures were incubated at 42°C for 90 min. RNA was degraded by the addition of 10 μl of 0.1 N NaOH, followed by incubation at 70°C for 10 min. The mixtures were neutralized by the addition of 15 μl of 1 M Tris-HCl (pH 7.5). The products of the two reactions (one with Cy3 labeling and the other with Cy5 labeling) were mixed, and unincorporated fluorescent nucleotides were removed with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) or the Illustra CyScribe GFX purification kit (GE Healthcare). The labeled cDNA was recovered in 29 μl of 10 mM Tris-HCl (pH 8.5), added to 10 μl of 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), denatured at 95°C for 5 min, allowed to cool to room temperature, and mixed with 1 μl of 10% SDS. The microarrays were hybridized and washed as described previously (25). The arrays were scanned with the Typhoon 9410 variable-mode imager (GE Healthcare), and the background-subtracted median fluorescence intensities (nMedianDens values) were calculated with the ArrayVision software (ver. 8.0; GE Healthcare). Global normalization was also applied to the Cy3-derived signals under LL conditions so that the average expression levels for all open reading frames (ORFs) within a microarray were equal across the replicate arrays, as described previously (7).
qRT-PCR.
For the nitrate deprivation experiments, the filaments of the wild-type strain and the hetR mutant strain DRhetRS (27), grown in BG-11 medium until they reached an OD750 of 0.4 to 0.5, were washed twice with water and then resuspended in BG-110 medium. The filaments were collected at the indicated times after nitrate deprivation. RNA extraction and the quantitative RT-PCR (qRT-PCR) analyses were performed as described previously (28), using the following primer pairs: RT1427-F (5′-TGCGAGTGTAGCAGAAGCAG-3′) and RT1427-R (5′-CGATACCGTAAGCATCAGCA-3′) for all1427 and RT1432-F (5′-TCTGAAGTCAGCGACGGTTT-3′) and RT1432-R (5′-CCCAATCATCCGTCATCAG-3′) for hesA.
Identification of circadian cycling genes.
The circadian indices, i.e., amplitude, correlation P value, and peak time of gene expression, were determined as described previously (7). Initially, global normalization was applied to the RNA signal profiles so that the averages of the expression levels for all ORFs within a microarray were equal across the replicate arrays. We identified statistically significant circadian cycling expression profiles for the Anabaena ORFs under LL using two criteria: the “correlation P value” to extract genes with periodic expression patterns and the “amplitude” to identify genes in which the changes were above the background level, as described by Ito et al. (7). Briefly, the correlation P value (here called the “P value”) is an index parameter that indicates the degree to which the gene expression profile has circadian periodicity, and it is defined as the significance of Pearson's correlation between each expression profile and a cosine wave for which the period is 24 h. The amplitude is an index parameter that indicates the extent to which the fluctuation in a gene expression profile is above the background level, and it is defined as the coefficient of variation (standard deviation divided by the mean). When we performed two experiments under nitrate-supplemented conditions, the minimum amplitude and maximum P value for each gene were used as the threshold values, as described previously (7). In our previous analysis of dark-acclimated samples of Synechococcus, we applied global normalization because the total raw signals were dramatically reduced in the dark (7). In contrast, in Anabaena, there was no statistically significant difference in the total fluorescence signals for the dark-collected samples (4, 8, or 12 h in the dark) and the light-collected samples (4, 8, or 12 h in the light), so global normalization was also applied to the dark samples in Anabaena. Detailed parameters extracted from the microarray data are summarized in Data Set S1 in the supplemental material.
Bioluminescence assays.
The bioluminescence profiles were measured with a photomultiplier tube on agar plates under LL after two cycles of 12-h/12-h LD alternations, as described previously (13).
Microarray data accession numbers.
The microarray data have been deposited in the KEGG Expression Database (accession numbers ex0001892 to ex001947.
RESULTS AND DISCUSSION
kai gene expression is not robustly rhythmic in Anabaena.
We first performed a Northern hybridization analysis of the expression of the kaiA, kaiB, and kaiC genes in Anabaena under LL, after two cycles of 12-h/12-h LD cycles, every 4 h for 48 h in the presence of combined nitrogen in the culture medium. As shown in Fig. 1A, in contrast to the high-amplitude kaiBC transcriptional rhythm in Synechococcus, we observed no reliably high-amplitude rhythmic expression of any of the kai genes in Anabaena, although they were likely expressed with a low-amplitude rhythm. In the filamentous cyanobacteria, including Anabaena, an additional kaiB-like (kaiBL) sequence is located at a different locus from the kaiABC gene cluster in the genome. It is three times longer than the primary kaiB gene (14), although its function remains unknown. We also analyzed this gene, and again, its expression was found to be almost constant over the circadian cycle (Fig. 1A). A very low-amplitude cycle in kai gene expression does not necessarily mean that circadian transcriptional control is less active in Anabaena. In another unicellular cyanobacterial strain, Synechocystis sp. strain PCC 6803, a DNA microarray analysis identified ∼50 significantly rhythmic genes, whereas rhythmic clock gene expression was limited to low-amplitude kaiA expression (29). Moreover, we recently found that in Synechococcus the expression rhythms of only a subset of genes are dampened in the dark, even when de novo kai gene expression is turned off (9).
Fig 1.
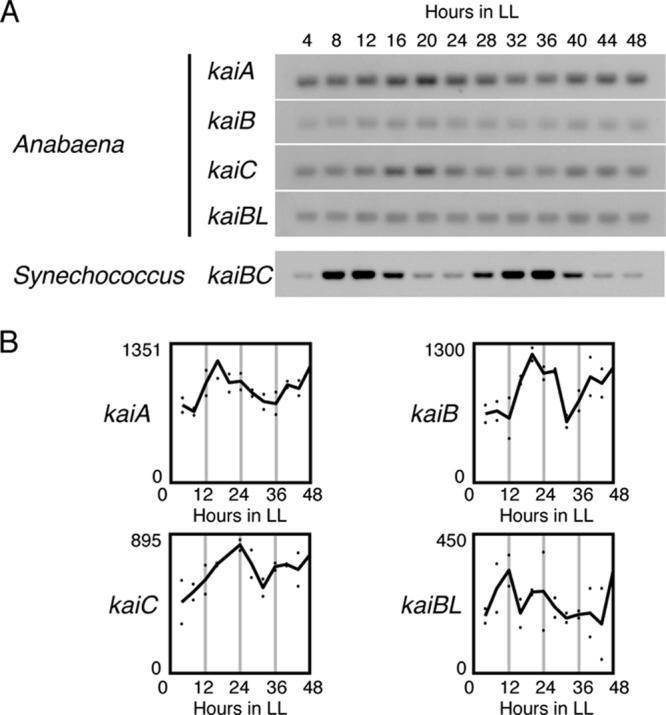
Expression of Anabaena kai genes is not robustly rhythmic under LL conditions. (A) Total RNAs were extracted from Anabaena cells that had been collected every 4 h from a continuous culture under LL after two LD cycles in the presence of sodium nitrate. They were subjected to Northern hybridization analysis using the extracted RNAs (1.0 μg) of each Anabaena kaiA, kaiB, kaiC, or kaiBL sequence as probes. As a reference, the high-amplitude kaiBC mRNA profile in Synechococcus is also shown. (B) Densitometric analysis of kai gene expression under LL, obtained with DNA microarray experiments. The dots represent the signals from two independent experiments, and the solid lines indicate average expression levels. The numbers on the ordinate indicate the relative expression levels.
Genome-wide circadian transcription profiles in Anabaena.
To examine whether circadian transcription functions in Anabaena in the absence of high-amplitude kai gene expression rhythms and to identify clock-controlled genes, if any, we performed a DNA microarray analysis using the Anabaena oligonucleotide microarray designed by Ehira and Ohmori (26), representing 5,336 ORFs from the 5,368 genes in the genome. Anabaena cells were cultured in a continuous culture system under LL after two LD cycles and were collected every 4 h (from 0 h in the dark in the second LD cycle to 48 h in LL) in the presence of combined nitrogen. The cells were collected from two independent cultures, their total RNA extracted, and a DNA microarray analysis performed (see Materials and Methods). On the basis of the filtration method, taking into account the oscillatory index parameters (amplitude and correlation P value, summarized in Data Set S1 and Table S2 in the supplemental material; for the standard filtering conditions, a P value of <0.05 and an amplitude of > 10−0.7) and the reproducibility over two independent experiments, as described previously (7) with slight modifications (see Materials and Methods), we identified 78 genes as “significantly rhythmic” under LL (Fig. 2A; see Data Set S1 and Table S2 in the supplemental material). This number may be larger or smaller, depending on the filtration cutoff values that are applied: 18 genes with more stringent conditions (P value of <0.01 and amplitude of > 10−0.6) or 192 genes with less stringent conditions (P value of <0.1 and amplitude of > 10−0.8) (Fig. 2A). The left panel of Fig. 2B shows the 78 cycling genes that we identified, in order of peak time under LL. As in Synechococcus (7), there are two peaks at around circadian time (CT) 8 to 12 and CT 20 to 24 (Fig. 2B and C) in Anabaena, but among the clock-controlled genes, there is a larger population of genes that peak in the intermediate phases (CT 4 to 8 and 12 to 20) than in Synechococcus. We performed a Northern blot analysis of 14 genes that exhibited relatively high amplitudes and different peak times, with results that resembled those of the microarray profiles (Fig. 3), validating our analysis. Our microarray analysis also confirmed that the kaiA and kaiC genes are not expressed in a significantly circadian manner under our filtration conditions, whereas kaiB was identified as a low-amplitude gene (Fig. 1B). This is similar in Synechocystis, in which only the expression of the kaiA gene is rhythmic, with a low amplitude, as described above (29).
Fig 2.
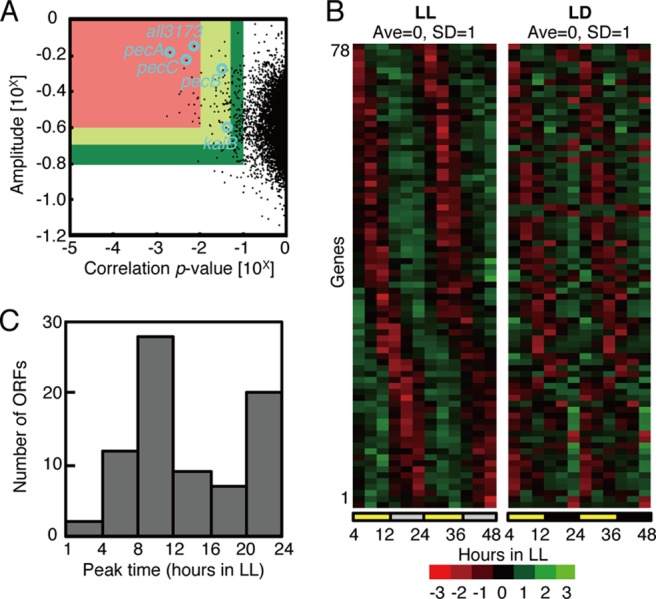
Genome-wide circadian transcription profiles under LL conditions. (A) Variations in the oscillatory indices, amplitude (defined as standard deviation [SD] normalized to the mean value; ordinate), and a cosine-fitting correlation score (correlation P value; abscissa) for each transcript under LL conditions. Each index is defined in the Materials and Methods. Briefly, a higher amplitude represents larger fluctuations, and a lower P value means a better correlation to a periodic (sinusoidal) waveform with a 24-h period. Seventy-eight “cycling genes” were extracted, with P values of <0.05 and amplitudes of >10−0.7 (within the red and yellow regions). For more stringent filtration, the lower amplitude and the higher P value from two independent experiments were used to ensure reproducibility. The circles indicate the all3173 and pecBAC (alr0523 to -0525) genes used for the bioluminescence reporter analysis and the kaiB gene. (B) Expression profiles of the 78 cycling genes sorted by peak time under LL conditions (left). The panel on the right shows a double plot of the expression profiles of the corresponding genes in one LD cycle reconstructed by connecting data from hour 4 to 12 in LL and hour 4 to 12 in the dark. The colors in descending order from red to black to green represent normalized data. The average and SD over two cycles are 0.0 and 1.0, respectively. The average levels in two independent experiments were used for the analysis. (C) Phase distributions of the peak expression times of 78 circadianly expressed genes. The numbers of ORFs from two independent experiments are plotted on the ordinate.
Fig 3.
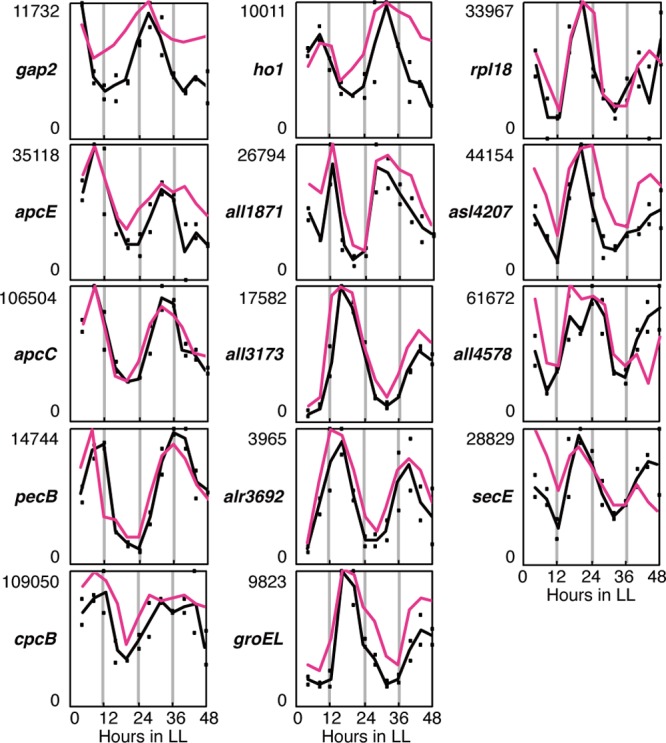
Temporal expression profiles of representative clock-controlled genes. The expression of photosynthesis-associated gap2 (all5062), encoding glyceraldehyde-3-phosphate dehydrogenase, and phycobilisome-related genes apcE (alr0020), apcC (asr0023), pecB (alr0523), cpcB (alr0528), and ho1 (all1897) peaked in subjective daytime, whereas rpl18 (all4200) and all4207 from a ribosomal gene cluster, secE (all5298), which is involved in protein secretion, and all1871, all3173, alr3692, groEL (alr3662), and all4578, encoding proteins with unknown functions, peaked in subjective night. For pecB and secE, we performed a Northern blot analysis with specific probes covering pecBAC (alr0523 to -0525) and all5298 and -5299, respectively. Note that pecB and all3173 were used for the bioluminescence reporter analysis. The pink and black lines represent the average expression levels calculated with Northern blot and microarray analyses, respectively. The dots represent the signals from two independent microarray experiments. The numbers on the ordinate indicate the relative expression levels based on the microarray analysis.
Clock-controlled genes with related functions.
As summarized in Fig. S1 and Table S2 in the supplemental material, the proportions of rhythmically expressed genes among those regulating the central metabolism, such as those involved in photosynthesis and respiration (9.2%, 14 of 153 genes), translation (3.7%, seven of 187 genes), amino acid biosynthesis (3.6%, four of 111 genes), cellular processes (3.3%, three of 92 genes), and energy metabolism (3.1%, three of 98 genes), were relatively higher than the proportion of rhythmic genes in the total genome (1.5%, 78 of 5336 genes). The cycling genes categorized as involved in photosynthesis/respiration/cell envelope and cellular processes/energy metabolism peaked in subjective day and night, respectively. Furthermore, most of the genes involved in translation and amino acid biosynthesis peaked in subjective night. Some gene clusters or operons consist of several ORFs. For example, the photosynthesis-associated phycobilisome-related genes, such as apcEABC (alr0020 to asr0023), pecBACEF (alr0523 to -0527), and the cpcG cluster (alr0534 to -0537), peaked in subjective daytime, whereas a ribosomal gene cluster (all4198 to all4215) and groEL (alr3662) peaked in subjective night (Fig. 3 shows the expression profiles of some representative ORFs). Interestingly, the homologues of these genes are not rhythmic in Synechococcus (7), revealing further differences in the circadian transcriptomic pathways of the two species. However, it should be noted that circadian rhythm in a transcript does not always give rise to cyclic protein induction or accumulation, and nonrhythmic gene expression does not necessarily mean that the encoded protein is arrhythmic, as discussed previously (7). Therefore, to validate the physiological relevance of the individual clock-controlled and/or diurnally controlled genes, more detailed analyses, including proteomic/metabolomic and physiological analyses, are required.
Gene expression profiles under LD cycles.
We have previously demonstrated in Synechococcus that the total mRNA level, which was estimated from the sum of the raw hybridization signals, was rapidly downregulated in the dark to ∼20% of the initial level at dark onset (after 12 h in the light, or zeitgeber time [ZT] 12) within 12 h, whereas it remained almost constant under LL conditions (7). Note that zeitgeber time is used to indicate the time in an LD cycle, where ZT 0 indicates dawn. In the present study, we also examined the expression profiles in the dark during the second LD cycle. In contrast to the case for Synechococcus, we observed no dramatic changes in the total raw fluorescent signals in Anabaena throughout the dark-to-dark transition (from hour 4 to 12 in the dark and subsequently from hour 4 to 12 in the light). This is not surprising, because such a dramatic difference in the total amount of expression over the LD cycles has been reported only for Synechococcus and has not been observed in other cyanobacterial strains, such as Cyanothece sp. strain ATCC 51142 (30). The right panel of Fig. 2B shows the double-plotted genome-wide expression profiles of the 78 clock-controlled genes under LD cycles from the data obtained for hour 4 to 12 under LL (ZT 4 to 12) and hour 12 to 24 under continuous-dark (DD) conditions (ZT 12 to 24). This result indicates that many of the subjective-daytime-expressed genes remain cyclic with the same phase angle under LL and LD conditions, whereas only a subset of subjective-night-expressed genes exhibit nocturnal expression under LD. Again, as for the circadian transcriptomic profiles, the physiological relevance of the diurnal/nocturnal control of Anabaena must be clarified with further proteomic analyses.
Confirmation of bona fide circadian regulation controlled by the kai-based oscillator in Anabaena.
To analyze the Anabaena circadian system in more detail, we constructed bioluminescent reporter strains in which a promoterless bacterial luciferase gene set from Vibrio harveyi was fused to the 5′-USRs of two high-amplitude genes alr0523 (pecB) and all3173, encoding a phycobilisome-related protein and a hypothetical protein, respectively. These genes were selected because of the large peak-to-trough ratios of their transcripts (Fig. 3) and their different peak times under LL: the pecBACEF cluster and all3173 peak at CT 8 to 12 and CT 16 to 20, respectively. The reporter constructs were introduced into Anabaena cells on a shuttle vector system by conjugal gene transfer (see Materials and Methods). The resulting reporter strains were cultured on solid medium in the presence of combined nitrogen and subjected to real-time bioluminescence assays with photomultiplier tubes under LL after two LD cycles, as described previously (13) with some modifications (see Materials and Methods). The results shown in Fig. 4A clearly demonstrate that both the pec and all3173 promoters display robust bioluminescent cycling, with periods of ∼24 h for at least 6 days under LL, maintaining an antiphasic relationship similar to that of the mRNA profiles (Fig. 3, pink lines). In addition to this free-running property, circadian oscillations are defined by the robustness of the period length against ambient constant temperature within a physiological range (the temperature compensation of the period), which allows the clock to keep time through all seasons. We confirmed that the Anabaena clock also satisfied the temperature compensation criterion, using the all3173reporter strain at 25, 30, and 35°C (Fig. 4B). The circadian-time-dependent photic entrainment property is also an important characteristic of circadian rhythms. In Synechococcus, 5-h dark pulses affect the phase of circadian gene expression differently, depending on the time at which the pulses are applied, and can be drawn as a phase response curve (PRC) (31). We performed the same experiment for the all3173 reporter strain by acclimating the cells to 5-h pulses at different circadian times, which is summarized as a PRC in Fig. 4C. The results are similar to those observed for Synechococcus (31) and clearly indicate that the Anabaena clock is also set by a 5-h dark period in a circadian-time-dependent manner. These results clearly confirm that the Anabaena transcription rhythms are driven by a bona fide circadian oscillator.
Fig 4.
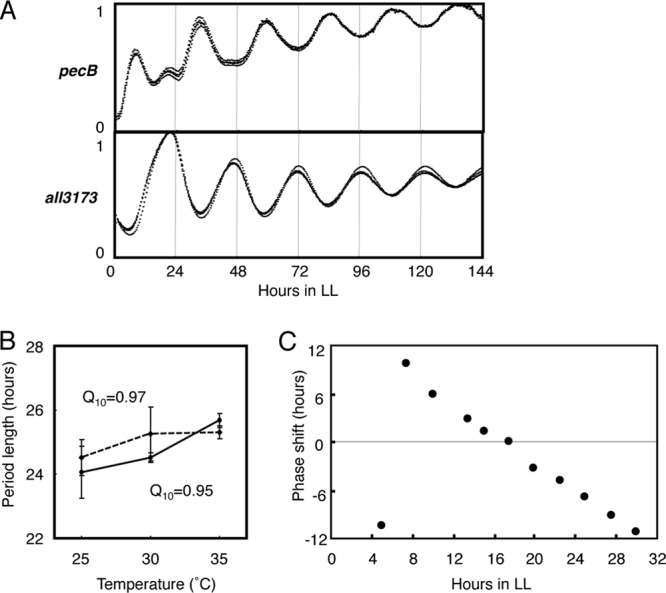
Circadian expression properties revealed with a bioluminescence reporter analysis. (A) Bioluminescence rhythms represent the clock-controlled promoter activities of the pecBACEF cluster (upper panel) and the all3173 gene (lower panel) from cells cultured on solid medium containing sodium nitrate in 35-mm culture plates under LL after two LD cycles. The bioluminescence profiles from four independent culture plates are shown (black traces). (B) Temperature compensation of the period. The bioluminescence profiles were monitored for the all3173 reporter strain, which was cultured on solid medium in the presence (solid line) or absence (dashed line) of sodium nitrate under LL after two LD cycles at 25, 30, or 35°C. The period length was plotted against temperature (n = 7 for each condition). The temperature coefficient Q10 values (the rate of change of a period length as a consequence of increasing the temperature by 10°C) in the presence and absence of combined nitrogen were 0.95 and 0.97, respectively. (C) Phase resetting of the all3173 bioluminescence rhythm in response to a 5-h dark pulse. At the circadian times indicated on the abscissa, the samples underwent 5 h of dark incubation, and they were then returned to LL to monitor the bioluminescence rhythm. The ordinate for each data point indicates the offset of the phase of the peaks after the treatment, relative to a control not pulsed with darkness, indicating a phase advance (positive values) or phase delay (negative values).
Although the expression profiles of the kai genes in Synechococcus and Anabaena are strikingly different, it seems plausible that the Anabaena circadian transcription rhythms are regulated by the kai gene products. To test this, we genetically eliminated the entire kaiABC gene cluster from the chromosome of the Anabaena cells by replacing it with a streptomycin/spectinomycin resistance gene. No accumulation of kai mRNA was observed by Northern blot analysis (Fig. 5A). We then introduced a reporter cassette to monitor the all3173 promoter activity. As expected, the kaiABC-nullified reporter strain failed to exhibit a circadian rhythm in its bioluminescence under LL after two LD cycles, as shown in Fig. 5B, whereas the average level of bioluminescence was unaffected. Moreover, Northern analysis confirmed that kaiABC disruption nullified the robust oscillations in pecBAC, all3173, and groEL transcripts observed in the wild type strains (Fig. 5C). Interestingly, the expression levels of pecBAC and all3173 were reduced in the kaiABC mutant strain, while in Synechococcus no rhythmic genes have been found to be dramatically downregulated by kaiABC nullification (7). Therefore, we conclude that the circadian transcription cycle shown in the present study is regulated by the Kai protein-based oscillator. It has been suggested that the transcription-translation feedback for the kai gene expression cycle is a slave oscillatory cycle in Synechococcus, whereas the posttranslational enzymatic oscillation produced by the Kai proteins is considered to be the core timing process (8, 12, 32, 33), represented by the rhythms in KaiC phosphorylation (8, 13), ATPase activity (34), and protein complex formation (35, 36). Although Anabaena KaiA is reported to form a homodimer and enhanced the rate of phosphorylation of a recombinant Thermosynechococcus KaiC protein (15) in vitro, no information is currently available on Anabaena KaiC phosphorylation or protein complex formation profiles, either in vitro or in vivo.
Fig 5.
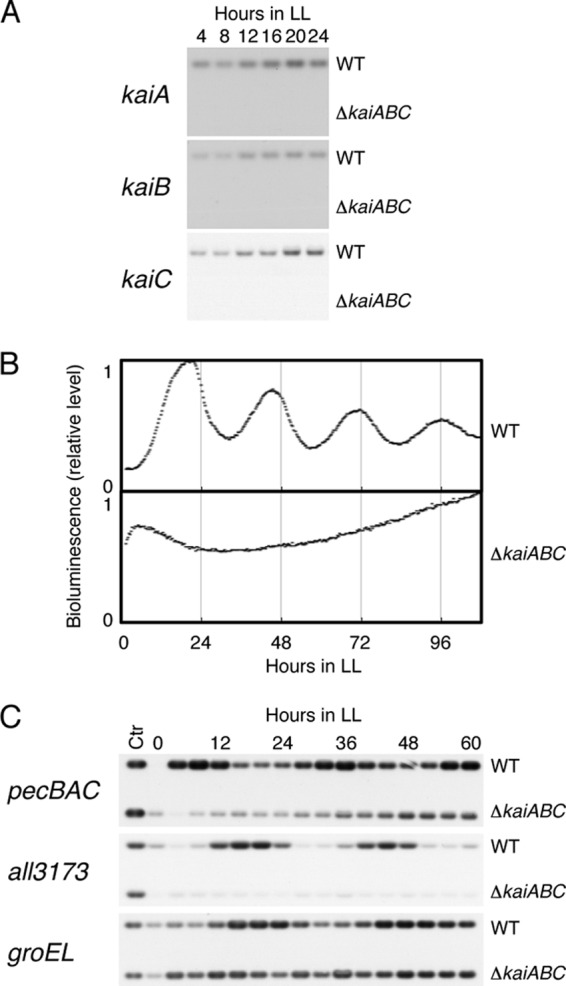
Nullification of transcriptional rhythms by genetic disruption of the kaiABC cluster. (A) Northern blot analysis using the total RNAs extracted from wild-type (WT) and kaiABC-null mutant (ΔkaiABC) cells with each kai gene-specific probe. (B) Bioluminescence rhythms to monitor the all3173 promoter activity, comparing the wild-type strain with the kaiABC-disrupted strain, in the presence of sodium nitrate. Results from a representative of multiple experiments are shown. (C) Circadian expression of the pecBAC, all3173, and groEL genes was nullified in the kaiABC-null mutant strains examined by Northern blot analysis. “Ctr” indicates a control RNA extracted from the wild-type strain at hour 64 in LL.
Circadian regulation in the absence of combined nitrogen.
We wanted to know whether circadian control acts in the heterocyst to unveil a possible link among metabolism, developmental program, and circadian regulation. Therefore, we analyzed the circadian profiles under nitrate-deprived conditions, which trigger heterocyst differentiation. Samples collected from one series of cells continuously cultured in the absence of combined nitrogen (BG-110 medium) under LL after two LD cycles were subjected to microarray analysis. Using the standard filtration conditions (P value of <0.05 and amplitude of >10−0.7), we found that in the absence of combined nitrogen, 39 of the 78 previously identified clock-controlled genes, including pecBAC and all3173, retained their circadian expression profiles with essentially similar peak times and amplitudes (see Fig. S2A and B and Table S2 in the supplemental material). Most of the other 39 genes retained low-amplitude rhythmic patterns, while their P value and/or amplitude scores were below our filtration threshold values.
Northern analysis confirmed that kaiABC disruption also nullified the pecBAC and all3173 mRNA rhythms in the absence of combined nitrogen (see Fig. S3 in the supplemental material). These results are not surprising given that the majority of cells in the bacterial filament remain vegetative cells, in which the intracellular metabolism may be similar in the presence or absence of combined nitrogen. Therefore, we looked for genes that were more specifically upregulated by nitrate depletion and analyzed their circadian expression profiles.
Ehira and colleagues (25) reported the genomic expression profiles in heterocyst-enriched samples (80% of the cells were estimated to be heterocysts) at 48 h after nitrate deprivation. However, at that time, they used a different version of the DNA microarray and large genomic clones as probes, each of which covered approximately 3 kbp, and contained 1 to 8 ORFs of various sizes. Therefore, it was difficult to precisely identify genes with upregulated or downregulated expression in the heterocysts. Consequently, we reevaluated the heterocyst-specific genes using the improved version of the oligonucleotide array (26) and heterocyst-enriched fractions (80% of the cells were confirmed to be heterocysts by microscopic observation) collected 48 h after nitrate deprivation (for details, see Materials and Methods). Initially, we compared the ratios [termed Sum(N−)/Sum(N+)] of the average gene expression levels in the nitrate-deprived medium at LL 4 to 48 to that in nitrate-containing medium and the ratios (Ri) of the gene expression levels in the heterocyst-enriched samples to those in intact filaments containing both vegetative cells and heterocysts (before heterocyst extraction) at 48 h after nitrate deprivation. As shown in Fig. 6A, highly heterocyst-enriched genes tended to be upregulated under nitrate-deprived conditions, as expected. Table S3 in the supplemental material shows 23 representative heterocyst-upregulated genes with both ratio values [Sum(N−)/Sum(N+) and Ri]of > 3, which are consistent with our previous analysis (25). Note that the genes downregulated in the heterocyst-enriched fraction were not always downregulated in the circadianly collected sample without combined nitrogen, because the expression of these genes may not be reduced in vegetative cells, which constitute the majority of cells in individual bacterial filaments.
Fig 6.
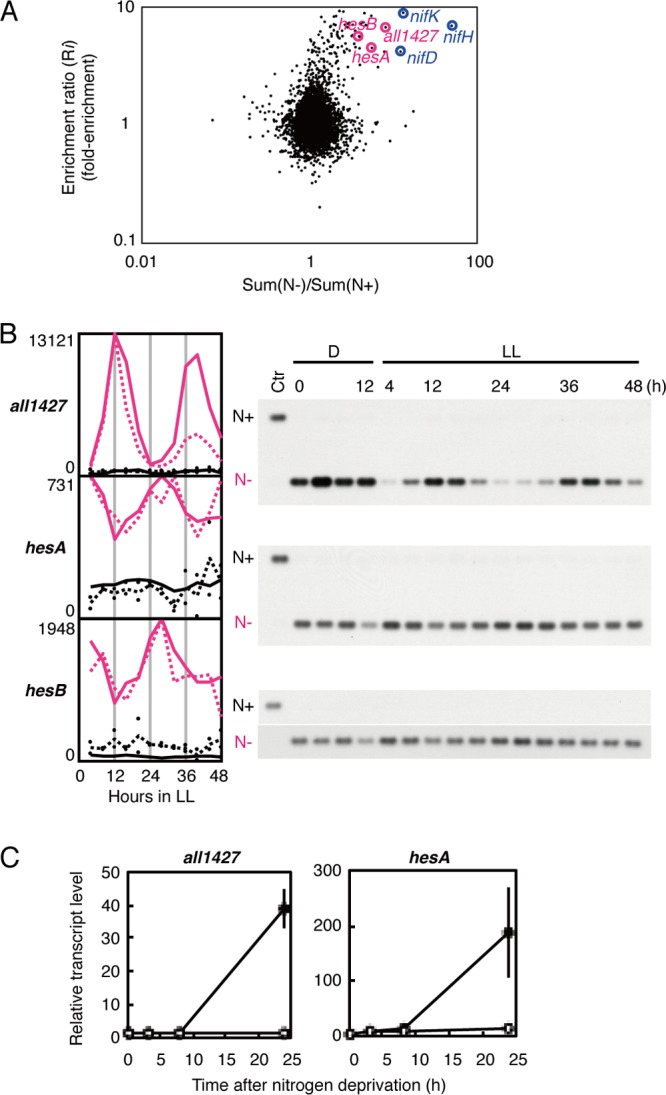
Circadian gene expression in heterocysts without combined nitrogen. (A) Variations in the changes in gene expression levels induced by nitrate deprivation and heterocyst enrichment. The abscissa shows the ratio of the average expression level for each gene throughout two circadian cycles under LL (hour 4 to 48) under nitrate deprivation (N−) to that in the presence of sodium nitrate (N+) (n = 2). The ordinate indicates the ratio of the average expression level for each gene 48 h after nitrate deprivation in heterocyst-enriched cells to that in intact bacterial filaments (n = 8). (B) Rhythmic expression of heterocyst-specific genes in LL under nitrate deprivation. Expression profiles of the all1427 and hesAB genes in the presence (black lines) or absence (pink lines) of sodium nitrate. Densitometric data from the Northern hybridization analysis (right panels) and microarray analysis are indicated by solid and dotted lines, respectively. For Northern blotting we loaded samples collected at hour 0 in the dark (D0) under different (N− for N+, N+ for N]) conditions as control samples, indicated by “Ctr.” (C) Expression of the all1427 and hesA genes in the wild-type strain (filled circles) and the hetR-null mutant strain (open circles) at 0, 3, 8, and 48 h after nitrate depletion. A densitometric analysis of the RT-PCR experiments is shown.
Circadian transcription in heterocysts.
Among the genes highly upregulated in heterocysts, all1427 showed a high-amplitude expression rhythm under LL, peaking at subjective dusk, which was confirmed with a Northern hybridization analysis (Fig. 6B). The all1427 gene was reported to be induced during heterocyst maturation in a previous transcriptomic analysis (26), and a proteome analysis found its encoded protein All1427 in the heterocyst cell wall in Anabaena (37). The physiological function of All1427 remains unknown, although it contains two cystathionine-β-synthase domains, which are found in cytosolic and membrane proteins with diverse functions (37). We also realized that members of the all1432-all1431 gene set, encoding the HesA and HesB proteins, show expression rhythms that peak at subjective dawn (Fig. 6B). The functions of HesA and HesB are also unknown, although insertional inactivation of hesA impairs the cells' nitrogen fixation activity by ∼55% (38).
To exclude the possibility that circadian transcription under nitrate depletion is active not in heterocyst cells but only in vegetative cells, the cyclic genes of interest must be expressed exclusively in heterocysts. Therefore, based on the Ri values (Rall1427 = 6.74; RhesA = 5.66; RhesB = 4.57), we indirectly estimated that the levels of all1427, hesA, and hesB in a single heterocyst were 27-, 15-, and 10-fold higher, respectively, than those in a single vegetative cell (see Table S3 in the supplemental material and Materials and Methods). hetR is the well-characterized master regulator essential for heterocyst differentiation and is responsible for the induction of many heterocyst-specific genes (4, 5). Therefore, we also examined the expression profiles of the all1427 and hesA genes in the wild type and the hetR-null mutant strain after nitrate depletion. As shown in Fig. 6C, none of these genes was expressed in the hetR-null strain, but all were exclusively expressed in the wild type within 24 h of nitrate deprivation. All these results strongly support the inference that both the all1427 and hesAB genes are specifically induced in heterocysts. We also confirmed that in the kaiABC-null mutant strain in the absence of combined nitrogen, which also formed heterocysts, rhythmic transcription of these genes was nullified (see Fig. S3 in the supplemental material). Therefore, we conclude that there is also active circadian transcriptional output in the differentiated cells.
The magnitude of kaiABC expression did not differ greatly between the heterocyst-enriched samples and the control (1.09 < Rkai < 1.17), indicating that the kai genes are expressed in heterocysts at levels equivalent to those in vegetative cells. Thus, we speculate that the rhythmic heterocyst-specific gene expression is driven by the Kai-based clock inside each heterocyst. However, we cannot exclude the possibility that the rhythmic transcription is indirectly induced in the heterocyst by time-dependent intercellular signals from oscillators in neighboring vegetative cells. Single-cell imaging analysis in Synechococcus has demonstrated that the synchronization among cells is negligible, and instead, the intracellular timing mechanism is extremely robust and precise (39, 40). In contrast, it has been suggested that in multicellular eukaryotes, cell-to-cell communication is important in synchronizing each clock cell into a robust oscillatory system at the tissue or organ level (41, 42). Therefore, it is also relevant to ask whether intercellular synchronization occurs between neighboring cells within the Anabaena filament. The development of fluorescence reporter strains to monitor the spatiotemporal expression profiles of clock-controlled genes at the single-cell level will be important in addressing these questions.
What is the role of circadian regulation in heterocysts? In several nonheterocystous, diazotrophic cyanobacterial strains, circadian rhythms in nitrogen fixation and the nifHDK genes encoding nitrogenase subunits have been reported. In the unicellular diazotrophic species, such as Cyanothece sp. PCC 8801 (previously known as Synechococcus sp. RF-1) (43), Cyanothece sp. ATCC 51142 (44, 45), and Crocosphaera watsonii WH 8501 (46), both nitrogenase activity and nif gene expression peak in subjective night. The nocturnal nitrogen fixation is compatible with a model that oxygenic photosynthesis and oxygen-sensitive nitrogen fixation are temporally separated by the circadian clock, while the nonheterocystous, filamentous strain Trichodesmium sp. strain IMS 101 shows nitrogenase activity and nif gene expression rhythms peaking during subjective daytime (47). In contrast, under our experimental conditions, the expression of most of the major heterocyst-enriched genes, such as nifHDK and fdxN, encoding a heterocyst-specific ferredoxin subunit, was not clearly rhythmic under LL conditions. However, considering partial involvement of hesAB genes in regulating nitrogenase activity (38), it is of interest to examine if nitrogenase activity in Anabaena is modulated by the circadian clock. The physiological relevance of the rhythmic expression of the all1427 and hesAB genes remains to be resolved, for instance, by the construction of strains in which their promoters are replaced by constitutive promoters with expression levels similar to those of the original promoters.
Another question to be addressed is whether the circadian system affects heterocyst differentiation/patterning. Circadian regulation has been proposed to modify developmental programs in eukaryotes, such as the changes from vegetative to reproductive organs in plants (48) and the initiation of differentiation from embryonic stem cells in mammals (49). Since the kaiABC-null Anabaena mutant forms heterocysts without combined nitrogen with semiregular intervals, circadian regulation is not an essential requirement of patterned heterocyst formation. However, a more careful quantitative analysis should be undertaken to examine whether the timing of heterocyst differentiation is modified by the circadian program in Anabaena.
Conclusion.
Together, these results provide a basis for molecular studies of the circadian system in filamentous, heterocyst-forming cyanobacteria. We have demonstrated that the Anabaena circadian clock drives genome-wide gene expression rhythms of genes that encode a variety of functions during LL, under both vegetative and differentiation-inducing conditions. Although it is strongly suggested that these rhythms are driven by the Kai-based oscillator, our study has shown that the expression of the Anabaena kai gene is not highly rhythmic, in contrast to that of Synechococcus. Another striking difference identified here is that the total RNA levels remain almost constant throughout the LD cycle in Anabaena, whereas in Synechococcus, the amount of total RNA is dramatically reduced in the night. Finally, we also found a subset of heterocyst-specific genes, including all1427 and hesAB, expressed in a robust circadian fashion, strongly suggesting the presence of circadian control. Anabaena sp. PCC 7120 is the best-established molecular genetic model organism not only for studies of heterocyst differentiation/patterning but also for the investigation of biological hydrogen and biofuel production. The present study provides fundamental insights into the spatiotemporal properties of the metabolic, physiological, and developmental processes in filamentous cyanobacterial species.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Iwasaki laboratory (Waseda University) for their valuable comments and advice.
This study was supported in part by Grants-in-Aid from the Japanese Society for the Promotion of Science (KAKENHI no. 23657138 and 23687002 to H. Iwasaki and 23603005 to S. Ehira), the Waseda University Grant for Special Research Projects (2010A-503) to H. Iwasaki, and the Asahi Glass Foundation to H. Iwasaki.
H. Iwasaki designed the research; H. Kushige, H. Kugenuma, M. Matsuoka, S. Ehira, and H. Iwasaki performed the study; S. Ehira and M. Ohmori provided the microarrays; H. Kushige, H. Kugenuma, S. Ehira, and H. Iwasaki analyzed the data; and H. Iwasaki and H. Kushige wrote the paper.
Footnotes
Published ahead of print 11 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02067-12.
REFERENCES
- 1. Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O'Neill JS, Reddy AB. 2012. Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee DY, Rhee GY. 1999. Circadian rhythm in growth and death of Anabaena flos-aquae. J. Phycol. 35:694–699 [Google Scholar]
- 3. Wolk CP, Ernst A, Elhai J. 1994. Heterocyst metabolism and development, p 769–823 In Bryant DA. (ed), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 4. Flores E, Herrero A. 2010. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8:39–50 [DOI] [PubMed] [Google Scholar]
- 5. Kumar K, Mella-Herrera RA, Golden JW. 2010. Cyanobacterial heterocysts. Cold Spring Harbor Perspect. Biol. 2:a000315 doi:10.1101/cshperspect.a000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, Tanabe A, Golden SS, Johnson CH, Kondo T. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281:1519–1523 [DOI] [PubMed] [Google Scholar]
- 7. Ito H, Mutsuda M, Murayama Y, Tomita J, Hosokawa N, Terauchi K, Sugita C, Sugita M, Kondo T, Iwasaki H. 2009. Cyanobacterial daily life with Kai-based circadian and diurnal genome-wide transcriptional control in. Synechococcus elongatus. Proc. Natl. Acad. Sci. U. S. A. 106:14168–14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomita J, Nakajima M, Kondo T, Iwasaki H. 2005. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science 307:251–254 [DOI] [PubMed] [Google Scholar]
- 9. Hosokawa N, Hatakeyama TS, Kojima T, Kikuchi Y, Ito H, Iwasaki H. 2011. Circadian transcriptional regulation by the posttranslational oscillator without de novo clock gene expression in Synechococcus. Proc. Natl. Acad. Sci. U. S. A. 108:15396–15401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson CR, Tsinoremas NF, Shelton J, Lebedeva NV, Yarrow J, Min H, Golden SS. 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 305:527–542 [DOI] [PubMed] [Google Scholar]
- 11. Mackey SR, Ditty JL, Clerico EM, Golden SS. 2007. Detection of rhythmic bioluminescence from luciferase reporters in cyanobacteria. Methods Mol. Biol. 362:115–129 [DOI] [PubMed] [Google Scholar]
- 12. Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. 2005. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308:414–415 [DOI] [PubMed] [Google Scholar]
- 13. Iwasaki H, Nishiwaki T, Kitayama Y, Nakajima M, Kondo T. 2002. KaiA-stimulated KaiC phosphorylation in circadian timing loops in cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 99:15788–15793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dvornyk V, Vinogradova O, Nevo E. 2003. Origin and evolution of circadian clock genes in prokaryotes. Proc. Natl. Acad. Sci. U. S. A. 100:2495–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uzumaki T, Fujita M, Nakatsu T, Hayashi F, Shibata H, Itoh N, Kato H, Ishiura M. 2004. Crystal structure of the C-terminal clock-oscillator domain of the cyanobacterial KaiA protein. Nat. Struct. Mol. Biol. 11:623–631 [DOI] [PubMed] [Google Scholar]
- 16. Garces RG, Wu N, Gillon W, Pai EF. 2004. Anabaena circadian clock proteins KaiA and KaiB reveal a potential common binding site to their partner KaiC. EMBO J. 23:1688–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ivleva NB, Gao T, LiWang AC, Golden SS. 2006. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc. Natl. Acad. Sci. U. S. A. 103:17468–17473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rust MJ, Golden SS, O'Shea EK. 2011. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science 331:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YI, Vinyard DJ, Ananyev GM, Dismukes GC, Golden SS. 2012. Oxidized quinones signal onset of darkness directly to the cyanobacterial circadian oscillator. Proc. Natl. Acad. Sci. U. S. A. 109:17765–17769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elhai J, Wolk CP. 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium. Anabaena. EMBO J. 9:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prentki P, Krisch HM. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313 [DOI] [PubMed] [Google Scholar]
- 22. Horton RM. 1993. In vitro recombination and mutagenesis of DNA: SOEing together tailor-made genes. Methods Mol. Biol. 15:251–261 [DOI] [PubMed] [Google Scholar]
- 23. Black TA, Cai Y, Wolk CP. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77–84 [DOI] [PubMed] [Google Scholar]
- 24. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 25. Ehira S, Ohmori M, Sato N. 2003. Genome-wide expression analysis of the responses to nitrogen deprivation in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 10:97–113 [DOI] [PubMed] [Google Scholar]
- 26. Ehira S, Ohmori M. 2006. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 59:1692–1703 [DOI] [PubMed] [Google Scholar]
- 27. Ehira S, Ohmori M. 2012. The pknH gene restrictively expressed in heterocysts is required for diazotrophic growth in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 158:1437–1443 [DOI] [PubMed] [Google Scholar]
- 28. Ehira S, Ohmori M. 2011. NrrA, a nitrogen-regulated response regulator protein, controls glycogen catabolism in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. J. Biol. Chem. 286:38109–38114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kucho K, Okamoto K, Tsuchiya Y, Nomura S, Nango M, Kanehisa M, Ishiura M. 2005. Global analysis of circadian expression in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 187:2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stöckel J, Welsh EA, Liberton M, Kunnvakkam R, Aurora R, Pakrasi HB. 2008. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl. Acad. Sci. U. S. A. 105:6156–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS. 2000. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289:765–768 [DOI] [PubMed] [Google Scholar]
- 32. Kitayama K, Nishiwaki T, Terauchi K, Kondo T. 2008. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev. 22:1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qin X, Byrne M, Xu Y, Mori Y, Johnson CH. 2010. Coupling of a core post-translational pacemaker to a slave transcription/translation feedback loop in a circadian system. PLoS Biol. 8:e1000394 doi:10.1371/journal.pbio.1000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terauchi K, Kitayama Y, Nishiwaki T, Miwa K, Murayama Y, Oyama T, Kondo T. 2007. ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 104:16377–16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kageyama H, Kondo T, Iwasaki H. 2003. Circadian formation of clock protein complexes by KaiA, KaiB, KaiC, and SasA in cyanobacteria. J. Biol. Chem. 278:2388–2395 [DOI] [PubMed] [Google Scholar]
- 36. Kageyama H, Nishiwaki T, Nakajima M, Iwasaki H, Oyama T, Kondo T. 2006. Cyanobacterial circadian pacemaker: Kai protein complex dynamics in the KaiC phosphorylation cycle in vitro. Mol. Cell 23:161–171 [DOI] [PubMed] [Google Scholar]
- 37. Moslavac S, Reisinger V, Berg M, Mirus O, Vosyka O, Plöscher M, Flores E, Eichacker LA, Schleiff E. 2007. The proteome of the heterocyst cell wall in Anabaena sp. PCC 7120. Biol. Chem. 388:823–829 [DOI] [PubMed] [Google Scholar]
- 38. Borthakur D, Basche M, Buikema WJ, Borthakur PB, Haselkorn R. 1990. Expression, nucleotide sequence and mutational analysis of two open reading frames in the nif gene region of Anabaena sp. strain PCC 7120. Mol. Gen. Genet. 221:227–234 [DOI] [PubMed] [Google Scholar]
- 39. Mihalcescu I, Hsing W, Leibler S. 2004. Resilient circadian oscillator revealed in individual cyanobacteria. Nature 430:81–85 [DOI] [PubMed] [Google Scholar]
- 40. Amdaoud M, Vallade M, Weiss-Schaber C, Mihalcescu I. 2007. Cyanobacterial clock, a stable phase oscillator with negligible intercellular coupling. Proc. Natl. Acad. Sci. U. S. A. 104:7051–7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. 2004. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14:2289–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 21:1408–1412 [DOI] [PubMed] [Google Scholar]
- 43. Grobbelaar N, Huang T-C, Lin H-Y, Chow T-J. 1986. Dinitrogen-fixing endogenous rhythm in Synechococcus RF-1. FEMS Microbiol. Lett. 37:173–177 [Google Scholar]
- 44. Schneegurt MA, Sherman DM, Nayar S, Sherman LA. 1994. Oscillating behavior of carbohydrate granule formation and dinitrogen fixation in the cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 176:1586–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toepel J, Welsh E, Summerfield TC, Pakrasi HB, Sherman LA. 2008. Differential transcriptional analysis of the cyanobacterium Cyanothece sp. strain ATCC 51142 during light-dark and continuous-light growth. J. Bacteriol. 190:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pennebaker K, Mackey KR, Smith RM, Williams SB, Zehr JP. 2010. Diel cycling of DNA staining and nifH gene regulation in the unicellular cyanobacterium Crocosphaera watsonii strain WH 8501 (Cyanophyta). Environ. Microbiol. 12:1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen YB, Dominic B, Mellon MT, Zehr JP. 1998. Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101. J. Bacteriol. 180:3598–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Imaizumi T, Kay SA. 2006. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 11:550–558 [DOI] [PubMed] [Google Scholar]
- 49. Yagita K, Horie K, Koinuma S, Nakamura W, Yamanaka I, Urasaki A, Shigeyoshi Y, Kawakami K, Shimada S, Takeda J, Uchiyama Y. 2010. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc. Natl. Acad. Sci. U. S. A. 107:3846–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


