Abstract
Protection of telomeres protein 1 (Pot1) binds to single-stranded telomere overhangs and protects chromosome ends. RecQ helicases regulate homologous recombination at multiple stages, including resection, strand displacement, and resolution. Fission yeast pot1 and RecQ helicase rqh1 double mutants are synthetically lethal, but the mechanism is not fully understood. Here, we show that the synthetic lethality of pot1Δ rqh1Δ double mutants is due to inappropriate homologous recombination, as it is suppressed by the deletion of rad51+. The expression of Rad51 in the pot1Δ rqh1Δ rad51Δ triple mutant, which has circular chromosomes, is lethal. Reduction of the expression of Rqh1 in a pot1 disruptant with circular chromosomes caused chromosome missegregation, and this defect was partially suppressed by the deletion of rad51+. Taken together, our results suggest that Rqh1 is required for the maintenance of circular chromosomes when homologous recombination is active. Crossovers between circular monomeric chromosomes generate dimers that cannot segregate properly in Escherichia coli. We propose that Rqh1 inhibits crossovers between circular monomeric chromosomes to suppress the generation of circular dimers.
INTRODUCTION
DNA double-strand break (DSB) ends are repaired by nonhomologous end joining (NHEJ), homologous recombination (HR), or single-strand annealing (SSA) (1, 2). In yeasts, HR is the preferred pathway (3). It uses the sister chromatid as a template for repair and requires a homology search and strand invasion, mediated by Rad52 and the RecA homologue Rad51, to form a D loop. The invading strand is extended, using the homologous sequence as a template, and then displaces and reanneals to the original strand, a process known as synthesis-dependent strand annealing, or the second end of the DSB is captured to form a double Holliday junction. The repair is completed by the resolution of the Holliday junctions by resolvase, Yen1, or the structure-specific nuclease, Mus81/Eme1, or by dissolution by the RecQ helicase-topoisomerase III complex (4, 5). If regions of homology are exposed by resection of the DSB, SSA can be used for repair. This requires Rad52 but is independent of Rad51 (6). The initial step of both HR and SSA is the resection of DSB ends, and several nucleases are suggested to be involved in this. In Saccharomyces cerevisiae, the Mre11-Rad50-Xrs2 complex with Sae2 initiates resection. Then, longer single-stranded DNAs (ssDNAs) are generated by two nucleases, Exo1 and Dna2, the latter acting in conjunction with the RecQ helicase-topoisomerase complex Sgs1-Top3-Rmi1 (7–10). The exo1Δ sgs1Δ double mutant has very low HR and SSA efficiency (10). S. cerevisiae Sgs1 and Schizosaccharomyces pombe RecQ helicase rqh1 mutants have a high frequency of recombination, suggesting that Sgs1 and Rqh1 inhibit unscheduled recombinogenic events (11–16).
Telomeres protect chromosome ends from DNA repair activities, such as HR, NHEJ, and SSA (17). Protection of telomeres protein 1 (Pot1) binds to single-stranded telomere DNA to inhibit DNA repair activity at telomeres (18). In S. pombe, the deletion of pot1+ causes rapid telomere loss and chromosome circularization by SSA, suggesting that the chromosome ends are resected in the absence of Pot1 (19, 20). However, the nuclease(s) responsible for the resection remains unknown. In S. pombe, pot1Δ is synthetically lethal with deletion of the RecQ helicase rqh1 gene (rqh1Δ) (20). As the S. cerevisiae homologue Sgs1 is involved in SSA (10), one explanation for the lethality of the pot1Δ rqh1Δ double mutant might be its inefficient SSA. To test this hypothesis and to understand the mechanism of synthetic lethality of the pot1Δ rqh1Δ double mutant, we sought to identify the genes that suppress its lethality. We found that its lethality is suppressed by the deletion of either rad51+ or exo1+. Moreover, Rqh1 was not required for chromosome circularization when the expression of Pot1 was reduced. An analysis of the phenotypes of the pot1Δ rqh1Δ rad51Δ triple mutant suggests that Rqh1 is required for the maintenance of circular chromosomes.
MATERIALS AND METHODS
Strain construction and growth media.
The strains used in this report are listed in Table 1. The pot1Δ rqh1Δ double mutant (pot1::KanMX rqh1::hphMX) expressing Pot1 from a plasmid (pPC27-pot1+-hemagglutinin [HA], containing the ura4 gene, a gift from Peter Baumann) was created by the transformation of pot1Δ cells (YI002) expressing Pot1 from the plasmid pPC27-pot1+-HA, with the rqh1::hphMX disruption fragment, in which the complete rqh1 open reading frame (ORF) is replaced by the hphMX gene. The pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants, containing the Pot1 plasmid pPC27-pot1-3HA, were created by transforming the pot1Δ rqh1Δ double mutant expressing Pot1 from pPC27-pot1-3HA with the rad51::LEU2 or exo1::aur1 (aureobasidin A resistance gene) disruption fragments. The pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants that do not have pPC27-pot1-3HA were selected on yeast extract-agar (YEA) plates containing 2 g/liter 5-fluoroorotic acid (FOA). The promoter of rqh1 was replaced by the nmt81 promoter, according to the previously described procedure, resulting in nmt-rqh1 (KTA030, h− leu1-32 ura4-D18 ade6-704 rqh1::sup3-5-nmt81-rqh1+) (21). The pot1Δ nmt-rqh1 strain expressing Pot1 from the plasmid pPC27-pot1+-HA (KTA031) was created by mating nmt-rqh1 (KTA030) with the pot1Δ rqh1-hd double mutant expressing Pot1 from the plasmid pPC27-pot1+-HA (GT000). The loss of pPC27-pot1-3HA (KTA032) was selected on YEA plates containing FOA. The loss of telomeric repeats and chromosome circularization were confirmed by Southern hybridization and pulsed-field gel electrophoresis (PFGE) (data not shown). To tag the Rad11 protein in pot1Δ nmt-rqh1 cells with monomeric red fluorescent protein (mRFP) at the C terminus, pFA6a-mRFP-natMX6-rad11 was linearized with NspV and used in the transformation of KTA032, resulting in strain KTA035 (22, 23). The auxin-inducible (24) nmt-pot1-aid strain was created by the same procedure. Cells were grown in YEA medium (0.5% yeast extract, 3% glucose, and 40 μg/ml adenine) or Edinburgh minimal medium (EMM) with required supplements at the indicated temperature (25).
Table 1.
Schizosaccharomyces pombe strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| JY741 | h− leu1-32 ura4-D18 ade6-M216 | M. Yamamoto |
| JY746 | h+ leu1-32 ura4-D18 ade6-M210 | M. Yamamoto |
| MGF809 | h− leu1-32 ura4-D18 ade6-M216 rad11-GFP::kanMX6 | M. Ferreira |
| RY004 | h+ leu1-32 ura4-D18 ade6-M210 rqh1::hphMX6 | This study |
| GT000 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pPC27-pot1+-HA | 32 |
| YI002 | h− leu1-32 ura4-D18 ade6 pot1::kanMX6 pPC27-pot1+-HA | This study |
| TK115 | h+ leu1-32 ura4-D18 cdc25-22 exo1::aur1 | This study |
| FY18537 | h− leu1-32 ura4-D18 rad51::hphMX6 | NBRP |
| 501 | h− leu1-32 ura4-D18 ade6-704 | A. Carr |
| KTA023 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 pPC27-pot1+-HA | This study |
| KTA024 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 rad51::LEU2 pPC27-pot1+-HA | This study |
| KTA025 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 rad51::LEU2 | This study |
| KTA026 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 exo1::aur1 pPC27-pot1+-HA | This study |
| KTA027 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 exo1::aur1 | This study |
| KTA028 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 pPC27-pot1+-HA | This study |
| KTA029 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 rad51::hphMX6 pPC27-pot1+-HA | This study |
| KTA030 | h− leu1-32 ura4-D18 ade6-704 rqh1::sup3-5-nmt81-rqh1+ | This study |
| KTA031 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ pPC27-pot1+-HA | This study |
| KTA032 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ | This study |
| KTA033 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ rad51::LEU2 pPC27-pot1+-HA | This study |
| KTA034 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ rad51::LEU2 | This study |
| KTA035 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ rad11-mRFP::natMX6 | This study |
| KTA036 | h− leu1-32 ura4-D18 ade6-704 pot1::kanMX6 rqh1::sup3-5-nmt81-rqh1+ rad51::LEU2 rad11-mRFP::natMX6 | This study |
| KTA037 | h− leu1-32 ura4-D18 ade6 pot1::kanMX6 | This study |
| KTA038 | h− leu1-32 ura4-D18 ade6 pot1::kanMX6 rad11-mRFP::natMX6 | This study |
| KTA028 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 pPC27-pot1+-HA | This study |
| KTA029 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 rad51::hphMX6 pPC27-pot1+-HA | This study |
| TN001 | h− leu1-32 ura4-D18 ade6-704 rqh1::sup3-5-nmt81-rqh1+ rad11-mRFP::natMX6 | This study |
| TN004 | h+ rad11-mRFP::natMX6 | This study |
| NH001 | h− leu1-32 ura4-D18 pot1::sup3-5-nmt81-pot1+-IAA17::ura4+ ade6::ade6+-Padh15-skp1-AtTIR1-2NLS-9myc | This study |
| NH002 | h− ura4-D18 pot1::sup3-5-nmt81-pot1+-IAA17::ura4+ rqh1::hphMX6 ade6::ade6+-Padh15-skp1-AtTIR1-2NLS-9myc | This study |
| KTA039 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 rad51::hphMX6 | This study |
| KTA040 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A top3::LEU2 rad51::hphMX6 | This study |
| TN040 | h+ ura4 leu1 pot1::kanMX rqh1::hphMX6 rad51::his3 pPC27-pot1+-HA | This study |
| TN042 | h+ ade6 ura4 leu1 pot1::kanMX rqh1::hphMX6 rad51::his3 | This study |
| KTA041 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pPC27-pot1+-HA rad11-mRFP::natMX6 | This study |
| KTA042 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pPC27-pot1+-HA rad11-mRFP::natMX6 pREP41-Top3 | This study |
| KTA044 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A rad11-mRFP::natMX6 pREP41-Top3 | This study |
| KTA043 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pPC27-pot1+-HA rad11-mRFP::natMX6 pREP41-Top3-Y330F | This study |
| KTA045 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A rad11-mRFP::natMX6 pREP41-Top3-Y330F | This study |
| KTA046 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pPC27-pot1+-HA pREP41-HA | This study |
| KTA047 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1-K547A pREP41-HA | This study |
| KTA048 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 pPC27-Leu-pot1+-HA | This study |
| TN076 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 pPC27-Leu-pot1+-HA sfr1::ura4 | This study |
| TN077 | h+ leu1-32 ura4-D18 ade6-M210 pot1::kanMX6 rqh1::hphMX6 pPC27-Leu-pot1+-HA rad57::ura4 | This study |
Measurement of telomere length.
Telomere length was measured using Southern hybridization with an AlkPhos direct kit module (GE Healthcare), according to a previously described procedure (26).
Pulsed-field gel electrophoresis.
PFGE was performed as described by Baumann et al. (27). For the detection of NotI-digested chromosomes, NotI-digested S. pombe chromosomal DNA was fractionated in a 1% agarose gel with 0.5× TBE (50 mM Tris-HCl, 5 mM boric acid, and 1 mM EDTA [pH 8.0]) buffer at 14°C using the CHEF Mapper PFGE system at 6 V/cm (200 V) and a pulse time of 60 to 120 s for 24 h. DNA was visualized by staining with ethidium bromide (1 μg/ml) for 30 min.
Microscopy.
Microscope images of living cells were obtained using an AxioCam digital camera (Zeiss) connected to an Axio Observer.Z1 microscope (Zeiss) with a Plan-Apochromat 63× objective lens (numerical aperture, 1.4). Pictures were captured and analyzed using AxioVision Rel. 4.8.2 software (Zeiss). A glass-bottom dish (Iwaki) was coated with 5 mg/ml lectin from Bandeiraea simplicifolia BS-I (Sigma).
Western blot analysis.
Cells were lysed in 10% trichloroacetic acid (TCA) with glass beads at 4°C. After centrifugation at 15,000 × g for 10 min at 4°C, the precipitate was washed with acetone and suspended in SDS sample buffer. For the detection of Rqh1, the lysate was subjected to Western blot analysis using anti-Rqh1 as a primary antibody at a dilution of 1:5,000 and anti-rabbit IgG–horseradish peroxidase (GE Healthcare) as a secondary antibody at a dilution of 1:5,000. Signals were detected using the ECL Plus detection system (GE Healthcare).
RESULTS
The synthetic lethality of the pot1Δ rqh1Δ double mutant is suppressed by deletion of rad51+ or exo1+.
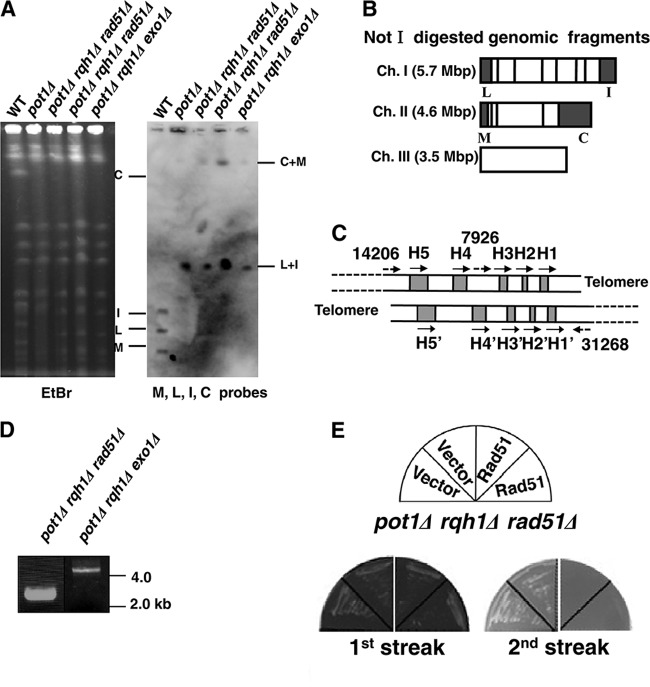
As published previously, the pot1Δ rqh1Δ double mutant is lethal (20). The lethality of the rad60-1 rqh1Δ and rad3Δ rqh1Δ double mutants is suppressed by the deletion of rad51+ (28, 29) (Rad51 is encoded by rhp51 in S. pombe, but for clarity, the new designation rad51 is used throughout). The deletion of rad51+ also improves cell growth in the srs2Δ rqh1Δ double mutant (30). We thus investigated whether the synthetic lethality of the pot1Δ rqh1Δ double mutant was suppressed by the deletion of rad51+, and we created a pot1Δ rqh1Δ rad51Δ triple mutant carrying a plasmid containing pot1+ and the ura4+ gene, which is a negative selection marker. Loss of the plasmid was selected on plates containing 5-fluorodeoxyuridine (FOA), where only cells that did not express ura4+ were viable. The pot1Δ rqh1Δ rad51Δ triple mutant was able to grow on the plate containing FOA, demonstrating that the deletion of rad51+ suppressed the synthetic lethality of the pot1Δ rqh1Δ double mutant (Fig. 1A). We also found that the pot1Δ rqh1Δ exo1Δ triple mutant was viable (Fig. 1A), although its colony formation efficiency was very low compared to that of the pot1Δ rqh1Δ rad51Δ triple mutant.
Fig 1.
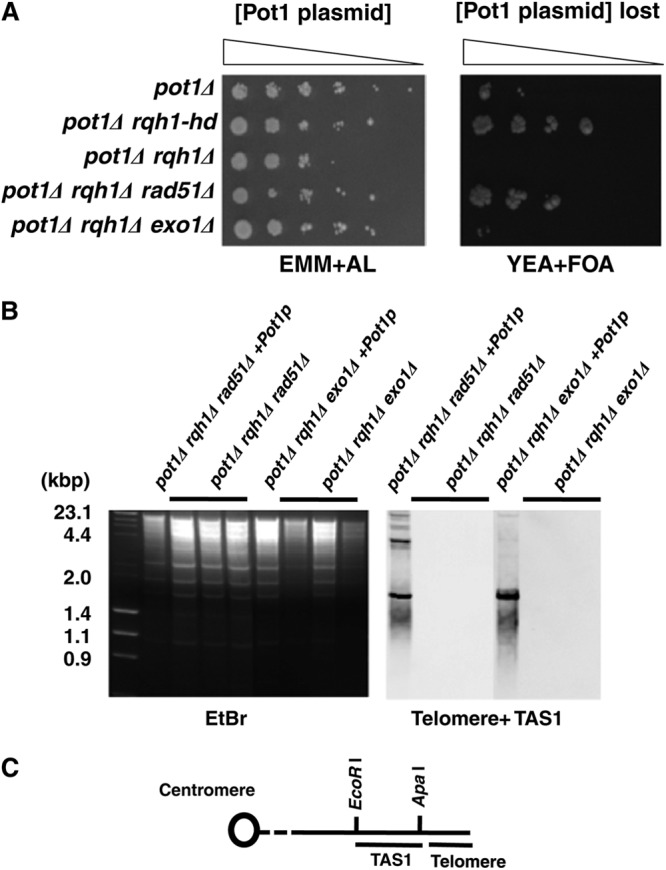
pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants are viable and lose telomeric DNA. (A) Spotting assay of 10-fold serial dilutions of cells. The pot1Δ, pot1Δ rqh1-hd, pot1Δ rqh1Δ, pot1Δ rqh1Δ rad51Δ, and pot1Δ rqh1Δ exo1Δ cells expressing Pot1 from plasmid (YI002, GT000, KTA023, KTA024, and KTA026) were plated on EMM plus adenine and leucine (EMM+AL) and YEA+FOA at 25°C. The Pot1 plasmid is retained on EMM+AL, and cells that lost the plasmid were selected against on YEA+FOA at 25°C. (B) The telomere lengths of the three independent pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ strains were analyzed using Southern hybridization at 25°C. pot1Δ rqh1Δ rad51Δ cells and pot1Δ rqh1Δ exo1Δ cells that express Pot1 from plasmid (+Pot1p) were used as controls that have telomeric DNA. Genomic DNA was digested with EcoRI and separated by 1.5% agarose gel electrophoresis. A telomere fragment (Telomere) plus telomere-associated sequence (TAS1) derived from pNSU70 (72) was used as a probe. To assess the total amount of DNA, the gel was stained with ethidium bromide (EtBr) before blotting onto the membrane was performed. (C) Restriction enzyme sites around the telomere and TAS1 of 1 chromosome arm cloned in the plasmid pNSU70 (72).
Both pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants lose telomeric DNA completely, and the chromosomes are circularized.
The pot1 disruptant loses telomeric DNA completely, and survival depends on the circularization of chromosomes via SSA (20). Since Rad51 is not required for SSA and resection can still occur, albeit inefficiently, in the absence of exo1 and sgs1 (Rqh1 homologue) (7–10), the above data suggest that SSA can still occur in these backgrounds and that the lack of chromosome circularization is not the cause of lethality in the pot1Δ rqh1Δ double mutant. We therefore analyzed telomere length in the triple mutants by using Southern blotting. Both the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants had completely lost telomeric DNA (Fig. 1B and C). Next, we analyzed the chromosome structure by pulsed-field gel electrophoresis (PFGE) (Fig. 2A and B). The NotI-digested fragments M, L, I, and C, which are located at the ends of chromosomes I and II, were detected in wild-type cells. In contrast, the M+I and C+L bands can be detected in both the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants. This is similar to the pot1Δ single mutant, which has circular chromosomes (Fig. 2A and B). These results demonstrate that the chromosomes of both the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants are circularized.
Fig 2.
Chromosomes of the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ cells are circularized by single-strand annealing. (A) NotI-digested S. pombe chromosomal DNA from the wild type, a pot1Δ isolate, two independent pot1Δ rqh1Δ rad51Δ isolates, and one pot1Δ rqh1Δ exo1Δ isolate were analyzed using PFGE. Probes specific for the telomeric NotI fragments (M, L, I, and C) were used (66). (B) NotI restriction site map of S. pombe chromosomes. Chromosomes I, II, and III (Ch. I, Ch. II, and Ch. III) are shown. (C) Schematic of two chromosome ends in head-to-head orientation. Five homology regions (H1 to H5 and H1′ to H5′) are shown in gray. The matching orientations of repeats for single-strand annealing are shown by the arrows. The positions and orientations for PCR primers 7926, 14206, and 31268 used to amplify the junctions are shown by dotted arrows. (D) Amplification of DNA fragments flanking the junctions of circular chromosomes. Primers 7926 and 31268 and primers 14206 and 31268 were used in the PCR, as described previously (20), with genomic DNAs from pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ cells, respectively. (E) Expression of Rad51 in the pot1Δ rqh1Δ rad51Δ cells is lethal. Empty vector (pREP1) or plasmid expressing Rad51 (pREP81x-Rhp51, a gift from Greg A. Freyer) was introduced into the pot1Δ rqh1Δ rad51Δ cells with circular chromosomes and was selected on EMM plus adenine and uracil (EMM+AU) in the presence of thiamine (30 μg/ml) to suppress Rad51 expression. Then, two independent pot1Δ rqh1Δ rad51Δ isolates with either empty vector or plasmid expressing Rad51 were streaked on EMM+AU to induce the expression of Rad51.
Chromosomes of the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants are circularized by single-strand annealing.
Chromosomes in the pot1Δ single mutant are circularized by SSA. There are five subtelomeric homology regions (H1 to H5) and these regions, apart from H4, can be used for SSA in the pot1 disruptant (Fig. 2C) (20). We first attempted to amplify DNA sequences surrounding junctions using two primers that were 8 kb (primer 7226) and 31 kb (primer 31268) from the intact chromosome ends (Fig. 2C). PCR products were obtained from two independent pot1Δ rqh1Δ rad51Δ triple mutants (Fig. 2D and data not shown). Sequencing of the PCR products shows the junction to be in H3, suggesting that circularization results from SSA in the two independent pot1Δ rqh1Δ rad51Δ triple mutants (data not shown; see Fig. S1 in the supplemental material). A PCR product was not obtained from the pot1Δ rqh1Δ exo1Δ triple mutant when two primers, 8 kb (7226) and 31 kb (31268), were used but was obtained when primers 15 kb (14206) and 31 kb (31268), from the intact chromosome ends, were used (Fig. 2C and D). Sequencing of the PCR product suggests that H5 is used for SSA in the pot1Δ rqh1Δ exo1Δ triple mutant (see Fig. S2 in the supplemental material). These results suggest that the chromosomes of the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants are circularized by SSA. Although the pot1Δ rqh1Δ rad51Δ and pot1Δ rqh1Δ exo1Δ triple mutants analyzed here used different homology regions for SSA, the difference might be due simply to the isolates characterized here and might not reflect differences in the genotypes.
Expression of Rad51 in the pot1Δ rqh1Δ rad51Δ triple mutant with circular chromosomes is lethal.
The fact that the pot1Δ rqh1Δ rad51Δ triple mutant is viable, but the pot1Δ rqh1Δ double mutant is not, implies that Rad51 creates toxic intermediates or recombination products in the latter. This might occur after circularization. If this were the case, expression of Rad51 in the pot1Δ rqh1Δ rad51Δ triple mutant with circular chromosomes would be lethal. To test this possibility, we transformed the plasmid expressing Rad51 from the nmt81 promoter or empty vector to the pot1Δ rqh1Δ rad51Δ triple mutant (31). The pot1Δ rqh1Δ rad51Δ triple mutant with circular chromosomes was able to maintain both empty vector and the plasmid expressing Rad51 from the nmt81 promoter in the presence of thiamine (data not shown). Then two independent pot1Δ rqh1Δ rad51Δ triple mutants with the empty vector or the plasmid expressing Rad51 were streaked on a plate without thiamine (Fig. 2E). The pot1Δ rqh1Δ rad51Δ triple mutants with the empty vector can grow on the plate, but the pot1Δ rqh1Δ rad51Δ triple mutants with the plasmid expressing Rad51 lost their viability when streaked two times (Fig. 2E). This suggests that Rad51 creates toxic intermediates or recombination products in the pot1Δ rqh1Δ double mutant with circular chromosomes, resulting in cell death.
Reduction of Rqh1 expression in pot1Δ cells with circular chromosomes inhibits growth.
To study the primary defects associated with rqh1 loss of function in pot1Δ cells with circular chromosomes, the rqh1 promoter was replaced with the nmt81 thiamine-repressible promoter (21). The pot1Δ cells in which the rqh1 promoter was replaced with the nmt81 promoter, designated pot1Δ nmt-rqh1, had circular chromosomes, similarly to pot1Δ cells (Fig. 3A). pot1Δ nmt-rqh1 cells lost their telomeric DNA completely (data not shown). The reduction in Rqh1 protein levels in the presence of thiamine was checked by Western blotting using an antibody against Rqh1. Rqh1 protein levels were reduced after 12 h incubation in the presence of thiamine and were almost undetectable after 24 h incubation (Fig. 3B). Next, nmt-rqh1, pot1Δ, pot1Δ nmt-rqh1, and pot1Δ nmt-rqh1 rad51Δ cells were grown in liquid culture in the presence or absence of thiamine (30 μg/ml), and the cell numbers were measured every 24 h. nmt-rqh1 and pot1Δ cells did not lose viability. In contrast, the nmt-rqh1 pot1Δ cells lost viability in the presence of thiamine (Fig. 3C). This indicates that Rqh1 is required for maintaining the viability of cells with circular chromosomes. Interestingly, pot1Δ nmt-rqh1 rad51Δ cells did not lose viability in the presence of thiamine (Fig. 3C). These data further support our model in which Rqh1 is required for circular chromosomes, but this requirement is canceled by the deletion of rad51+.
Fig 3.
Reduction of rqh1 expression in pot1Δ cells causes growth defects, and the defect is suppressed by deletion of rad51+. (A) NotI-digested S. pombe chromosomal DNA from the pot1Δ and pot1Δ nmt-rqh1 strains was analyzed using PFGE. (B) Anti-Rqh1 Western blotting of protein extracts from pot1Δ nmt-rqh1 and pot1Δ nmt-rqh1 rad51Δ cells collected at time zero, 12 h, and 24 h following promoter repression in the presence of thiamine (30 μg/ml) at 25°C. The arrowhead indicates nonspecific bands that serve as loading controls. (C) Growth characteristics of nmt-rqh1, pot1Δ, pot1Δ nmt-rqh1, and pot1Δ nmt-rqh1 rad51Δ cells (KTA030, KTA037, KTA032, and KTA034) in liquid culture. Cells were diluted to 1.25 × 106 cells/ml in 10 ml of EMM plus adenine, leucine, and uracil in the absence (−Thiamine) or presence (+Thiamine) of thiamine (30 μg/ml). These cultures were grown for 24 h at 25°C, at which point the cell density was determined by counting in a hemocytometer, and the cells were diluted to a cell density of 1.25 × 106 cells/ml in 10 ml of the same medium and were incubated at 25°C. These procedures were repeated every 24 h for 3 days.
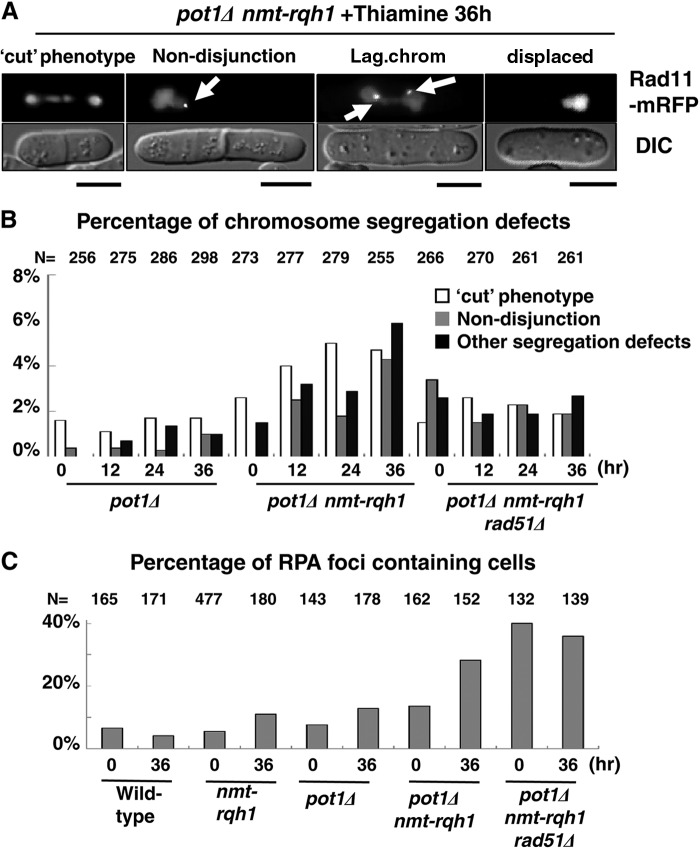
Reduction of Rqh1 expression in pot1Δ cells induces chromosome segregation defects and DNA damage foci.
Next, replication protein A (RPA)-mRFP (Rad11-mRFP)-expressing cells were used to monitor simultaneously the chromosome segregation defects and the DNA damage foci. rad11 encodes the large subunit of RPA, a single-stranded DNA-binding protein complex that is present in the nucleus and localizes to a DNA damage site (32). The percentage of chromosome segregation defects, including the cut phenotype where the septum bisects the nucleus, chromosome nondisjunction, and other defects, including lagging chromosome and displaced nuclei (displace), increased in pot1Δ nmt-rqh1 cells but not in the pot1Δ single mutant when cells were grown in liquid medium in the presence of thiamine (Fig. 4A and B); this indicates that Rqh1 is required for the proper segregation of circular chromosomes. The deletion of rad51+ partially suppressed the chromosome segregation defects, suggesting that the major cause of lethality of the circular chromosome-containing Rqh1 shutoff and rqh1Δ strains is chromosome segregation defects. The percentage of RPA focus-containing cells increased in pot1Δ nmt-rqh1 cells but not in wild-type, nmt-rqh1, or pot1Δ cells 36 h after promoter repression in the presence of thiamine (Fig. 4C). This suggests that Rqh1 prevents DNA damage in circular chromosomes. The deletion of rad51+ did not reduce the percentage of RPA foci, suggesting that the suppression of chromosome segregation defects by the deletion of rad51+ is not due to the prevention of DNA damage (Fig. 4C).
Fig 4.
Chromosome segregation defects of pot1Δ nmt-rqh1 cells in the presence of thiamine. (A) Examples of the chromosome segregation defects in pot1Δ nmt-rqh1 cells, in which RPA (Rad11) is endogenously tagged with mRFP, 36 h following promoter repression in the presence of thiamine at 25°C. Fluorescence micrograph images of Rad11-mRFP and differential interference contrast (DIC) images are shown. Each bar under the diagram represents 5 μm. Examples of cut, nondisjunction, lagging chromosome (Lag.chrom), and displaced nuclei are shown. The arrows indicate RPA foci. (B) Percentages of defects in chromosome segregation in cells at time zero and 12 h, 24 h, and 36 h following promoter repression in the presence of thiamine are shown. pot1Δ, pot1Δ nmt-rqh1, and pot1Δ nmt-rqh1 rad51Δ cells, in which RPA (Rad11) is endogenously tagged with mRFP, were observed at 25°C. Other segregation defects include lagging chromosomes and displaced nuclei. The y axis indicates the percentage of cells that show chromosome segregation defects in the total number of cells. The numbers of cells examined (N) are shown at the top. (C) Percentages of RPA focus-containing cells at time zero and 36 h of the total number of cells following promoter repression in the presence of thiamine are shown. Wild-type, nmt-rqh1, pot1Δ, pot1Δ nmt-rqh1, and pot1Δ nmt-rqh1 rad51Δ cells, in which RPA (Rad11) is endogenously tagged with mRFP (TN004, TN001, KTA038, KTA035, and KTA036), were observed at 25°C.
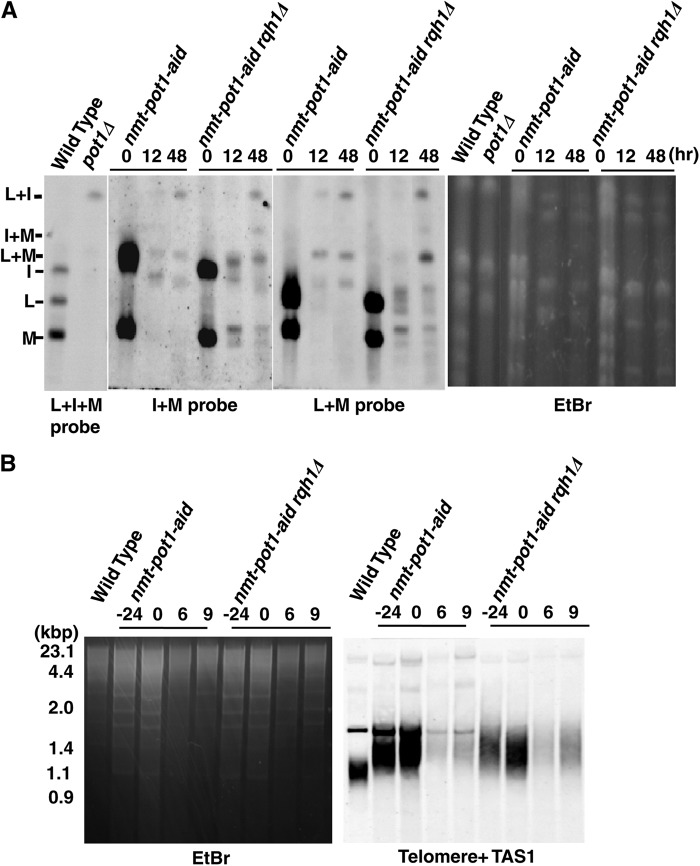
Reduction of Pot1 expression in rqh1Δ cells causes chromosome fusions.
Our results clearly demonstrate that Rqh1 is required for the maintenance of circular chromosomes. However, it remains unclear whether Rqh1 is required for chromosome circularization. To test this, we created the nmt-pot1-aid strain by adding the auxin-inducible degron (aid) tag to the C terminus of the Pot1 to deplete Pot1 efficiently (33). The pot1 promoter was replaced by the nmt81 thiamine-repressible promoter to achieve a more efficient depletion by the addition of both auxin and thiamine (24). We first preincubated both nmt-pot1-aid cells and nmt-pot1-aid rqh1Δ cells in the presence of thiamine for 24 h to reduce the expression of pot1 (0 h). Then, both thiamine and auxin were added and cells were incubated for 12 h and 48 h. The chromosomal structures of these cells were analyzed by PFGE (Fig. 5A). The NotI-digested fragments M, L, and I were detected in both nmt-pot1-aid cells and nmt-pot1-aid rqh1Δ cells in the presence of only thiamine for 24 h (0 h in Fig. 5A). Telomere signals were detected under these conditions (0 h) using a Southern hybridization assay (Fig. 5B). In contrast, most of the M, L, and I fragments disappeared in both kinds of cells after 12 h in the presence of both thiamine and auxin (Fig. 5A). The L+I bands were detected in both strains in the presence of both thiamine and auxin for 48 h, demonstrating that Rqh1 was not required for chromosome circularization, when the expression of pot1 was reduced. We also detected I+M and L+M bands in nmt-pot1-aid rqh1Δ cells and an L+M band in nmt-pot1-aid cells in the presence of both thiamine and auxin for 48 h, suggesting that interchromosome fusions also occur under these conditions. The telomere signals at 6 h and 9 h in both strains became very weak, suggesting the reduction of pot1 expression (Fig. 5B). Although we have not succeeded in detecting Pot1 using Western blotting, the loss of telomeric DNA and chromosome fusion strongly suggest the reduction of pot1 expression in the presence of both thiamine and auxin.
Fig 5.
Rqh1 is not required for chromosome fusion when Pot1 expression is reduced. (A) NotI-digested S. pombe chromosomal DNA from the wild-type, pot1Δ, nmt-pot1-aid, and nmt-pot1-aid rqh1Δ strains (JY746, KTA037, NH001, and NH002) was analyzed using PFGE. Probes specific for the telomeric NotI fragments (I, L, and M) were used (66). Cells were preincubated in the presence of thiamine (15 μg/ml) for 24 h to reduce the expression of pot1 (0 h) at 30°C. Then, both thiamine and auxin (0.5 mM 1-naphthaleneacetic acid) were added and incubated for 12 h and 48 h. (B) The telomere lengths of the wild-type, nmt-pot1-aid, and nmt-pot1-aid rqh1Δ strains were analyzed using Southern hybridization at 30°C. Cells were incubated as described for panel A.
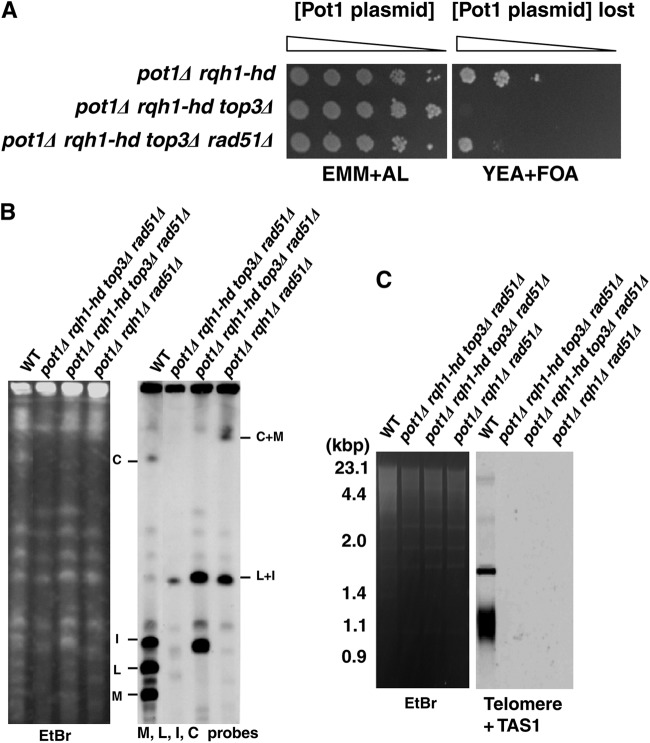
pot1Δ rqh1-hd top3Δ triple mutant is lethal but is suppressed by deletion of rad51+.
Rqh1 binds to type 3 topoisomerase (Top3) and functions as a Rqh1-Top3-Rmi1 complex (34, 35). We asked whether Top3 was also required for the viability of pot1Δ cells and, if so, whether this requirement was also canceled by the deletion of rad51+. The top3 disruptant is lethal, but this lethality can be suppressed by a deletion or mutation of rqh1+ (34, 36). We used a top3 disruptant in an rqh1-hd mutant background because both the pot1Δ rqh1-hd and top3Δ rqh1-hd double mutants are viable, which enabled us to test whether the pot1Δ rqh1-hd top3Δ triple mutant was viable (32, 37). We first created a pot1Δ rqh1-hd top3Δ triple mutant expressing Pot1 from a plasmid, and cells that lost the plasmid were selected on FOA. The pot1Δ rqh1-hd top3Δ triple mutant was not viable, demonstrating that Top3 is required for the viability of pot1Δ cells in the rqh1-hd background (Fig. 6A). Interestingly, lethality of the pot1Δ rqh1-hd top3Δ triple mutant was also suppressed by the deletion of rad51+ (Fig. 6A). These genetic interactions are very similar to those of the pot1Δ rqh1Δ double mutant. Our results suggest that Top3 and Rqh1 function together to maintain viability under these conditions.
Fig 6.
Top3 is required for viability of pot1Δ when Rad51 is active. (A) Spotting assay of 10-fold serial dilutions of cells. pot1Δ rqh1-hd, pot1Δ rqh1-hd top3Δ, and pot1Δ rqh1-hd top3Δ rad51Δ cells expressing Pot1 from a plasmid (GT000, KTA028, and KTA029) were plated on EMM+AL and YEA+FOA at 25°C. The Pot1 plasmid is retained on EMM+AL, and cells that lost the plasmid were selected for on YEA+FOA at 25°C. (B) NotI-digested S. pombe chromosomal DNA from the wild type, two independent pot1Δ rqh1-hd top3Δ rad51Δ isolates, and one pot1Δ rqh1Δ rad51Δ isolate (JY746, KTA039, KTA040, and TN042) was analyzed using PFGE at 25°C. (C) The telomere lengths of the same strains were analyzed using Southern hybridization at 25°C.
The chromosome structures of two independent pot1Δ rqh1-hd top3Δ rad51Δ strains were analyzed by PFGE. Similar to the pot1Δ rqh1Δ rad51Δ triple mutant, which has circular chromosomes, the NotI-digested L+I fragment bands were detected in both strains (Fig. 6B). Moreover, M, L, I, and C bands disappeared in both strains. However, the C+M band was not detected in either strain. One of the pot1Δ rqh1-hd top3Δ rad51Δ strains had a new band, which has a mobility similar to that of the I band. These results suggest that, in these cells, at least chromosome I is circularized. But additional chromosome rearrangements might happen in these cells. Telomere and subtelomere signals were completely gone in two independent pot1Δ rqh1-hd top3Δ rad51Δ strains, further supporting the notion that the chromosomes were circularized in these cells (Fig. 6C). The different PFGE patterns between pot1Δ rqh1Δ rad51Δ cells and pot1Δ rqh1-hd top3Δ rad51Δ cells might suggest that Top3 plays a more important role in maintaining chromosome integrity in the absence of both Pot1 and Rad51.
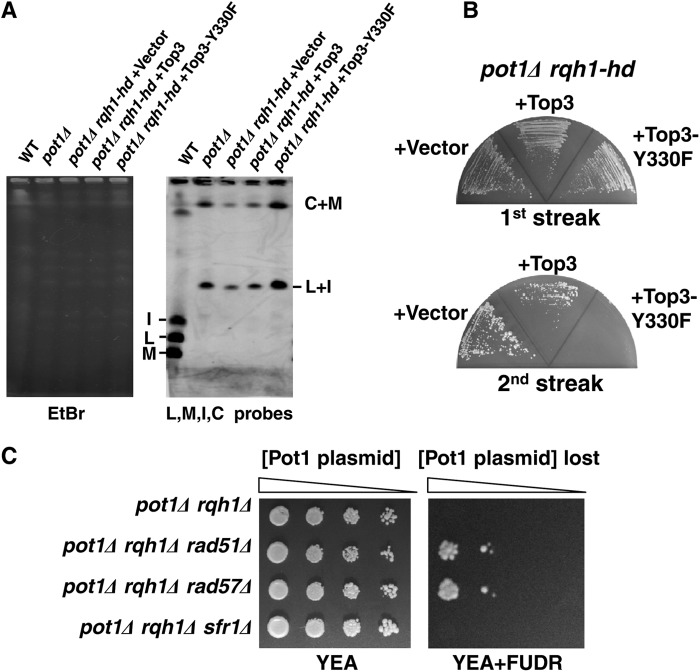
Inactivation of Top3 by overexpression of a Top3-Y330F mutant in pot1Δ rqh1-hd cells, which have circular chromosomes, causes cell death.
Reduction of Rqh1 expression in pot1Δ cells with circular chromosomes inhibits growth. If Rqh1 functions together with Top3, inactivation of Top3 in the cells with circular chromosomes would also inhibit growth. When overexpressed in wild-type cells, the Top3-Y330F active-site mutant displays a dominant negative phenotype, including a growth defect, which is suppressed by the deletion of rqh1 (37). To test whether the inactivation of Top3 in the pot1Δ rqh1-hd double mutant causes cell death, we overexpressed Top3-Y330F in the pot1Δ rqh1-hd double mutant. First, we checked whether the chromosomes in the pot1Δ rqh1-hd double mutant are linear in the presence of empty vector, Top3 vector, or Top3-Y330F vector in the presence of thiamine. Under these conditions, only a very small amount of Top3 or Top3-Y330F is expressed. The chromosomes of the pot1Δ rqh1-hd double mutant are not circularized when they are grown on YEA medium (32). Surprisingly, the chromosomes in the pot1Δ rqh1-hd double mutant were circularized in all three cases (Fig. 7A). To maintain the plasmid, which has the LEU2 marker, cells were grown on minimum medium. Although the mechanism is unclear, we assume that minimum medium makes the pot1Δ rqh1-hd double mutant, which has circular chromosomes, viable. In contrast, the pot1Δ rqh1Δ double mutant is not viable even on minimum medium (data not shown). Next, the pot1Δ rqh1-hd double mutants that have circular chromosomes and also the empty vector, Top3 vector, or Top3-Y330F vector were streaked on the plates in the absence of thiamine. Under these conditions, the expression of either Top3 or Top3-Y330F is highly induced from the nmt-41 promoter. The expression of Top3-Y330F caused a significant growth defect on the second streak in the absence of thiamine (Fig. 7B). This demonstrates that Top3 is required for the viability of cells with circular chromosomes. Both Rqh1 and Top3 are required for the viability of cells with circular chromosomes, strongly suggesting that the Rqh1-Top3 complex plays an important role in the maintenance of circular chromosomes.
Fig 7.
Top3 is required for viability of pot1Δ rqh1-hd double mutants with circular chromosomes, and reduction of crossover frequency significantly suppressed the lethality of a pot1Δ rqh1Δ double mutant. (A) Chromosomes of the pot1Δ rqh1-hd double mutant were circularized when cells were incubated in EMM medium. NotI-digested S. pombe chromosomal DNA from the wild type, a pot1Δ mutant, a pot1Δ rqh1-hd double mutant plus pREP41 empty vector, a pot1Δ rqh1-hd double mutant plus pREP41-Top3 vector, and a pot1Δ rqh1-hd double mutant plus pREP41-Top3-Y330F vector (JY746, KTA037, KTA047, KTA044, and KTA045) was analyzed using PFGE at 25°C. Cells were cultivated throughout in EMM medium in the presence of thiamine (30 μg/ml) to suppress Rad51 expression. (B) Expression of Top3-Y330F in the pot1Δ rqh1-hd cells with circular chromosomes is lethal. The pot1Δ rqh1-hd double mutant plus pREP41 empty vector, pot1Δ rqh1-hd double mutant plus pREP41-Top3 vector, and pot1Δ rqh1-hd double mutant plus pREP41-Top3-Y330F vector were streaked on EMM plus adenine and uracil (EMM+AU) to induce the expression of Top3 or Top3-Y330F. (C) Spotting assay of 10-fold serial dilutions of cells. pot1Δ rqh1Δ, pot1Δ rqh1Δ rad51Δ, pot1Δ rqh1Δ rad57Δ, and pot1Δ rqh1Δ sfr1Δ cells expressing Pot1 from plasmid (KTA048, TN040, TN077, and TN076) were plated on YEA and YEA plus 5-fluorodeoxyuridine (YEA+FUDR) at 25°C. The Pot1 plasmid is retained on YEA medium, and cells that lost the plasmid were selected against on YEA plus 50 μM FUDR at 25°C.
Deletion of rad57+, but not swi5+, suppresses cell death in pot1Δ rqh1Δ cells.
In S. pombe, Rad51-dependent HR has two subpathways, Rad55-Rad57 (Rad55 and Rad57 are encoded by rhp55 and rhp57 in S. pombe, but for clarity, the new designations Rad55 and Rad57 are used throughout) and Swi5-Sfr1. Deletion of either rad57+ or swi5+ does not completely block homologous recombination (38, 39). High crossover frequency in rqh1Δ cells is decreased to 0% with the deletion of rad57+. In contrast, the deletion of swi5+ only slightly decreases the crossover frequency in rqh1Δ cells (40). Crossing-over between sister chromatids in circular chromosomes creates circular chromosome dimers, which inhibit chromosome segregation (41, 42). If this were a reason for the lethality of the pot1Δ rqh1Δ double mutant, deletion of the Rad57 pathway would suppress this lethality. To test this possibility, we created the pot1Δ rqh1Δ rad57Δ and pot1Δ rqh1Δ sfr1Δ triple mutants, which carry the plasmid expressing Pot1. The pot1Δ rqh1Δ rad57Δ triple mutant and the pot1Δ rqh1Δ sfr1Δ triple mutant, both of which carry the plasmid expressing Pot1, grow well on YEA plates (Fig. 7C). The pot1Δ rqh1Δ rad57Δ triple mutant that had lost the Pot1 plasmid was obtained very efficiently (Fig. 7C). In contrast, pot1Δ rqh1Δ sfr1Δ triple mutant cells that lost the Pot1 plasmid were not obtained. This suggests that crossing-over between sister chromatids in circular chromosomes is the reason for lethality.
DISCUSSION
In pot1Δ cells, survival of the loss of telomere function is due to chromosome circularization that is dependent on SSA. S. cerevisiae Sgs1 is involved in SSA, and it has been recently suggested that the S. pombe homologue Rqh1 has a similar involvement (43). This led us to test the hypothesis that the synthetic lethality of the pot1Δ rqh1Δ double mutant was due to a defect in SSA, which would result in the inability to form circular chromosome survivors. However, SSA is not completely blocked in an sgs1 disruptant (10). Moreover, SSA is suggested to occur in the rqh1Δ single mutant (43). These facts suggest that chromosomes might be circularized by SSA in the pot1Δ rqh1Δ double mutant. Indeed, we found that Rqh1 was not required for chromosome circularization when Pot1 expression was reduced (Fig. 5A). We also found that the deletion of rad51+ or exo1+ might suppress the lethality of the pot1Δ rqh1Δ double mutant (Fig. 1A). The chromosomes in both the pot1Δ rqh1Δ rad51Δ and the pot1Δ rqh1Δ exo1Δ triple mutants, as in the pot1Δ single mutant, were circularized, with the junctions in homologous regions consistent with generation by SSA (Fig. 2A and D); this demonstrates that Rqh1 is not essential for SSA. These results strongly suggest that chromosomes can be circularized in the pot1Δ rqh1Δ double mutant. Thus, the synthetic lethality of the pot1Δ rqh1Δ double mutant is not due to a defect in SSA but rather to an inability to maintain circular chromosomes.
The Rqh1 shut-off experiments demonstrate that Rqh1 is required for the viability of pot1Δ cells when Rad51 is active (Fig. 3C). We also found that the pot1Δ rqh1Δ rad51Δ triple mutant with circular chromosomes could not maintain viability when Rad51 was expressed (Fig. 2E), demonstrating that the expression of Rad51 is toxic in pot1Δ cells when Rqh1 is not present; this further supports the hypothesis that Rqh1 is required to maintain circular chromosomes. How does the circular chromosome cause Rad51-dependent lethality in the absence of Rqh1? Stable maintenance of a large circular minichromosome in S. pombe requires a high level of type II DNA topoisomerase (44), suggesting that circular chromosomes have topological stress, possibly during DNA replication, which might stall or collapse replication forks (45). In both S. cerevisiae and S. pombe, Sgs1 and Rqh1 have been implicated in the stabilization of stalled replication forks, the activation of the S phase checkpoint, and repair and replication restart (35). Loss of Rqh1 would lead to an increase in fork collapse under the conditions of replication stress and increased dependence on HR for replication restart. Consistently, we found that the reduction of Rqh1 protein levels in the pot1 disruptant increased the RPA foci, suggesting that Rqh1 prevents DNA damage in the absence of Pot1 (Fig. 4C).
HR between sister chromatids in circular chromosomes might cause crossing-over, creating a chromosome dimer and resulting in chromosome segregation defects. In E. coli, those dimeric chromosomes are converted to monomers by Xer site-specific recombination (46, 47). The defects of Xer mutants are suppressed by the inactivation of RecA (Rad51 homologue in E. coli), suggesting that RecA-dependent HR creates a chromosome dimer that is toxic in E. coli (48, 49). E. coli, S. cerevisiae, and human RecQ helicases suppress crossover recombination products both in vitro and in vivo (50–54). S. pombe Rqh1 also suppresses crossing-over (40). In addition, there is no GEN1 homologue in S. pombe (55), and therefore, in the absence of Rqh1, the resolution of recombination can only occur through the action of Mus81, which favors crossing-over (40, 56–58). Therefore, one cause of lethality in the pot1Δ rqh1Δ double mutant might be the formation of chromosome dimers by crossing-over and the resulting chromosome segregation problems. Consistently, a Rad51-dependent increase in chromosome missegregation was seen in the Rqh1 shut-off strain (Fig. 4B). Moreover, the deletion of rad57+, which should suppress the high crossover frequency in the pot1Δ rqh1Δ double mutant, significantly suppressed lethality, further supporting this possibility (Fig. 7C). The second cause of lethality in the pot1Δ rqh1Δ double mutant might be the accumulation of the recombination intermediates, as RecQ helicases promote the resolution of recombination intermediates (4, 35, 50, 59). These recombination intermediates might be removed by the Mus81-Eme1 complex, E. coli RusA, or human GEN1 (12, 60, 61). To test this possibility, we overexpressed the Mus81-Eme1 complex, E. coli RusA, or human GEN1 from the nmt promoter in the pot1Δ rqh1Δ double mutant. However, they did not suppress lethality (data not shown), suggesting that the second possibility is not likely. The third cause of lethality in the pot1Δ rqh1Δ double mutant might be the accumulation of catenanes between circular chromosomes, which might also lead to chromosome missegregation and lethality. DSBs and their repair by HR might create catenanes between two circular chromosomes if one DNA strand passes the broken DNA strand before DSB repair. These catenanes might be removed by type II topoisomerase (Top2) (62). To test this possibility, we overexpressed Top2 from the nmt81 promoter in the pot1Δ rqh1Δ double mutant (63). However, it did not suppress lethality (data not shown), suggesting that the third possibility is less likely. Thus, we propose that the main cause of lethality in the pot1Δ rqh1Δ double mutant is the formation of chromosome dimers. If the probability of dimer formation for each circular chromosome is 50%, we calculated the probability of cell survival to be 25% in each cell division (Fig. 8). This means that only about one cell of one million cells can survive after 10 cell divisions. We assume that the Mus81-Eme1 complex is responsible for generating crossovers and lethal chromosome dimers in the absence of Rqh1-Top3 complexes (40). However, this cannot be tested directly because the mus81Δ rqh1Δ double mutant is synthetically lethal (64). Further investigation is necessary to understand the function of the Mus81-Eme1 complex in the pot1Δ rqh1Δ double mutant.
Fig 8.
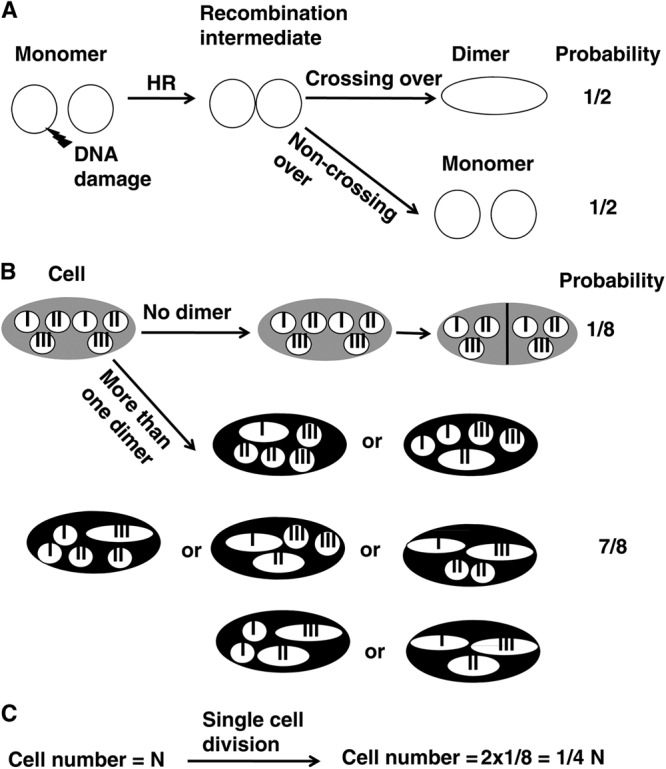
Probability of cell survival when a chromosome dimer is generated by crossing-over, resulting from Rad51-dependent HR in circular chromosomes in the absence of Rqh1. (A) Probability of chromosome dimer formation by crossing-over between one pair of circular sister chromatids. HR repair in one of the circular sister chromatids would convert the two monomers into a chromosome dimer with a probability of 50% in each cell cycle; the probability of dimer formation in each cell cycle is 50% for each chromosome. Open circles and the open oval represent chromosome monomers and a dimer, respectively. (B) Probability of cell survival in each cell cycle. When each circular sister chromatid in the three chromosomes is converted to a dimer with 50% probability as described for panel A, and at least one dimer-containing cell is killed by chromosome missegregation in each cell cycle, the probability of survival of the cell is only 1 in 8, or 12.5%, in each cell cycle. Gray and black ovals represent surviving and dead cells, respectively. Open circles and ovals represent chromosome monomers and dimers, respectively. I, II, and III in the open circles and ovals represent S. pombe chromosomes I, II, and III, respectively. (C) Change of cell number in a single cell division. When N cells undergo a single cell division, only living cells described for panel B can be duplicated, and the probability of a living cell is 1 in 8. As a result, N cells become 1/4 N cells in single cell division. Ten cell divisions will result in a reduction in cell numbers to (1/4)10 or 1/1,048,576. If in the absence of Rqh1, crossing-overs were dominant and/or if recombination occurred between homologous regions in different chromosomes instead of between the same sequences in sister chromatids, the probability of dimer formation increases, resulting in a lower chance of survival than is estimated here.
The next question is whether the requirement of Rqh1 for the maintenance of circular chromosomes is pot1 disruptant specific. In S. pombe, the catalytic subunit of telomerase is encoded by the trt1+ gene. The deletion of trt1+ produces circular survivors or survivors that maintain telomeres by HR (65, 66). We found that the trt1Δ rqh1Δ double mutant is not lethal, but the telomeres of the double mutant are maintained by HR, and we could not obtain a double mutant with circular chromosomes (23). This suggests that Rqh1 is required for the maintenance of any circular survivors. It remains unclear why no survivors of the pot1Δ rqh1 double mutant that maintain telomeres by HR were obtained. One possible explanation is that Pot1 inhibits SSA, and high SSA activity promotes chromosome circularization dominantly in the pot1Δ rqh1 double mutant. This idea is supported by the fact that survivors of the pot1Δ mutant that maintain telomeres by HR were not obtained.
Similar to the requirement for Rqh1, Top3 was also required for the viability of the pot1Δ rqh1-hd double mutant when Rad51 was active (Fig. 6A). Surprisingly, the helicase activity of Rqh1 was not required for the maintenance of circular chromosomes when cells were grown in minimum medium to select the cells that have plasmid, including the empty vector (Fig. 7A). This suggests that the helicase activity of Rqh1 is not required for the maintenance of circular chromosomes. This allowed us to test whether the inactivation of Top3 by overexpression of the dominant negative Top3-Y330F in the pot1Δ rqh1-hd double mutant, which has circular chromosomes, causes significant growth defects. Indeed, it caused significant growth defects (Fig. 7B). One possible explanation for these results is that the topoisomerase activity of the Rqh1-Top3-Rmi1 complex is essential for maintaining the viability of the pot1 disruptant. However, we cannot exclude the possibility that Top3 is required for the helicase-independent activity of Rqh1. The requirement of both Top3 and the helicase-independent activity of Rqh1 strongly suggests that Top3 and Rqh1 function together to maintain viability of the cells that have circular chromosomes. We detected chromosome fusion bands by PFGE when Pot1 expression was reduced in an rqh1-hd background (data not shown). These data demonstrate that the helicase-independent activity of Rqh1 does not inhibit chromosome circularization.
We also found that the deletion of exo1+ can suppress the lethality of the pot1Δ rqh1Δ double mutant (Fig. 1A). In S. cerevisiae, the exo1Δ sgs1Δ double mutant has very low efficiency of HR and SSA (10). In S. pombe, Exo1 is critical for extended resection, and the remaining resection activity in exo1Δ cells is attributable to Rqh1 (67). These facts suggest that there is no or little HR activity left in the absence of both Exo1 and Rqh1, similar to what occurs in the rad51 disruptant. This might be the reason for the suppression of lethality of the pot1Δ rqh1Δ double mutant by the deletion of exo1+. Interestingly, SSA can occur in the absence of both Exo1 and Rqh1 (Fig. 2D), suggesting that the resection of telomere ends also can occur in the absence of both Rqh1 and Exo1. However, the nuclease(s) responsible for the resection of telomere ends in the absence of both Rqh1 and Exo1 remains unclear.
Ring chromosomes have been identified for all human chromosomes. The phenotypes associated with ring chromosomes are variable, probably because of their instability (68). In the case of ring chromosome 22, which contains tumor suppressor gene NF2, the loss of the whole ring chromosome 22 has been suggested to increase the risk of cancer (69). Our results suggest that the suppression of crossing-over might stabilize human ring chromosomes and thus reduce the risk of cancer. The function of RecQ helicase is conserved from E. coli to humans (53, 70, 71). This implies that human RecQ helicases might be required for the maintenance of ring chromosomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Carr, T. Toda, R. Tesin, H. Iwasaki, J. Cooper, M. Whitby, G. Freyer, and the National Bioresource Project, Japan, for providing the plasmids and strains. We thank P. Baumann for providing strains and for the critical reading of the manuscript. We thank Shao-Win Wang for sharing unpublished results. We thank Akinori Awazu for helping with the calculation of the probability of survival.
This study was supported by Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to M.U. The Murray laboratory is supported by grants from the MRC (G0901011), Association for Cancer Research (10–273), and Cancer Research UK (C9601/A9484).
Footnotes
Published ahead of print 7 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01713-12.
REFERENCES
- 1. Kasparek TR, Humphrey TC. 2011. DNA double-strand break repair pathways, chromosomal rearrangements and cancer. Semin. Cell Dev. Biol. 22: 886– 897 [DOI] [PubMed] [Google Scholar]
- 2. Kass EM, Jasin M. 2010. Collaboration and competition between DNA double-strand break repair pathways. FEBS Lett. 584: 3703– 3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krejci L, Altmannova V, Spirek M, Zhao X. 2012. Homologous recombination and its regulation. Nucleic Acids Res. 40: 5795– 5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein KA, Gangloff S, Rothstein R. 2010. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 44: 393– 417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mimitou EP, Symington LS. 2009. Nucleases and helicases take center stage in homologous recombination. Trends Biochem. Sci. 34: 264– 272 [DOI] [PubMed] [Google Scholar]
- 6. Ivanov EL, Sugawara N, Fishman-Lobell J, Haber JE. 1996. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics 142: 693– 704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. 2010. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature 467: 112– 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gravel S, Chapman JR, Magill C, Jackson SP. 2008. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 22: 2767– 2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mimitou EP, Symington LS. 2008. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455: 770– 774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. 2008. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134: 981– 994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caspari T, Murray JM, Carr AM. 2002. Cdc2-cyclin B kinase activity links Crb2 and Rqh1-topoisomerase III. Genes Dev. 16: 1195– 1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doe CL, Dixon J, Osman F, Whitby MC. 2000. Partial suppression of the fission yeast rqh1− phenotype by expression of a bacterial Holliday junction resolvase. EMBO J. 19: 2751– 2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray JM, Lindsay HD, Munday CA, Carr AM. 1997. Role of Schizosaccharomyces pombe RecQ homolog, recombination, and checkpoint genes in UV damage tolerance. Mol. Cell. Biol. 17: 6868– 6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onoda F, Seki M, Miyajima A, Enomoto T. 2001. Involvement of SGS1 in DNA damage-induced heteroallelic recombination that requires RAD52 in Saccharomyces cerevisiae. Mol. Gen. Genet. 264: 702– 708 [DOI] [PubMed] [Google Scholar]
- 15. Stewart E, Chapman CR, Al-Khodairy F, Carr AM, Enoch T. 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16: 2682– 2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watt PM, Hickson ID, Borts RH, Louis EJ. 1996. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics 144: 935– 945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain D, Cooper JP. 2010. Telomeric strategies: means to an end. Annu. Rev. Genet. 44: 243– 269 [DOI] [PubMed] [Google Scholar]
- 18. Baumann P, Price C. 2010. Pot1 and telomere maintenance. FEBS Lett. 584: 3779– 3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitt CW, Cooper JP. 2010. Pot1 inactivation leads to rampant telomere resection and loss in one cell cycle. Nucleic Acids Res. 38: 6968– 6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Baumann P. 2008. Chromosome fusions following telomere loss are mediated by single-strand annealing. Mol. Cell 31: 463– 473 [DOI] [PubMed] [Google Scholar]
- 21. Werler PJ, Hartsuiker E, Carr AM. 2003. A simple Cre-loxP method for chromosomal N-terminal tagging of essential and non-essential Schizosaccharomyces pombe genes. Gene 304: 133– 141 [DOI] [PubMed] [Google Scholar]
- 22. Sato M, Dhut S, Toda T. 2005. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22: 583– 591 [DOI] [PubMed] [Google Scholar]
- 23. Ukimori S, Kawabata N, Shimada H, Imano R, Takahashi K, Yukawa M, Tsuchiya E, Ueno M. 2012. A double mutant between fission yeast telomerase and RecQ helicase is sensitive to thiabendazole, an anti-microtubule drug. Biosci. Biotechnol. Biochem. 76: 264– 269 [DOI] [PubMed] [Google Scholar]
- 24. Kanke M, Nishimura K, Kanemaki M, Kakimoto T, Takahashi TS, Nakagawa T, Masukata H. 2011. Auxin-inducible protein depletion system in fission yeast. BMC Cell Biol. 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreno S, Klar A, Nurse P. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795– 823 [DOI] [PubMed] [Google Scholar]
- 26. Cooper JP, Nimmo ER, Allshire RC, Cech TR. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385: 744– 747 [DOI] [PubMed] [Google Scholar]
- 27. Baumann P, Cech TR. 2000. Protection of telomeres by the Ku protein in fission yeast. Mol. Biol. Cell 11: 3265– 3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kearsey SE, Stevenson AL, Toda T, Wang SW. 2007. Fission yeast Cut8 is required for the repair of DNA double-strand breaks, ribosomal DNA maintenance, and cell survival in the absence of Rqh1 helicase. Mol. Cell. Biol. 27: 1558– 1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyabe I, Morishita T, Hishida T, Yonei S, Shinagawa H. 2006. Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol. Cell. Biol. 26: 343– 353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maftahi M, Hope JC, Delgado-Cruzata L, Han CS, Freyer GA. 2002. The severe slow growth of Δsrs2 Δrqh1 in Schizosaccharomyces pombe is suppressed by loss of recombination and checkpoint genes. Nucleic Acids Res. 30: 4781– 4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hope JC, Maftahi M, Freyer GA. 2005. A postsynaptic role for Rhp55/57 that is responsible for cell death in Δrqh1 mutants following replication arrest in Schizosaccharomyces pombe. Genetics 170: 519– 531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi K, Imano R, Kibe T, Seimiya H, Muramatsu Y, Kawabata N, Tanaka G, Matsumoto Y, Hiromoto T, Koizumi Y, Nakazawa N, Yanagida M, Yukawa M, Tsuchiya E, Ueno M. 2011. Fission yeast Pot1 and RecQ helicase are required for efficient chromosome segregation. Mol. Cell. Biol. 31: 495– 506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6: 917– 922 [DOI] [PubMed] [Google Scholar]
- 34. Ahmad F, Stewart E. 2005. The N-terminal region of the Schizosaccharomyces pombe RecQ helicase, Rqh1p, physically interacts with topoisomerase III and is required for Rqh1p function. Mol. Genet. Genomics 273: 102– 114 [DOI] [PubMed] [Google Scholar]
- 35. Ashton TM, Hickson ID. 2010. Yeast as a model system to study RecQ helicase function. DNA Repair (Amst.) 9: 303– 314 [DOI] [PubMed] [Google Scholar]
- 36. Goodwin A, Wang SW, Toda T, Norbury C, Hickson ID. 1999. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res. 27: 4050– 4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laursen LV, Ampatzidou E, Andersen AH, Murray JM. 2003. Role for the fission yeast RecQ helicase in DNA repair in G2. Mol. Cell. Biol. 23: 3692– 3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akamatsu Y, Dziadkowiec D, Ikeguchi M, Shinagawa H, Iwasaki H. 2003. Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc. Natl. Acad. Sci. U. S. A. 100: 15770– 15775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Akamatsu Y, Tsutsui Y, Morishita T, Siddique MS, Kurokawa Y, Ikeguchi M, Yamao F, Arcangioli B, Iwasaki H. 2007. Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J. 26: 1352– 1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hope JC, Cruzata LD, Duvshani A, Mitsumoto J, Maftahi M, Freyer GA. 2007. Mus81-Eme1-dependent and -independent crossovers form in mitotic cells during double-strand break repair in Schizosaccharomyces pombe. Mol. Cell. Biol. 27: 3828– 3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haber JE, Thorburn PC, Rogers D. 1984. Meiotic and mitotic behavior of dicentric chromosomes in Saccharomyces cerevisiae. Genetics 106: 185– 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClintock B. 1932. A correlation of ring-shaped chromosomes with variegation in Zea mays. Proc. Natl. Acad. Sci. U. S. A. 18: 677– 681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sofueva S, Osman F, Lorenz A, Steinacher R, Castagnetti S, Ledesma J, Whitby MC. 2011. Ultrafine anaphase bridges, broken DNA and illegitimate recombination induced by a replication fork barrier. Nucleic Acids Res. 39: 6568– 6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murakami S, Yanagida M, Niwa O. 1995. A large circular minichromosome of Schizosaccharomyces pombe requires a high dose of type II DNA topoisomerase for its stabilization. Mol. Gen. Genet. 246: 671– 679 [DOI] [PubMed] [Google Scholar]
- 45. Bermejo R, Doksani Y, Capra T, Katou YM, Tanaka H, Shirahige K, Foiani M. 2007. Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation. Genes Dev. 21: 1921– 1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barre FX, Søballe B, Michel B, Aroyo M, Robertson M, Sherratt D. 2001. Circles: the replication-recombination-chromosome segregation connection. Proc. Natl. Acad. Sci. U. S. A. 98: 8189– 8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sciochetti SA, Piggot PJ. 2000. A tale of two genomes: resolution of dimeric chromosomes in Escherichia coli and Bacillus subtilis. Res. Microbiol. 151: 503– 511 [DOI] [PubMed] [Google Scholar]
- 48. Kuempel PL, Henson JM, Dircks L, Tecklenburg M, Lim DF. 1991. dif, a recA-independent recombination site in the terminus region of the chromosome of Escherichia coli. New Biol. 3: 799– 811 [PubMed] [Google Scholar]
- 49. Michel B, Recchia GD, Penel-Colin M, Ehrlich SD, Sherratt DJ. 2000. Resolution of Holliday junctions by RuvABC prevents dimer formation in rep mutants and UV-irradiated cells. Mol. Microbiol. 37: 180– 191 [DOI] [PubMed] [Google Scholar]
- 50. Cejka P, Plank JL, Bachrati CZ, Hickson ID, Kowalczykowski SC. 2010. Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nat. Struct. Mol. Biol. 17: 1377– 1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ira G, Malkova A, Liberi G, Foiani M, Haber JE. 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115: 401– 411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oh SD, Lao JP, Hwang PY, Taylor AF, Smith GR, Hunter N. 2007. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130: 259– 272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sherratt DJ, Soballe B, Barre FX, Filipe S, Lau I, Massey T, Yates J. 2004. Recombination and chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359: 61– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu L, Hickson ID. 2003. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870– 874 [DOI] [PubMed] [Google Scholar]
- 55. Schwartz EK, Heyer WD. 2011. Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma 120: 109– 127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de los Santos T, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM. 2003. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164: 81– 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osman F, Dixon J, Doe CL, Whitby MC. 2003. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol. Cell 12: 761– 774 [DOI] [PubMed] [Google Scholar]
- 58. Smith GR, Boddy MN, Shanahan P, Russell P. 2003. Fission yeast Mus81. Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics 165: 2289– 2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vanoli F, Fumasoni M, Szakal B, Maloisel L, Branzei D. 2010. Replication and recombination factors contributing to recombination-dependent bypass of DNA lesions by template switch. PLoS Genet. 6: e1001205 doi:10.1371/journal.pgen.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gaskell LJ, Osman F, Gilbert RJ, Whitby MC. 2007. Mus81 cleavage of Holliday junctions: a failsafe for processing meiotic recombination intermediates? EMBO J. 26:1891– 1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lorenz A, West SC, Whitby MC. 2010. The human Holliday junction resolvase GEN1 rescues the meiotic phenotype of a Schizosaccharomyces pombe mus81 mutant. Nucleic Acids Res. 38: 1866– 1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang JC. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3: 430– 440 [DOI] [PubMed] [Google Scholar]
- 63. Germe T, Miller K, Cooper JP. 2009. A non-canonical function of topoisomerase II in disentangling dysfunctional telomeres. EMBO J. 28: 2803– 2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boddy MN, Lopez-Girona A, Shanahan P, Interthal H, Heyer WD, Russell P. 2000. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 20: 8758– 8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jain D, Hebden AK, Nakamura TM, Miller KM, Cooper JP. 2010. HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 467: 223– 227 [DOI] [PubMed] [Google Scholar]
- 66. Nakamura TM, Cooper JP, Cech TR. 1998. Two modes of survival of fission yeast without telomerase. Science 282: 493– 496 [DOI] [PubMed] [Google Scholar]
- 67. Langerak P, Mejia-Ramirez E, Limbo O, Russell P. 2011. Release of Ku and MRN from DNA ends by Mre11 nuclease activity and Ctp1 is required for homologous recombination repair of double-strand breaks. PLoS Genet. 7: e1002271 doi:10.1371/journal.pgen.1002271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guilherme RS, Meloni VF, Kim CA, Pellegrino R, Takeno SS, Spinner NB, Conlin LK, Christofolini DM, Kulikowski LD, Melaragno MI. 2011. Mechanisms of ring chromosome formation, ring instability and clinical consequences. BMC Med. Genet. 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zirn B, Arning L, Bartels I, Shoukier M, Hoffjan S, Neubauer B, Hahn A. 2012. Ring chromosome 22 and neurofibromatosis type II: proof of two-hit model for the loss of the NF2 gene in the development of meningioma. Clin. Genet. 81:82– 87 [DOI] [PubMed] [Google Scholar]
- 70. Chaganti RS, Schonberg S, German J. 1974. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 71: 4508– 4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hope JC, Mense SM, Jalakas M, Mitsumoto J, Freyer GA. 2006. Rqh1 blocks recombination between sister chromatids during double strand break repair, independent of its helicase activity. Proc. Natl. Acad. Sci. U. S. A. 103: 5875– 5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sugawara N. 1988. DNA sequences at the telomeres of the fission yeast S. pombe. Ph.D. dissertation. Harvard University, Cambridge, MA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.