Abstract
The Scl (Tal1) gene encodes a helix-loop-helix transcription factor essential for hematopoietic stem cell and erythroid development. The Scl +40 enhancer is situated downstream of Map17, the 3′ flanking gene of Scl, and is active in transgenic mice during primitive and definitive erythropoiesis. To analyze the in vivo function of the Scl +40 enhancer within the Scl/Map17 transcriptional domain, we deleted this element in the germ line. SclΔ40/Δ40 mice were viable with reduced numbers of erythroid CFU in both bone marrow and spleen yet displayed a normal response to stress hematopoiesis. Analysis of SclΔ40/Δ40 embryonic stem (ES) cells revealed impaired erythroid differentiation, which was accompanied by a failure to upregulate Scl when erythropoiesis was initiated. Map17 expression was also reduced in hematopoietic tissues and differentiating ES cells, and the Scl +40 element was able to enhance activity of the Map17 promoter. However, only Scl but not Map17 could rescue the SclΔ40/Δ40 ES phenotype. Together, these data demonstrate that the Scl +40 enhancer is an erythroid cell-specific enhancer that regulates the expression of both Scl and Map17. Moreover, deletion of the +40 enhancer causes a novel erythroid phenotype, which can be rescued by ectopic expression of Scl but not Map17.
INTRODUCTION
The basic helix-loop-helix transcription factor Scl (Tal1) is a key regulator of hematopoiesis (1–4), with additional important roles in the development of the vascular system (5) and central nervous system (CNS) (6). During development, Scl is expressed in sites of embryonic and fetal hematopoiesis, vascular endothelium, and specific regions of the CNS (7). Scl expression is maintained in adult endothelium and CNS (8); however, its expression in the adult hematopoietic system is restricted to hematopoietic stem cells (HSCs) and progenitors as well as the erythroid, megakaryocytic, and mast cell lineages (9, 10).
Scl null embryos die between days 8.5 and 10.5 of gestation due to the complete absence of primitive hematopoiesis (3, 4). Scl null embryonic stem (ES) cells fail to contribute to any hematopoietic lineages in chimeric mice (2) and are unable to generate hematopoietic colonies in vitro (1). Further studies in ES cells have uncovered that Scl is not required for the development of the hemangioblast but is essential for the formation of hemogenic endothelial cells (11). In the adult, Scl is not necessary for HSC maintenance (12–14), but loss of Scl severely affects the erythroid, megakaryocytic, and mast cell lineages (13, 15). Scl-deleted mice are anemic, display enlarged spleens, and show a shift toward more immature erythroid progenitors in bone marrow and spleen. While these animals have normal numbers of erythroid CFU (CFU-e), erythroid burst-forming units (BFU-e) were undetectable and the numbers of megakaryocyte-erythrocyte progenitors (MEP) were increased (16).
Scl is situated in a single transcriptional domain together with Map17 (17, 18). Map17, also known as Pdzk1ip1, encodes a transmembrane protein expressed in kidney cells (19), keratinocytes (20), early hematopoietic progenitors (21), and human adult reticulocytes (22). Not much is known about Map17 function; however, aberrant expression is observed in carcinomas arising from kidney, colon, lung, and breast (23). We have previously shown that Map17 has a role in zebrafish erythropoiesis, as morpholino-mediated knockdown caused a reduction in the number of circulating erythrocytes (24).
Systematic dissection of cis-regulatory mechanisms operating at the murine Scl locus has identified multiple cis-regulatory elements, each of which directs expression to a subdomain of the normal Scl expression pattern in transgenic mice (17, 25–29). One of these regulatory elements is the Scl +40 region, an enhancer situated 40 kb downstream of Scl exon 1a. We have shown previously that a 3.7-kb DNA fragment containing this element [Scl +40(3.7)] drives expression of a linked LacZ reporter gene to midbrain and primitive erythropoiesis in vivo (17), and extending this fragment to 5.0 kb [Scl +40(5.0)] resulted in additional expression of LacZ in definitive erythropoiesis (27).
To further analyze the function of the +40 enhancer in vivo, we have deleted the Scl +40 region in ES cells and generated SclΔ40/Δ40 knockout (KO) mice. Analysis of the knockout mice showed that the Scl +40 enhancer is functionally important for erythropoiesis since SclΔ40/Δ40 mice have reduced numbers of CFU-e in both bone marrow and spleen and in fetal liver during embryonic development. An erythroid defect was also observed during in vitro differentiation of SclΔ40/Δ40 ES cells and was accompanied by the failure of Scl upregulation at the later stages of erythroid differentiation. We also showed that the Scl +40 enhancer regulates Map17 expression but only ectopic expression of Scl can rescue the erythroid phenotype SclΔ40/Δ40 ES cells.
MATERIALS AND METHODS
Generation of knockout ES cells.
A 15-kb region spanning the Scl +40 enhancer (nucleotides [nt] 5758 to 20460 relative to Al731651) was retrieved onto the PL253 vector from bacterial artificial chromosome clone 175N02 using a recombineering protocol previously described (30). A LoxP-PGK-Neo-poly(A)-LoxP cassette was used to substitute the Scl +40(4.2) genomic region (nt 11716 to 15984 relative to Al731651). This generated a targeting vector containing 5′ and 3′ homologous regions of 6 kb and 4.7 kb, respectively, flanking the LoxP-PGK-Neo-poly(A)-LoxP cassette. Details of the cloning strategy are available on request.
AB2.2 ES cells were electroporated with the targeting construct and selected with 200 μg/ml of G418 (Sigma-Aldrich) and 2 μM ganciclovir (Sigma-Aldrich). Individual clones were picked, expanded, and genotyped by Southern blotting using internal 5′ (nt 8249 to 8845 relative to Al731651) and external 3′ (nt 21156 to 21833 relative to Al731651) probes. Positive clones (Scl +40/Neo′) were transiently transfected with a PGK-Cre construct for deletion of the Neo cassette. Clones were picked, expanded, and genotyped by Southern blotting. Clones in which the Neo cassette was deleted (SclΔ40/WT) were used for the generation of homozygous SclΔ40/Δ40 ES cells. An identical protocol was used for targeting of the second allele, with the generation of SclΔ40/Neo clones and subsequent deletion of the Neo cassette to generate SclΔ40/Δ40 ES clones.
Generation of knockout mice.
Two independent SclNeo/WT ES cell clones were injected into albino C57BL/6 blastocysts, and chimeras were bred with C57BL/6 mice to produce heterozygous SclNeo/WT mice. These mice were further bred with CMV-Cre transgenic mice (31) to remove the Neo cassette and generate SclΔ40/WT mice. Mice used in the experiments presented were backcrossed to C57BL/6 mice for at least 3 generations. The SclΔ40/Δ40 embryonic phenotype was analyzed at embryonic day 10.5 (E10.5), E12.5, and E14.5. Results shown here are from E12.5 embryos unless stated otherwise. The SclΔ40/Δ40 adult phenotype was analyzed in 6-, 12-, and 48-week-old mice. Results shown here are for 12-week-old animals unless stated otherwise. All mice were kept in specific-pathogen-free conditions, and all procedures were performed under the project license approved by the United Kingdom Home Office.
Peripheral blood analysis, fluorescence-activated cell sorting, colony assays, and stress hematopoiesis.
Peripheral blood was taken from tail vein into EDTA-coated tubes, and automated total and differential blood cell counts were determined using the ABC blood counter (Woodley). For analysis of mature hematopoietic lineages, single-cell suspensions from bone marrow, spleen, and fetal liver were stained with Gr-1 and Mac-1 for myeloid analysis, B220 for B-cell analysis, CD41 for megakaryocytic analysis, and CD71 and Ter119 for erythroid analysis (BD Biosciences). The concentration of erythropoietin (Epo) was measured using Quantikine mouse/rat Epo immunoassay (R&D systems).
For analysis of early hematopoietic progenitors, single-cell suspensions were lineage depleted using the MACS lineage cell depletion kit (Miltenyi Biotec), and depleted cells were stained for Sca1, cKit, CD34, FcγRIII, and interleukin-7R (IL-7R). For analysis of hematopoietic lineages during ES cell differentiation, cells were stained for cKit and Ter119. Populations of increasing erythroid maturity were defined as described previously (32). Flow cytometric analysis was performed with a CyAn ADP analyzer (Beckman Coulter). Cell sorting was performed with a MoFlo cell sorter (Beckman Coulter). Data were analyzed using FlowJo software (TreeStar). Granulocytes/macrophage CFU (CFU-GM), BFU-e, and CFU-e were grown using the methylcellulose-based medium kit Cameo-4 (HemoGenix), and megakaryocyte CFU (CFU-MK) were grown using collagen-based medium Megacult-C (Stem Cell Technologies) according to the manufacturers' instructions.
Mice were injected intraperitoneally at day 0 and day 1 with 12 μl/g body weight of 0.4% phenylhydrazine (PHZ) in phosphate-buffered saline (PBS) (Sigma) and analyzed at day 5.
Expression analysis.
Total RNA was isolated from tissues using Tri-reagent (Sigma-Aldrich) and from sorted cell populations using the ZR RNA MicroPrep kit (Zymo Research) according to the manufacturers' instructions. An identical quantity of total RNA (0.1 to 1 μg) was used to prepare cDNA [with either oligo(dT) or random hexamer primers] with the cDNA synthesis kit (Bioline) following the manufacturer's instructions. Real-time quantitative PCR was performed using the Brilliant SYBR green qPCR master mix (Stratagene). The following primers were used: Scl F, CATGTTCACCAACAACAACCG, and R, GGTGTGAGGACCATCAGAAATCTC; Map17 F, GTCCTTGTTGCAATCGTCTTC, and R, GAGGAGTATCTGCCATCCATTC; β-actin F, TCCTGGCCTCACTGTCCA, and R, GTCCGCCTAGAAGCACTTGC. Gata1 F, CAACAGTATGGAGGGAATTCCT, and R, GTGTCCAAGAACGTGTTGTTGC; Beta major F, ATGCCAAAGTGAAGGCCCAT, and R, CCCAGCACAATCACGATCAT. Expression was normalized to β-actin.
In vitro differentiation of ES cells.
For embryoid body generation, ES cells were seeded at 2 × 103 cell/ml in Iscove's modified Dulbecco's medium (IMDM; HyClone) supplemented with 15% fetal calf serum (FCS) (HyClone), 2 mM l-glutamine (PAA), 300 μg/ml transferrin (Sigma), 4 × 10−4 M monothioglycerol (MTG) (Sigma), 50 μg/ml ascorbic acid (Sigma), and 5% protein-free hybridoma medium II (PFHM-II) and allowed to differentiate up to 8 days (33). BFU-e colonies were grown by seeding 1.5 × 105 cells in 1.5 ml M3434 medium (Stem Cell Technologies). Colonies were counted after 14 days in culture.
F0 transgenic mouse assays and luciferase reporter assays.
All LacZ reporter constructs were generated by substitution of the luciferase gene with the lacZ gene. F0 transgenic mice were generated as described previously (27). Luciferase assays were performed as described previously (34). Map17P-Luc was generated by cloning a PCR-amplified 350-bp fragment containing the Map17 promoter into pGL2 vector (Stratagene). Map17P-Luc-+40 was generated by substitution of the simian virus 40 (SV40) promoter in SV40-Luc-+40(5.0) by the 350-bp Map17 promoter. Results were normalized to Map17P-Luc.
RESULTS
Deletion of the Scl +40 enhancer.
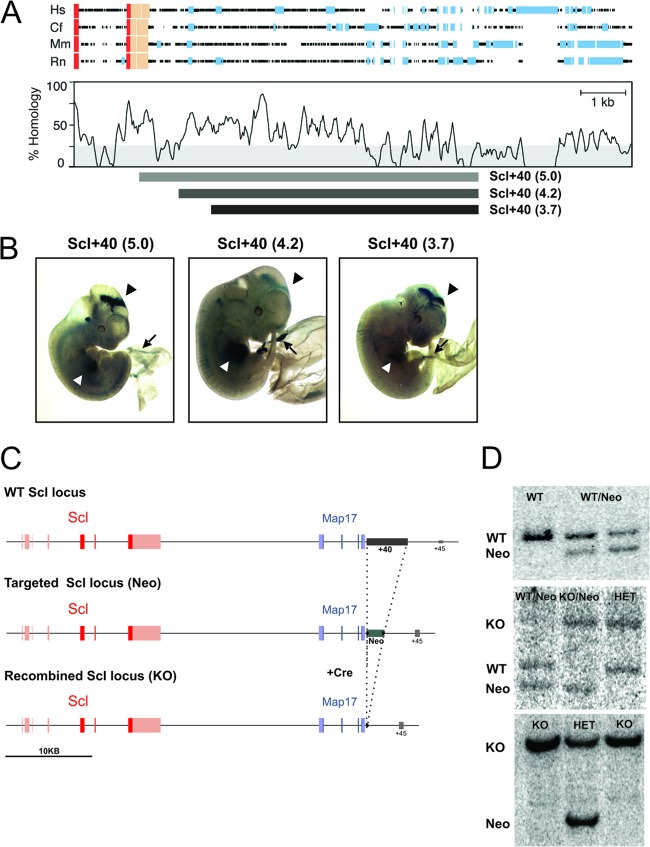
To define the function of the Scl +40 enhancer in vivo, we elected to delete this element in ES cells. Since the region corresponding to the Scl +40(5.0) included the poly(A) tail of the Map17 gene, we decided to delete a 4.2-kb fragment corresponding to the remaining sequence of the Scl +40(5.0) (Fig. 1A). To confirm that the deleted region was indeed a functional Scl +40 enhancer, the 4.2-kb fragment was inserted downstream of the SV40 promoter-LacZ cassette, and enhancer activity of the resultant construct was analyzed in transgenic embryos. The Scl +40(4.2) fragment drove LacZ expression to both primitive and definitive erythropoiesis and midbrain in E12.5 transgenic embryos, similarly to the Scl +40(5.0) fragment (Fig. 1B). The transgene therefore recapitulates the endogenous Scl and Map17 expression previously reported by us using in situ hybridization (24, 28).
Fig 1.
Generation of SclΔ40/Δ40 mice and ES cells. (A) Schematic representation of a four-way sequence alignment of the region downstream of Map17 in human (Hs), dog (Cf), mouse (Mm), and rat (Rn), showing peaks of sequence homology (modified from Delabesse et al. [17]). Red boxes, coding exons; beige boxes, untranslated exons; blue boxes, repeat sequences. The relative positions of the various +40 fragments incorporated into luciferase and lacZ reporter constructs are shown below the graph. (B) Representative E11.5 to E12.5 SV40-LacZ-Scl +40(5.0), SV40-LacZ-Scl +40(4.2), and SV40-LacZ-Scl +40(3.7) transgenic embryos stained for LacZ, showing expression in circulating blood (black arrow), fetal liver (white arrowhead), and midbrain (black arrowhead). (C) Schematic representation of the Scl locus with the Scl exons depicted in red and Map17 exons in blue. (Top) Structure of the WT locus, with the location of the Scl +40 enhancer indicated in gray. (Middle) Targeted Scl locus, where the Scl +40 enhancer was replaced by a LoxP-PGK-Neo-p(A)-LoxP cassette, indicated in green. (Bottom) Recombined Scl locus where the Neo cassette was removed by Cre-mediated recombination. (D) Southern blots used for genotyping of SclNeo/WT (top), SclΔ40/WT and SclΔ40/Neo (middle), and SclΔ40/Δ40 (bottom) ES cells.
Satisfied that the Scl +40(4.2) fragment is a bona fide Scl +40 enhancer, we proceeded with its deletion in ES cells. A targeting construct was generated using recombineering technology to replace the Scl +40(4.2) sequence by a LoxP-PGK-Neo-LoxP cassette (30), and this construct was used to target ES cells by homologous recombination (Fig. 1C). Correct targeting was confirmed by Southern blotting, and two independent clones were used to generate SclΔ40/Δ40 ES cells and mice for further analysis (Fig. 1D).
SclΔ40/Δ40 mice are viable and have normal blood counts.
Two independent clones of correctly targeted ES cells were used for the generation of SclNeo/WT mice. These mice were further bred with CMV-Cre transgenic mice (31) to remove the Neo cassette and generate SclΔ40/WT mice, which were interbred to generate homozygous SclΔ40/Δ40 mice. SclΔ40/Δ40 mice were born at normal Mendelian ratios and developed normally.
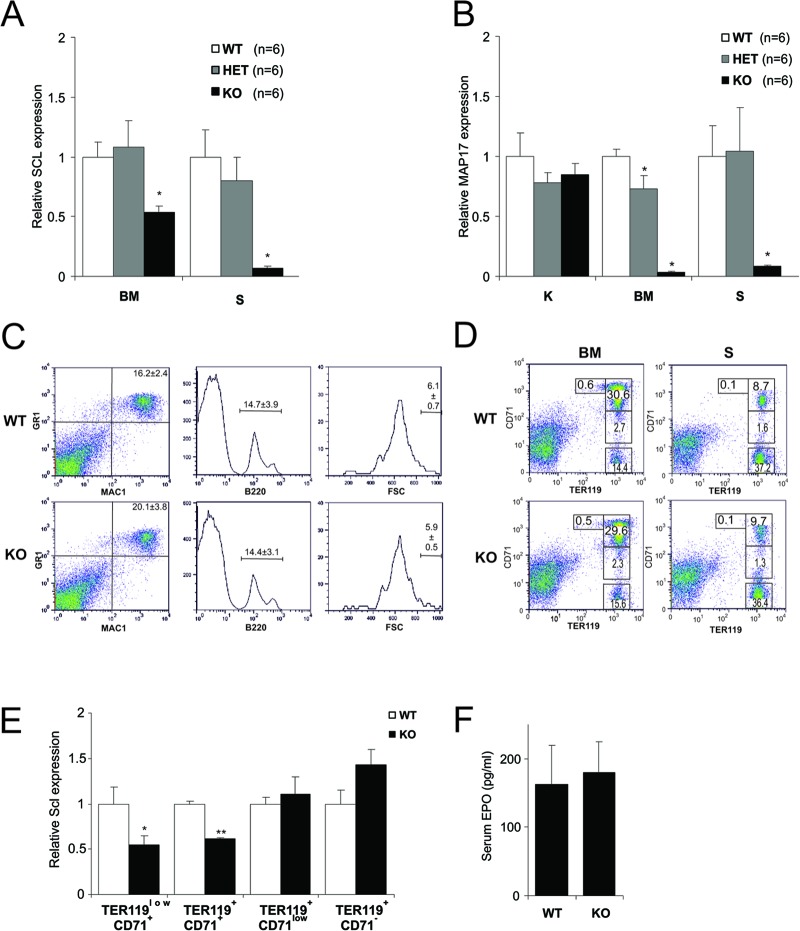
We checked the expression levels of Scl in the hematopoietic organs. Scl expression was severely reduced in both bone marrow and spleen (Fig. 2A). To confirm that Map17 expression was not affected due to the proximity of the Scl +40 deletion to the Map17 poly(A) site, we analyzed the expression of Map17 in kidney, where the Scl +40 enhancer is not active (17). Map17 was expressed at normal levels in SclΔ40/Δ40 kidneys, indicating that its poly(A) is intact (Fig. 2B). However, the expression of Map17 was severely reduced in both bone marrow and spleen (Fig. 2B), suggesting that Map17 may be regulated by the Scl +40 enhancer in hematopoietic cells.
Fig 2.
Mature hematopoietic lineages are normal in SclΔ40/Δ40 mice. (A and B) Relative Scl (A) and Map17 (B) expression in bone marrow (BM), spleen (S), and kidney (S). *, P ≤ 0.01. (C) Representative flow cytometry plots of bone marrow staining for granulocytes (Mac1+ Gr1+), B cells (B220+), and megakaryocytes (CD41+ FSChigh). (D) Terminal erythroid differentiation is normal in SclΔ40/Δ40 mice. Dot plots show CD71 and TER119 staining for the different stages of terminal erythroid differentiation in bone marrow and spleen. No difference was observed in early erythroid progenitors TER119low CD71+, during terminal erythroid differentiation (TER119+ CD71+ and TER119+ CD71low), or in erythrocytes (TER119+ CD71−). Numbers represent averages for at least 5 independent animals. (E) Relative Scl expression in erythroid progenitor populations purified from bone marrow. *, P ≤ 0.05; **, P ≤ 0.005. (F) Concentration of serum erythropoietin (pg/ml).WT, SclWT/WT; HET, SclWT/Δ40; KO, SclΔ40/Δ40.
Analysis of peripheral blood parameters in mice of different ages (6, 12, and 48 weeks) showed no statistically significant difference between SclWT/WT and SclΔ40/Δ40 mice (Table 1). Peripheral blood parameters can be within the normal range despite defects in hematopoietic progenitors within the bone marrow (35, 36). We therefore performed a more detailed analysis of adult hematopoietic organs by quantifying the different hematopoietic lineages in the bone marrow and spleen by flow cytometry. No difference was observed in the percentages of bone marrow granulocytes (Gr1+ Mac1+), B lymphocytes (B220+), and megakaryocytes (CD41+ FSChigh) between SclWT/WT and SclΔ40/Δ40 mice (Fig. 2C). More-detailed analysis of late erythroid progenitors using the CD71 and TER119 antibodies also failed to reveal any defect in terminal erythroid differentiation in both bone marrow and spleen, consistent with the normal peripheral blood parameters (Fig. 2D). To analyze Scl expression levels in SclΔ40/Δ40 mice during terminal erythroid differentiation, the populations described above were purified from bone marrow and used for RNA isolation. Levels of Scl were significantly reduced in SclΔ40/Δ40 mice at the earlier stages of differentiation (TER119low CD71+ and TER119+ CD71+) but increased at later stages of differentiation (TER119+ CD71low and TER119+ CD71−) (Fig. 2E). These results are consistent with the fact that blood counts are normal but suggest a defect during earlier stages of differentiation.
Table 1.
SclΔ40/Δ40 mice have normal hematological parametersa
| Genotype | WBC (103/μl) | RBC (103/μl) | Hgb (g/liter) | Hct (%) | MCV (fl) | MCH (pg) | MCHC (g/liter) | PLT (103/μl) | MPV (fl) |
|---|---|---|---|---|---|---|---|---|---|
| SclWT/WT | 10.0 ± 2.34 | 9.9 ± 0.69 | 159.2 ± 43.04 | 54.3 ± 2.28 | 53.5 ± 2.07 | 16.9 ± 1.28 | 315.4 ± 13.95 | 1,015.2 ± 147.25 | 5.0 ± 0.20 |
| SclWT/Δ40 | 9.8 ± 2.74 | 9.7 ± 1.24 | 162.0 ± 34.58 | 52.4 ± 6.41 | 53.3 ± 2.36 | 16.9 ± 1.37 | 290.4 ± 87.31 | 971.6 ± 265.53 | 5.0 ± 0.195 |
| SclΔ40/Δ40 | 12.3 ± 3.07 | 10.6 ± 0.68 | 172.8 ± 6.93 | 56.3 ± 2.88 | 53.5 ± 2.95 | 16.4 ± 1.09 | 307.5 ± 10.24 | 923.4 ± 307.36 | 5.1 ± 0.29 |
Data shown are for 12-week-old animals. Similar results were obtained for 6- and 48-week-old animals (not shown). WBC, leukocyte concentration; RBC, erythrocyte concentration; Hgb, hemoglobin concentration; Hct, hematocrit level; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin amount; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; MPV, mean platelet volume.
A possible explanation for the phenotype observed in early progenitors but not in terminal differentiated erythrocytes can be compensatory mechanisms via cytokines and growth factors. Therefore, we measured the concentration of erythropoietin in serum of SclΔ40/Δ40 mice. Although we observed a 10% increase in Epo concentration in SclΔ40/Δ40 mice, the variation between samples was high, and therefore the results were not statistically significant (Fig. 2F).
SclΔ40/Δ40 mice have reduced numbers of CFU-e and CFU-MK progenitors.
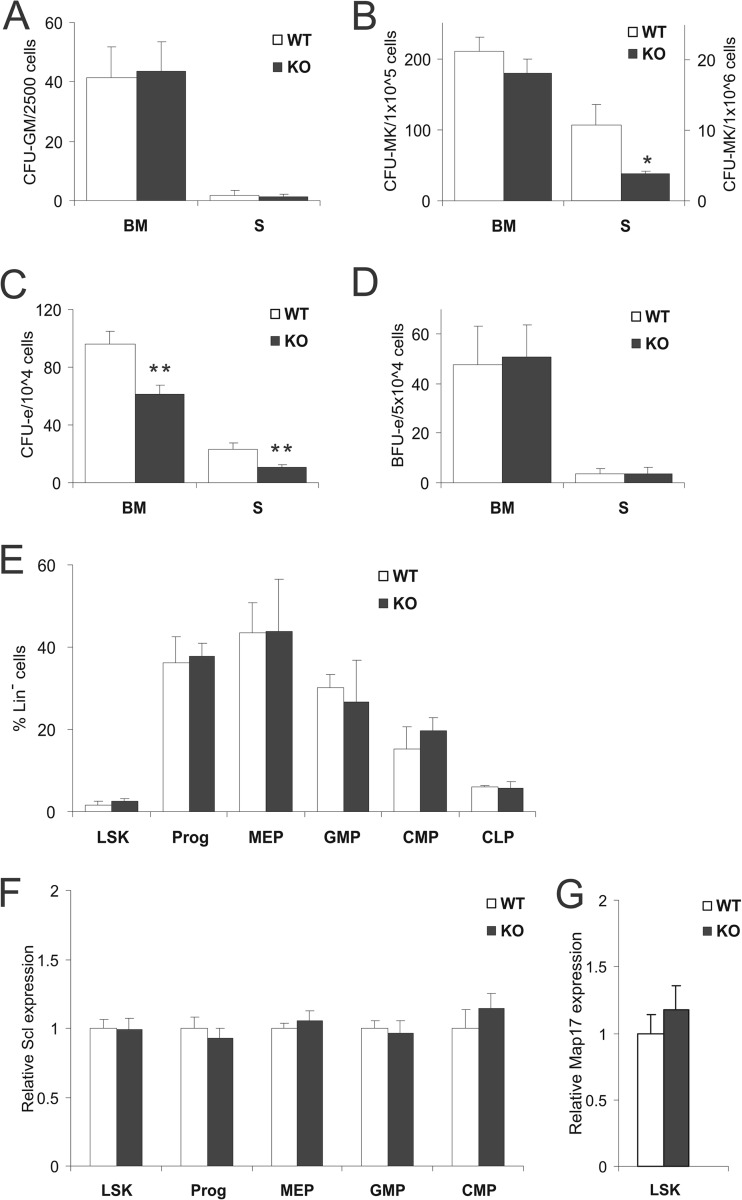
Hematopoietic progenitors in the bone marrow and spleen were also quantified using standard methylcellulose-based colony-forming methods. No difference was observed in the number of granulocyte/macrophage CFU (CFU-GM) (Fig. 3A). Megakaryocyte CFU (CFU-MK) were reduced in SclΔ40/Δ40 spleen but not in bone marrow (Fig. 3B). A reduction of erythroid CFU (CFU-e) (Fig. 3C) was observed in the bone marrow and spleen, but no such difference was observed in the earlier erythroid progenitor erythroid burst-forming units (BFU-e) (Fig. 3D).
Fig 3.
CFU-e numbers are reduced in SclΔ40/Δ40 bone marrow (BM) and spleen (S). (A to D) Histograms showing numbers of CFU-GM (A), CFU-MK(B), CFU-e (C), and BFU-e (D) in bone marrow and spleen. *, P ≤ 0.05; **, P ≤ 0.01. (E) Histogram showing percentages of hematopoietic stem and progenitor cells in lineage-negative bone marrow cells. (F) Relative expression of Scl in early stem and progenitor cells. (G) Relative expression of Scl in Lin− Sca1+ Kit+ cells. WT, SclWT/WT; KO, SclΔ40/Δ40.
Earlier hematopoietic stem/progenitor cells were quantified by flow cytometry using standard combinations of cell markers. No differences were observed in the Lin− Sca1+ Kit+ (LSK) stem cell-enriched population, common myeloid progenitor (CMP; Lin− Sca1− Kit+ CD34+ FcγR−), myeloid-erythroid progenitor (MEP; Lin− Sca1− Kit+ CD34− FcγR−), granulocyte-macrophage progenitor (GMP; Lin− Sca1− Kit+ CD34+ FcγR+), and common lymphocyte progenitor (CLP; IL-7R+ cKit+ Sca1+) populations (Fig. 3E). Moreover, Scl expression was unaltered in SclΔ40/Δ40 LSK, CMP, MEP, and GMP (Fig. 3F). Map17 expression was unaltered in the LSK population (Fig. 3G) and undetectable in the CMP, MEP, and GMP populations of both SclWT/WT and SclΔ40/Δ40 mice. Expression of Scl and Map17 in CLP was not analyzed. Taken together, these data show that expression changes are accompanied by functional defects in specific progenitor subsets in SclΔ40/Δ40 mice.
SclΔ40/Δ40 mice show a normal response to stress erythropoiesis.
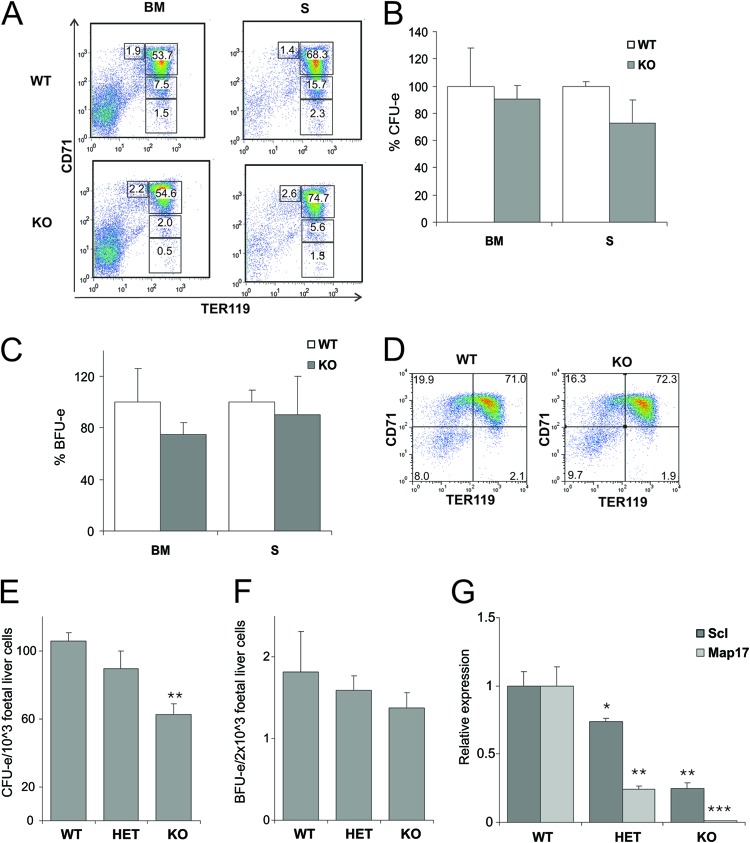
Given the specific defects under homeostatic conditions, we next asked how the hematopoietic system of SclΔ40/Δ40 mice responds under stress conditions. To test the ability of SclΔ40/Δ40 mice to respond to hematopoietic stress, we injected SclΔ40/Δ40 mice and SclWT/WT littermate controls with 0.4% phenylhydrazine (PHZ) and analyzed mice at day 5 postinjection. No differences were observed in blood parameters (Table 2) or in spleen weight or cellularity of the bone marrow and spleen (data not shown). Analysis of terminal erythroid differentiation in bone marrow and spleen also revealed no difference between SclΔ40/Δ40 and SclWT/WT controls (Fig. 4A). CFU-e (Fig. 4B) and BFU-e (Fig. 4C) numbers were also similar in SclΔ40/Δ40 and SclWT/WT mice after PHZ treatment, which was a surprising result given the difference in CFU-e numbers observed in SclΔ40/Δ40 mice under nonstress conditions (see previous section).
Table 2.
SclΔ40/Δ40 mice show normal response to stress erythropoiesisa
| Time after PHZ addition (days) | Genotype | RBC (103/μl) | Hgb (g/liter) | Hct (%) | MCV (fl) | MCH (pg) | MCHC (g/liter) | PLT (103/μl) | MPV(fl) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | SclWT/WT | 9.24 ± 1.84 | 147.7 ± 22.90 | 50.2 ± 8.39 | 55.0 ± 2.00 | 16.1 ± 0.75 | 294.3 ± 3.78 | 1,308.3 ± 235.97 | 5.3 ± 0.45 |
| SclΔ40/Δ40 | 10.42 ± 0.50 | 158.67 ± 3.21 | 53.9 ± 2.14 | 51.7 ± 2.08 | 15.3 ± 0.59 | 294.7 ± 6.35 | 1,148.3 ± 439.03 | 5.5 ± 0.66 | |
| 5 | SclWT/WT | 4.45 ± 0.50 | 117.67 ± 29.29 | 30.0 ± 2.00 | 69.3 ± 10.78 | 26.3 ± 4.25 | 393.0 ± 127.23 | 1,505.0 ± 192.59 | 6.07 ± 0.45 |
| SclΔ40/Δ40 | 4.32 ± 0.46 | 123.67 ± 19.03 | 29.6 ± 0.52 | 69.3 ± 8.50 | 28.6 ± 3.12 | 418.6 ± 71.50 | 1,719.7 ± 324.22 | 6.33 ± 0.50 |
RBC, erythrocyte concentration; Hgb, hemoglobin concentration; Hct, hematocrit level; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin amount; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; MPV, mean platelet volume.
Fig 4.
Response to stress hematopoiesis is normal, but CFU-e numbers are reduced in fetal liver of SclΔ40/Δ40 mice. (A) Dot plots showing representative staining of erythroid cells in bone marrow (BM) and spleen (S) 5 days after induction of stress hematopoiesis by PHZ. Values indicate averages for 4 animals. (B and C) CFU-e (B) numbers are reduced, but BFU-e (C) numbers are unaltered in SclΔ40/Δ40 mice. Results are shown as percentages of colonies compared to SclWT/WT mice. (D) Dot plots showing representative CD71/TER119 profile of E12.5 fetal liver cells. (E and F) CFU-e (E) and BFU-e (F) counts in E12.5 fetal livers. **, P ≤ 0.005. (G) Expression analysis of Scl and Map17 in E12.5 fetal livers. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. WT, SclWT/WT; HET, SclWT/Δ40; KO, SclΔ40/Δ40.
SclΔ40/Δ40 embryos display reduced numbers of CFU-e progenitors.
Since mild hematopoietic phenotypes in adult mice are sometimes exacerbated during early embryonic development (37, 38), we next analyzed SclΔ40/Δ40 embryos during development. SclWT/WT and SclΔ40/Δ40 embryos (E10.5, E12.5, and E14.5) were morphologically indistinguishable (not shown), and analysis of the erythroid lineage by flow cytometry using CD71 and TER119 markers showed no difference in terminal erythroid differentiation at E12.5 (Fig. 4D) and E14.5 (not shown). Similarly to the observations in adult tissues, a reduction in CFU-e numbers was observed in SclΔ40/Δ40 fetal livers (Fig. 4E) but the BFU-e numbers were not significantly different (Fig. 4F).
Analyses of Scl and Map17 expression levels showed a significant reduction in Scl expression at E12.5 (Fig. 4G) in SclΔ40/Δ40 fetal livers, but a recovery in Scl expression levels was observed at E14.5 (not shown). Map17 expression was almost absent in SclΔ40/Δ40 fetal livers at both E12.5 and E14.5 (Fig. 4G and data not shown). No significant difference in Scl expression was observed when expression was analyzed in the embryo head, where the Scl +40 enhancer is functional in mid- and hindbrain (not shown). The erythroid phenotype in fetal liver is therefore consistent with the adult Scl phenotype. The reduced Scl expression levels at E12.5 and a recovery of Scl expression levels observed at later stages of development can partially explain the mild phenotype observed.
Erythroid differentiation is impaired in SclΔ40/Δ40 ES cells.
Two independently targeted ES clones were used for the generation of SclΔ40/Δ40 ES cells (Fig. 1D). SclNeo/WT ES cells were transiently transfected with a PGK-Cre construct to remove the floxed Neo cassette and generate SclΔ40/WT ES cells. The second Scl allele, containing an intact Scl +40 enhancer, was then deleted using the same targeting vector to generate SclΔ40/Neo ES, and PGK-Cre was again used to remove the Neo cassette and generate SclΔ40/Δ40 ES cells. SclΔ40/Δ40 ES cells are indistinguishable from SclWT/WT ES cells when cultured under self-renewal conditions with serum and leukemia inhibitory factor (LIF). To investigate the differentiation potential of these ES cells, embryoid bodies (EBs) were generated from SclWT/WT and SclΔ40/Δ40 ES cells, and samples were collected for analysis at days 4, 6, and 8 of differentiation. These time points were selected because Scl expression initiates in EBs at day 3.75, at day 6 only primitive erythropoiesis is observed, and by day 8 definitive hematopoiesis is present.
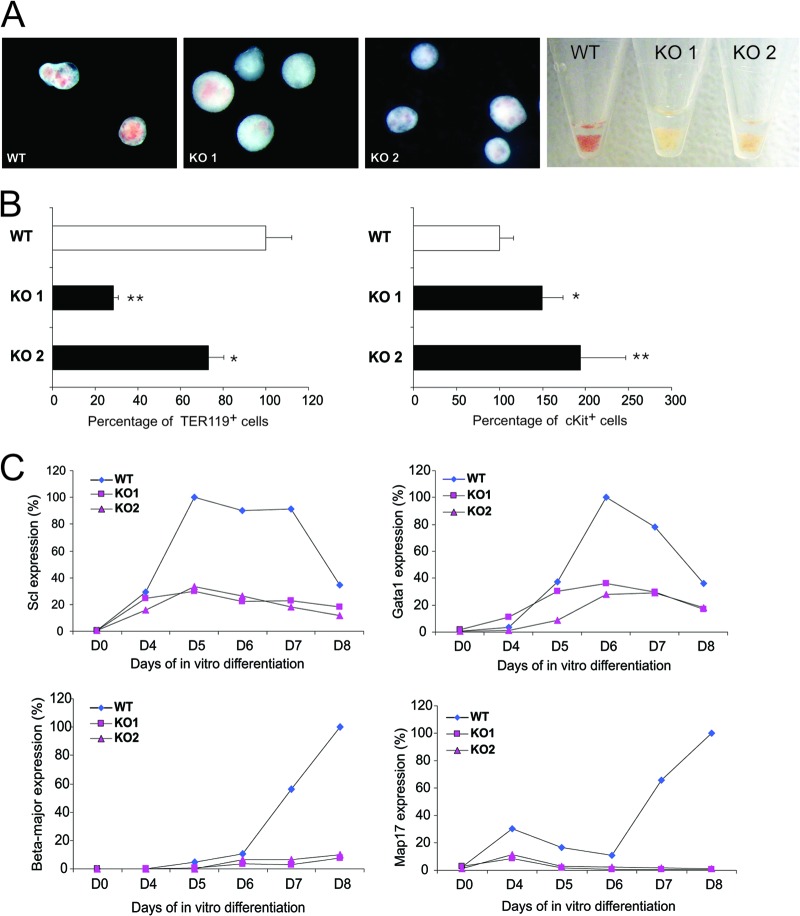
SclΔ40/Δ40 ES cells formed EBs similarly to SclWT/WT ES cells, but the SclΔ40/Δ40 EBs were pale compared to the SclWT/WT controls (Fig. 5A). Detailed flow cytometric analysis of relevant hematopoietic markers in day 8 EBs revealed a significant reduction in the percentage of TER119+ cells and an increase of cKit+ cells at day 8 (Fig. 5B). To evaluate the differentiation potential of the erythroid lineage in more detail, EBs were disrupted at both day 6 and day 8 and BFU-e were quantified by methylcellulose-based colony assays. BFU-e numbers were variable, and no statistically significant difference between SclWT/WT and SclΔ40/Δ40 cells was obtained. However, while BFU-e obtained from SclΔ40/Δ40 ES cells were hemoglobinized, the clones were significantly smaller and very compact, a characteristic of primitive BFU-e (data not shown). These observations, together with the pale appearance of the EBs, indicated a defect in the differentiation of erythroid cells from SclΔ40/Δ40 ES cells.
Fig 5.
Analysis of SclΔ40/Δ40 ES cells reveals an erythroid maturation defect. (A) Representative photographs of EBs generated from SclWT/WT (WT) and 2 independent SclΔ40/Δ40 ES clones (KO 1 and KO 2) as well as pelleted EBs (right). (B) Histograms showing the percentages of TER119-positive (left) and cKit-positive (right) cells in day 8 EBs. *, P ≤ 0.05; **, P ≤ 0.01. (C) Expression of Scl (top left), Gata1 (top right), Beta-major (bottom left), and Map17 (bottom right) during EB differentiation. WT, SclWT/WT; KO, SclΔ40/Δ40.
Expression time course analysis of the EBs revealed that Scl is expressed at normal levels at day 4 but fails to be upregulated at later days (Fig. 5C). Since Scl upregulation is essential for terminal erythroid differentiation, we also analyzed the expression of several other genes known to be involved in this process. Similarly to Scl, Gata1 levels failed to be upregulated from day 6 to day 8 (Fig. 5C). Moreover, there was no induction of Beta-major, the beta-globin gene expressed in the definitive erythroid lineage, in SclΔ40/Δ40 EBs after day 6. Due to the genomic location of the Scl +40 enhancer, we also checked the expression of the Map17 gene, situated immediately upstream of the +40 enhancer. Similarly to Scl, Map17 was not expressed in ES cells and was induced by day 4, with its expression remaining low until day 6 but being upregulated thereafter. Expression of Map17 in SclΔ40/Δ40 EBs was already reduced by day 4 and remained absent after day 6, the time its expression is upregulated in SclWT/WT EBs (Fig. 5C).
Together, these results indicate that deletion of the Scl +40 enhancer prevents the upregulation of Scl and Map17 during terminal erythroid differentiation. This is accompanied by a reduction in the percentage of mature erythrocytes in day 8 EBs.
The erythroid differentiation phenotype of SclΔ40/Δ40 ES cells is rescued by restoration of Scl expression.
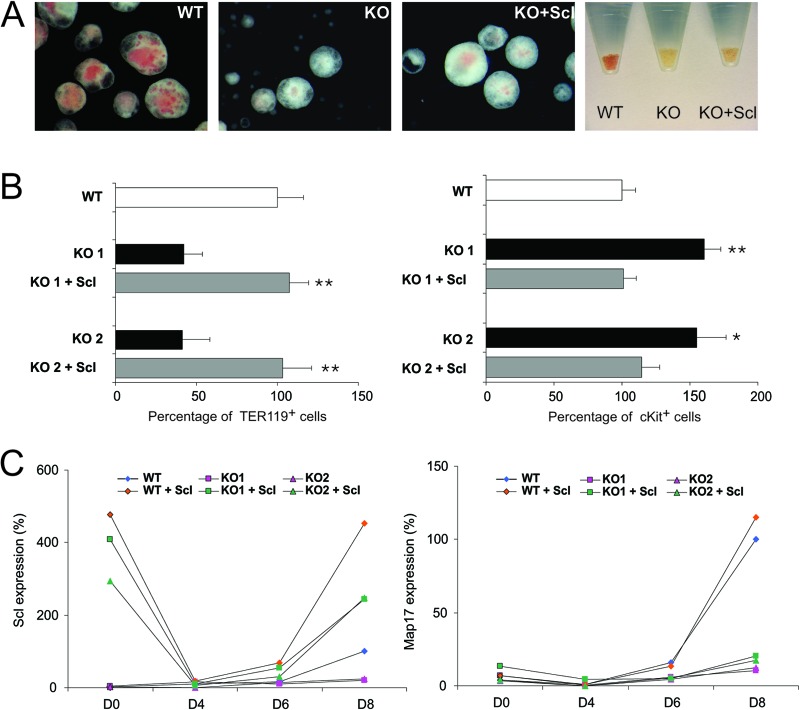
To confirm that the observed phenotype was indeed due to the defect in Scl expression, we transduced the SclΔ40/Δ40 ES cells with a retrovirus containing a Flag-tagged cDNA of Scl, which has been previously used to rescue the phenotype of Scl knockout (KO) ES cells (1, 39). Expression of Flag-Scl in SclΔ40/Δ40 ES cells was able to rescue the erythroid phenotype during ES differentiation. In the presence of Flag-Scl, hemoglobinization was significantly restored in SclΔ40/Δ40 EBs (Fig. 6A). More-detailed analysis showed that the percentage of TER119+ cells was increased while the percentage of cKit+ progenitors was decreased, indicating a restoration of terminal erythroid differentiation (Fig. 6B). Moreover, expression time course analysis confirmed that Scl was indeed overexpressed in the transduced SclΔ40/Δ40 EBs (Fig. 6C). Scl overexpression had no effect on Map17 expression, which remained unaltered in both SclWT/WT and SclΔ40/Δ40 ES cells expressing Flag-Scl.
Fig 6.
Rescue of the SclΔ40/Δ40 ES cells phenotype by Flag-Scl. (A) Representative photographs of EBs generated from SclWT/WT (WT), SclΔ40/Δ40 (KO), and SclΔ40/Δ40 mice overexpressing Flag-Scl (KO + Scl) and pelleted EBs (right). (B) Histograms showing the percentages of Ter119-positive (left) and cKit-positive (right) cells in day 8 EBs. *, P ≤ 0.05; **, P ≤ 0.01 (compared to KO mice). (C) Expression of Scl (left) and Map17 (right) during embryoid body differentiation. Data are normalized to the expression at day 8 in SclWT/WT EBs. WT, SclWT/WT; KO, SclΔ40/Δ40.
The Scl +40 enhancer is shared by Scl and Map17 genes.
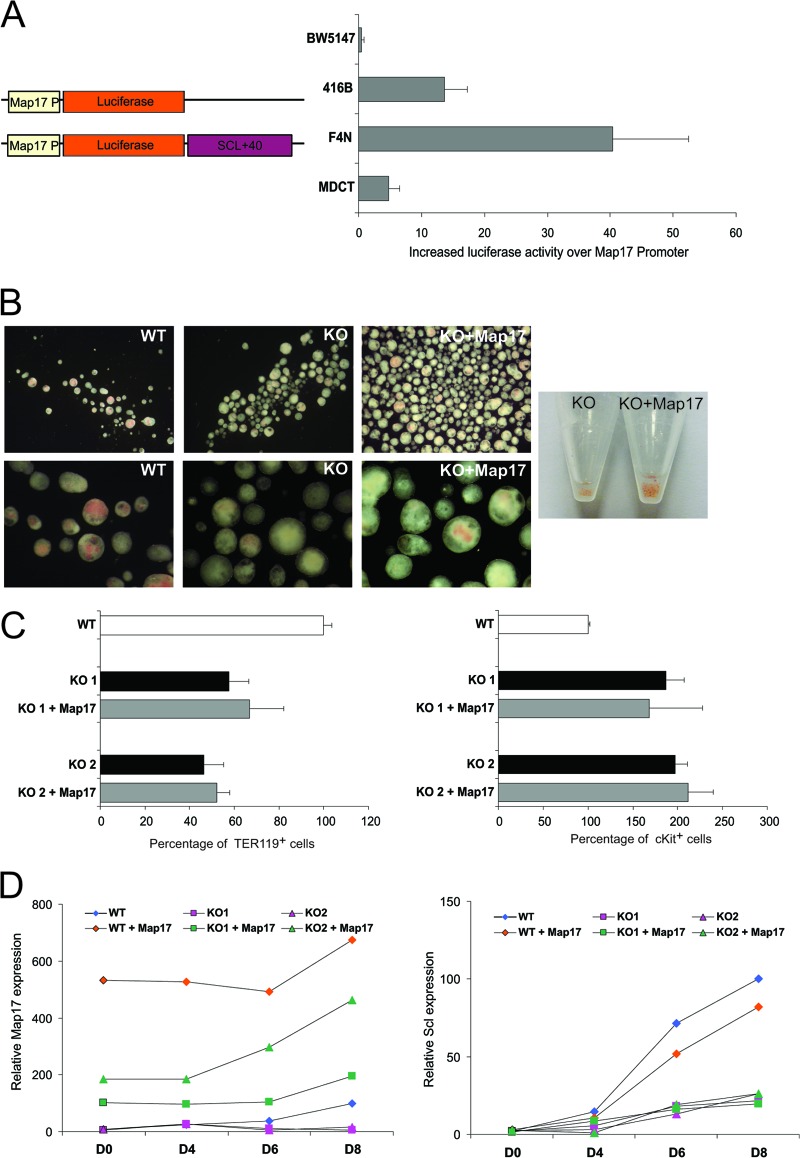
To confirm that the Scl +40 enhancer is a hematopoietic cell-specific enhancer not only of the Scl gene but also of Map17, constructs were generated wherein the luciferase reporter gene was placed under the control of the Map17 promoter. Addition of the Scl +40(5.0) enhancer downstream of the luciferase reporter gene resulted in enhanced expression in a cell-type-dependent matter (Fig. 7A). No enhanced activity was detected in the BW5147 T-cell line, in which the Scl +40 enhancer is known to be inactive. A moderate increase in luciferase activity was detected in the 416B hematopoietic progenitor cell line, with much more substantial enhancement in the F4N erythroid cell line, thus once more demonstrating that the Scl +40 enhancer is mostly erythroid cell specific. Only a very minor increase in luciferase activity was detected in the MDCT kidney cell line, where Map17 is highly expressed, thus suggesting that the +40 enhancer is not active in kidney, consistent with the lack of any loss of Map17 expression in the kidney in SclΔ40/Δ40 mice.
Fig 7.
The Scl +40 element can enhance the Map17 promoter, but Map17 cannot rescue the SclΔ40/Δ40 ES cell phenotype. (A) (Left) Schematic representation of constructs used for luciferase assays. (Right) Luciferase activity in BW5147, 416B, F4N, and MDCT cell lines. Data are normalized to Map17P-Luc. (B) Representative photographs of EBs generated from SclWT/WT, SclΔ40/Δ40, and SclΔ40/Δ40 mice overexpressing Flag-Map17 and pelleted EBs (right). Magnification, ×1.5 (top) or ×5 (bottom). (C) Histograms showing the percentages of TER119-positive (left) and cKit-positive (right) cells in day 8 EBs. (D) Graphs showing expression of Map17 (left) and Scl (right) during embryoid body differentiation. Data are normalized to the expression at day 8 in SclWT/WT EBs. WT, SclWT/WT; KO, SclΔ40/Δ40.
The fact that expression of both Scl and Map17 is altered in SclΔ40/Δ40 EBs raised the question as to which gene is responsible for the observed phenotype. To address this question, we infected the SclΔ40/Δ40 ES cells with a retrovirus containing a Flag-tagged cDNA for Map17. Overexpression of Flag-Map17 in SclΔ40/Δ40 ES cells did not have a significant impact on hemoglobinization of the SclΔ40/Δ40 EBs; however, an increased generation of EBs was observed, indicating a potential function for Map17 in the early stages of EB formation and/or differentiation (Fig. 7B). Map17 overexpression had no effect on the erythroid differentiation phenotype of the SclΔ40/Δ40 ES cells, with the percentage of TER119+ erythroid cells or cKit+ progenitors remaining unaltered (Fig. 7C). Expression analysis of transduced EBs during in vitro differentiation confirmed that Map17 was overexpressed (Fig. 7D). Map17 overexpression had no effect on Scl expression, which remained unaltered in both SclWT/WT and SclΔ40/Δ40 ES cells expressing Flag-Map17.
Together, these data clearly demonstrate that the Scl +40 enhancer regulates Map17 expression but reduced Map17 expression is not the cause of the erythroid phenotype observed in SclΔ40/Δ40 ES cells.
DISCUSSION
Concerted research efforts over the past 20 years have established the Scl gene as a paradigm gene locus for the study of hematopoietic transcriptional control mechanisms (17, 18, 25, 26, 40–48). We have shown previously that the Scl +40 enhancer can drive expression of a linked reporter gene in the erythroid lineage in transgenic assays (17, 27). By deleting this element in the germ line, we now confirm that this enhancer indeed is important for the accurate spatiotemporal expression of Scl in the erythroid lineage. Deletion of the Scl +40 enhancer caused defects in the erythroid lineage, both in vitro and in vivo. No difference in Scl expression was detected in the brain, presumably because neuronal expression of Scl is also driven by the promoter (28). Furthermore, we show that the +40 enhancer regulates the expression of Map17 as well as Scl in hematopoietic cells but only Scl is able to rescue the erythroid defect in ES cell differentiation assays.
The data obtained from analysis of SclΔ40/Δ40 mice and ES cells confirm that indeed the Scl +40 enhancer, in the hematopoietic system, is specific for the erythroid lineage. Deletion of this element leads to reduced Scl expression in whole bone marrow and spleen, containing large numbers of erythroid cells, and specifically in the erythroid lineage, with the highest reduction observed in the progenitor populations containing BFU-e, CFU-e, and proerythroblasts (Ter119low CD71+ and Ter119+ CD71+). This is also confirmed by a reduction in CFU-e numbers in bone marrow spleen and fetal liver. No difference was observed in early progenitors like LSK, CMP, and MEP, where Scl is known to be functionally important (1–4, 12–14, 16). Furthermore, expression analysis of SclΔ40/Δ40 ES cells during in vitro differentiation also showed that Scl expression is normal during early stages of differentiation corresponding to the hemangioblast (day 4) but fails to be upregulated in later stages, when erythroid differentiation occurs. These observations contrast with the absence of differentiation defects in Scl+/− ES cells (2, 7, 49). Although little is known about the function of Map17 in the hematopoietic system, reports have indicated that Map17 is highly expressed in long-term repopulating HSCs (21) and is upregulated in leukemic stem cells (50), suggesting functional importance in HSCs. Our observation of normal Scl expression levels in HSCs and early hematopoietic progenitors are not unexpected, since we have shown previously that alternative regulatory elements such as the Scl +19 and −4 enhancers are active in the progenitor compartment (18, 25, 26, 28). Similarly, we have shown recently that Map17 can be regulated via the Scl +19 enhancer in early hematopoietic cells (24), thus providing a potential explanation for normal Map17 levels in immature cells.
SclΔ40/Δ40 mice are mostly normal, showing only a transient defect in erythroid differentiation at the level of the CFU-e, with no phenotype observed at earlier or later stages of erythroid differentiation, both in adults and during embryonic development. This mild phenotype is surprisingly different from the ES cell phenotype, whereby EBs derived from SclΔ40/Δ40 ES cells showed reduced hemoglobinization and reduced numbers or TER119+ cells. A potential explanation for the different phenotypes could lie in the increased complexity of the in vivo whole-animal setting, where, for example, the erythropoietin (Epo) concentrations as well as other growth factors in the bone marrow can be adjusted to stimulate erythroid differentiation (51, 52). In an attempt to understand if that is the case, we measured the concentration of Epo in the sera of SclΔ40/Δ40 mice. Although we observed a slight increase in the Epo concentration, it failed to reach statistical significance. These data do not, however, dismiss the possibility that increased levels of Epo or other growth factors could be the explanation for the mild phenotype. The fact that the induction of stress erythropoiesis by PHZ did not result in an exacerbated phenotype further emphasizes that such regulation exists in vivo.
The SclΔ40/Δ40 phenotype is also mild in comparison to the phenotype observed when the Scl gene is deleted in adult mice (13). Mice with Scl deletion are anemic, display enlarged spleens, and show a shift toward more immature erythroid progenitors in bone marrow and spleen (16), while the SclΔ40/Δ40 mice show only a reduction in CFU-e numbers. In contrast, Scl heterozygous mice display a defect in the repopulation capacity of short-term (ST)-HSCs but no defect in erythropoiesis was reported (12, 53). We have, however, recently reported a similar ST-HSC phenotype upon the deletion of the Scl stem cell enhancer, thus emphasizing functional differences between the various Scl enhancers (36). The mild phenotype observed in SclΔ40/Δ40 mice is, however, similar to what is observed in adult mice homozygous for a mutant Scl protein with a nonfunctional DNA binding domain (37). The comparatively mild phenotype in our SclΔ40/Δ40 mice may be a reflection of the fact that Scl is still normally expressed in earlier progenitors and also by possible compensation from the other Scl enhancers still functional in these animals. Analysis of the Scl locus in SclΔ40/Δ40 mice by chromatin immunoprecipitation (data not shown) did not suggest a compensatory mechanism by any previously described regulatory element (Scl-4, Promoter, and Scl +19). However, considering that the in vivo phenotype is restricted to a particular stage of erythroid differentiation, it is possible that the effect is diluted in the tissues analyzed by the presence of cells with normal Scl expression. It is also possible that such compensation may be driven by an as-yet-unidentified regulatory element. Due to our extensive study of the Scl/Map17 locus, it is unlikely that such an element resides within the locus; however, the existence of a shadow enhancer situated in a more distant location cannot be excluded (54). Shadow enhancers that can drive expression in patterns similar to that of the primary enhancer have been identified in Drosophila, where they confer robustness to the expression pattern of developmentally important genes (55). Of notice, mild phenotypes upon deletion of regulatory elements are a common occurrence and have been frequently reported before (36, 56–59).
Our data strongly indicate that the Scl +40 enhancer can regulate the expression of Map17 in the erythroid lineage. The +40 element enhances the expression of the Map17 promoter in luciferase reporter assays in erythroid cells and to a lower extent in hematopoietic progenitors but not in kidney cells. This connection between the +40 element and Map17 is reinforced by the fact that in SclΔ40/Δ40 ES cells Map17 is downregulated. The +40 enhancer contains two functionally important Gata/E-box motifs that are bound in erythroid cells by Scl and Gata1 (25). Our results are therefore consistent with a model whereby binding of Scl to the +40 E-box contributes to Map17 expression in the erythroid lineage. Furthermore, we showed that ectopic expression of Map17 does not alter endogenous Scl expression and argue against the possibility of Map17 protein regulating Scl.
Rescue of the SclΔ40/Δ40 ES phenotype by ectopic expression of Flag-Scl without upregulation of Map17 expression suggests that Map17 expression is not necessary for terminal erythroid differentiation, which would also be consistent with the observed failure of Map17 ectopic expression to rescue the phenotype in SclΔ40/Δ40 ES cells. Surprisingly, ectopic overexpression of Map17 led to an increase in the number of EBs, presumably through an expansion of multipotent cells during the earliest stages of differentiation. This suggests that Map17 may perform important functions during the early stages of EB differentiation, which future experiments will need to confirm through comprehensive loss- and gain-of-function studies during early embryo development. It is, however, attractive to speculate that the elevated expression of Map17 seen in many cancers may be related to the apparent increase in cells with EB formation ability.
ACKNOWLEDGMENTS
We thank Michelle Hammett and Tina Hamilton for the generation of the knockout mice, Amy Chaney and Dean Pask for mouse husbandry, and Simon McCallum, Thurka Poobalasingam, and Anna Petrunkina for support with cell sorting. We are also in debt to Pentau Liu and Alan Bradley for the recombineering protocol and reagents.
This work was supported by the Wellcome Trust, the MRC, BBSRC, Leukemia and Lymphoma Research, CRUK, and the Leukemia and Lymphoma Society.
Footnotes
Published ahead of print 14 January 2013
REFERENCES
- 1.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. 1996. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86:47–57 [DOI] [PubMed] [Google Scholar]
- 2.Robb L, Elwood NJ, Elefanty AG, Kontgen F, Li R, Barnett LD, Begley CG. 1996. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15:4123–4129 [PMC free article] [PubMed] [Google Scholar]
- 3.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP, Metcalf D, Begley CG. 1995. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl. Acad. Sci. U. S. A. 92:7075–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivdasani RA, Mayer EL, Orkin SH. 1995. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373:432–434 [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Fujiwara Y, Orkin SH. 1998. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 12:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley CK, Takano EA, Hall MA, Gothert JR, Harvey AR, Begley CG, van Eekelen JA. 2006. The essential haematopoietic transcription factor Scl is also critical for neuronal development. Eur. J. Neurosci. 23:1677–1689 [DOI] [PubMed] [Google Scholar]
- 7.Elefanty AG, Begley CG, Hartley L, Papaevangeliou B, Robb L. 1999. SCL expression in the mouse embryo detected with a targeted lacZ reporter gene demonstrates its localization to hematopoietic, vascular, and neural tissues. Blood 94:3754–3763 [PubMed] [Google Scholar]
- 8.van Eekelen JA, Bradley CK, Gothert JR, Robb L, Elefanty AG, Begley CG, Harvey AR. 2003. Expression pattern of the stem cell leukaemia gene in the CNS of the embryonic and adult mouse. Neuroscience 122:421–436 [DOI] [PubMed] [Google Scholar]
- 9.Green AR, Lints T, Visvader J, Harvey R, Begley CG. 1992. SCL is coexpressed with GATA-1 in hemopoietic cells but is also expressed in developing brain. Oncogene 7:653–660 [PubMed] [Google Scholar]
- 10.Visvader J, Begley CG, Adams JM. 1991. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene 6:187–194 [PubMed] [Google Scholar]
- 11.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. 2009. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457:892–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis DJ, Hall MA, Van Stekelenburg LJ, Robb L, Jane SM, Begley CG. 2004. SCL is required for normal function of short-term repopulating hematopoietic stem cells. Blood 103:3342–3348 [DOI] [PubMed] [Google Scholar]
- 13.Hall MA, Curtis DJ, Metcalf D, Elefanty AG, Sourris K, Robb L, Gothert JR, Jane SM, Begley CG. 2003. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc. Natl. Acad. Sci. U. S. A. 100:992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421:547–551 [DOI] [PubMed] [Google Scholar]
- 15.Salmon JM, Slater NJ, Hall MA, McCormack MP, Nutt SL, Jane SM, Curtis DJ. 2007. Aberrant mast-cell differentiation in mice lacking the stem-cell leukemia gene. Blood 110:3573–3581 [DOI] [PubMed] [Google Scholar]
- 16.Hall MA, Slater NJ, Begley CG, Salmon JM, Van Stekelenburg LJ, McCormack MP, Jane SM, Curtis DJ. 2005. Functional but abnormal adult erythropoiesis in the absence of the stem cell leukemia gene. Mol. Cell. Biol. 25:6355–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delabesse E, Ogilvy S, Chapman MA, Piltz SG, Gottgens B, Green AR. 2005. Transcriptional regulation of the SCL locus: identification of an enhancer that targets the primitive erythroid lineage in vivo. Mol. Cell. Biol. 25:5215–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottgens B, Ferreira R, Sanchez MJ, Ishibashi S, Li J, Spensberger D, Lefevre P, Ottersbach K, Chapman M, Kinston S, Knezevic K, Hoogenkamp M, Follows GA, Bonifer C, Amaya E, Green AR. 2010. cis-Regulatory remodeling of the SCL locus during vertebrate evolution. Mol. Cell. Biol. 30:5741–5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocher O, Cheresh P, Brown LF, Lee SW. 1995. Identification of a novel gene, selectively up-regulated in human carcinomas, using the differential display technique. Clin. Cancer Res. 1:1209–1215 [PubMed] [Google Scholar]
- 20.Jaeger C, Schaefer BM, Wallich R, Kramer MD. 2000. The membrane-associated protein pKe#192/MAP17 in human keratinocytes. J. Invest. Dermatol. 115:375–380 [DOI] [PubMed] [Google Scholar]
- 21.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. 2005. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 1:e28 doi:10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh SH, Josleyn M, Lee YT, Danner RL, Gherman RB, Cam MC, Miller JL. 2007. The human reticulocyte transcriptome. Physiol. Genomics 30:172–178 [DOI] [PubMed] [Google Scholar]
- 23.Guijarro MV, Leal JF, Fominaya J, Blanco-Aparicio C, Alonso S, Lleonart M, Castellvi J, Ruiz L, Ramon YCS, Carnero A. 2007. MAP17 overexpression is a common characteristic of carcinomas. Carcinogenesis 28:1646–1652 [DOI] [PubMed] [Google Scholar]
- 24.Tijssen MR, Cvejic A, Joshi A, Hannah RL, Ferreira R, Forrai A, Bellissimo DC, Oram SH, Smethurst PA, Wilson NK, Wang X, Ottersbach K, Stemple DL, Green AR, Ouwehand WH, Gottgens B. 2011. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell 2011:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottgens B, Broccardo C, Sanchez MJ, Deveaux S, Murphy G, Gothert JR, Kotsopoulou E, Kinston S, Delaney L, Piltz S, Barton LM, Knezevic K, Erber WN, Begley CG, Frampton J, Green AR. 2004. The scl +18/19 stem cell enhancer is not required for hematopoiesis: identification of a 5′ bifunctional hematopoietic-endothelial enhancer bound by Fli-1 and Elf-1. Mol. Cell. Biol. 24:1870–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottgens B, Nastos A, Kinston S, Piltz S, Delabesse EC, Stanley M, Sanchez MJ, Ciau-Uitz A, Patient R, Green AR. 2002. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. EMBO J. 21:3039–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogilvy S, Ferreira R, Piltz SG, Bowen JM, Gottgens B, Green AR. 2007. The SCL +40 enhancer targets the midbrain together with primitive and definitive hematopoiesis and is regulated by SCL and GATA proteins. Mol. Cell. Biol. 27:7206–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair AM, Gottgens B, Barton LM, Stanley ML, Pardanaud L, Klaine M, Gering M, Bahn S, Sanchez M, Bench AJ, Fordham JL, Bockamp E, Green AR. 1999. Distinct 5′ SCL enhancers direct transcription to developing brain, spinal cord, and endothelium: neural expression is mediated by GATA factor binding sites. Dev. Biol. 209:128–142 [DOI] [PubMed] [Google Scholar]
- 29.Silberstein L, Sanchez MJ, Socolovsky M, Liu Y, Hoffman G, Kinston S, Piltz S, Bowen M, Gambardella L, Green AR, Gottgens B. 2005. Transgenic analysis of the stem cell leukemia +19 stem cell enhancer in adult and embryonic hematopoietic and endothelial cells. Stem Cells 23:1378–1388 [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Jenkins NA, Copeland NG. 2003. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su H, Mills AA, Wang X, Bradley A. 2002. A targeted X-linked CMV-Cre line. Genesis 32:187–188 [DOI] [PubMed] [Google Scholar]
- 32.Wood AD, Chen E, Donaldson IJ, Hattangadi S, Burke KA, Dawson MA, Miranda-Saavedra D, Lodish HF, Green AR, Gottgens B. 2009. ID1 promotes expansion and survival of primary erythroid cells and is a target of JAK2V617F-STAT5 signaling. Blood 114:1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. 2004. Development of definitive endoderm from embryonic stem cells in culture. Development 131:1651–1662 [DOI] [PubMed] [Google Scholar]
- 34.Bockamp EO, Fordham JL, Gottgens B, Murrell AM, Sanchez MJ, Green AR. 1998. Transcriptional regulation of the stem cell leukemia gene by PU. 1 and Elf-1. J. Biol. Chem. 273:29032–29042 [DOI] [PubMed] [Google Scholar]
- 35.Magnusson M, Brun AC, Lawrence HJ, Karlsson S. 2007. Hoxa9/hoxb3/hoxb4 compound null mice display severe hematopoietic defects. Exp. Hematol. 35:1421–1428 [DOI] [PubMed] [Google Scholar]
- 36.Spensberger D, Kotsopoulou E, Ferreira R, Broccardo C, Scott LM, Fourouclas N, Ottersbach K, Green AR, Gottgens B. 2012. Deletion of the Scl +19 enhancer increases the blood stem cell compartment without affecting the formation of mature blood lineages. Exp. Hematol. 40:588–598 e581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassouf MT, Chagraoui H, Vyas P, Porcher C. 2008. Differential use of SCL/TAL-1 DNA-binding domain in developmental hematopoiesis. Blood 112:1056–1067 [DOI] [PubMed] [Google Scholar]
- 38.Maetens M, Doumont G, Clercq SD, Francoz S, Froment P, Bellefroid E, Klingmuller U, Lozano G, Marine JC. 2007. Distinct roles of Mdm2 and Mdm4 in red cell production. Blood 109:2630–2633 [DOI] [PubMed] [Google Scholar]
- 39.Chan WY, Follows GA, Lacaud G, Pimanda JE, Landry JR, Kinston S, Knezevic K, Piltz S, Donaldson IJ, Gambardella L, Sablitzky F, Green AR, Kouskoff V, Gottgens B. 2007. The paralogous hematopoietic regulators Lyl1 and Scl are coregulated by Ets and GATA factors, but Lyl1 cannot rescue the early Scl-/- phenotype. Blood 109:1908–1916 [DOI] [PubMed] [Google Scholar]
- 40.Aplan PD, Nakahara K, Orkin SH, Kirsch IR. 1992. The SCL gene product: a positive regulator of erythroid differentiation. EMBO J. 11:4073–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton LM, Gottgens B, Gering M, Gilbert JG, Grafham D, Rogers J, Bentley D, Patient R, Green AR. 2001. Regulation of the stem cell leukemia (SCL) gene: a tale of two fishes. Proc. Natl. Acad. Sci. U. S. A. 98:6747–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bockamp EO, McLaughlin F, Gottgens B, Murrell AM, Elefanty AG, Green AR. 1997. Distinct mechanisms direct SCL/tal-1 expression in erythroid cells and CD34 positive primitive myeloid cells. J. Biol. Chem. 272:8781–8790 [DOI] [PubMed] [Google Scholar]
- 43.Bockamp EO, McLaughlin F, Murrell AM, Gottgens B, Robb L, Begley CG, Green AR. 1995. Lineage-restricted regulation of the murine SCL/TAL-1 promoter. Blood 86:1502–1514 [PubMed] [Google Scholar]
- 44.Chapman MA, Donaldson IJ, Gilbert J, Grafham D, Rogers J, Green AR, Gottgens B. 2004. Analysis of multiple genomic sequence alignments: a web resource, online tools, and lessons learned from analysis of mammalian SCL loci. Genome Res. 14:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gothert JR, Gustin SE, Hall MA, Green AR, Gottgens B, Izon DJ, Begley CG. 2005. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105:2724–2732 [DOI] [PubMed] [Google Scholar]
- 46.Gottgens B, Barton LM, Gilbert JG, Bench AJ, Sanchez MJ, Bahn S, Mistry S, Grafham D, McMurray A, Vaudin M, Amaya E, Bentley DR, Green AR, Sinclair AM. 2000. Analysis of vertebrate SCL loci identifies conserved enhancers. Nat. Biotechnol. 18:181–186 [DOI] [PubMed] [Google Scholar]
- 47.Gottgens B, McLaughlin F, Bockamp EO, Fordham JL, Begley CG, Kosmopoulos K, Elefanty AG, Green AR. 1997. Transcription of the SCL gene in erythroid and CD34 positive primitive myeloid cells is controlled by a complex network of lineage-restricted chromatin-dependent and chromatin-independent regulatory elements. Oncogene 15:2419–2428 [DOI] [PubMed] [Google Scholar]
- 48.Sanchez M, Gottgens B, Sinclair AM, Stanley M, Begley CG, Hunter S, Green AR. 1999. An SCL 3′ enhancer targets developing endothelium together with embryonic and adult haematopoietic progenitors. Development 126:3891–3904 [DOI] [PubMed] [Google Scholar]
- 49.Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, Choi K. 2002. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development 129:5511–5520 [DOI] [PubMed] [Google Scholar]
- 50.Forsberg EC, Passegue E, Prohaska SS, Wagers AJ, Koeva M, Stuart JM, Weissman IL. 2010. Molecular signatures of quiescent, mobilized and leukemia-initiating hematopoietic stem cells. PLoS One 5:e8785 doi:10.1371/journal.pone.0008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minamishima YA, Kaelin WG., Jr 2010. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waskow C, Terszowski G, Costa C, Gassmann M, Rodewald HR. 2004. Rescue of lethal c-KitW/W mice by erythropoietin. Blood 104:1688–1695 [DOI] [PubMed] [Google Scholar]
- 53.Lacombe J, Herblot S, Rojas-Sutterlin S, Haman A, Barakat S, Iscove NN, Sauvageau G, Hoang T. 2010. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood 115:792–803 [DOI] [PubMed] [Google Scholar]
- 54.Barolo S. 2012. Shadow enhancers: frequently asked questions about distributed cis-regulatory information and enhancer redundancy. Bioessays 34:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frankel N, Davis GK, Vargas D, Wang S, Payre F, Stern DL. 2010. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466:490–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahituv N, Zhu Y, Visel A, Holt A, Afzal V, Pennacchio LA, Rubin EM. 2007. Deletion of ultraconserved elements yields viable mice. PLoS Biol. 5:e234 doi:10.1371/journal.pbio.0050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anguita E, Sharpe JA, Sloane-Stanley JA, Tufarelli C, Higgs DR, Wood WG. 2002. Deletion of the mouse alpha-globin regulatory element (HS −26) has an unexpectedly mild phenotype. Blood 100:3450–3456 [DOI] [PubMed] [Google Scholar]
- 58.Bender MA, Byron R, Ragoczy T, Telling A, Bulger M, Groudine M. 2006. Flanking HS-62.5 and 3′ HS1, and regions upstream of the LCR, are not required for beta-globin transcription. Blood 108:1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snow JW, Trowbridge JJ, Johnson KD, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, Bresnick EH. 2011. Context-dependent function of “GATA switch” sites in vivo. Blood 117:4769–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]