Abstract
P bodies are cytoplasmic RNA granules containing the Dcp1-Dcp2 decapping enzymes where mRNA decay can occur. Here, we describe the characterization of P bodies in the fission yeast Schizosaccharomyces pombe. Most information on the property and function of P bodies stems from studies in the distantly related budding yeast Saccharomyces cerevisiae, and Edc3 was identified as a scaffold protein required for P-body assembly. However, we found that, unlike in S. cerevisiae, fission yeast Edc3 was dispensable for P-body formation. Pdc1, a novel partner of the fission yeast decapping enzyme, with a limited similarity to plant Edc4/Varicose that is required for the assembly of P bodies, was identified (tandem affinity purification–matrix-assisted laser desorption ionization tandem mass spectrometry [TAP-MALDI MS/MS]). Pdc1 interacts with Dcp2 through its C terminus and contains a coiled-coil region for self-interaction to mediate P-body formation. In line with the model that Pdc1 cross-bridges different proteins, additional interactions can be demonstrated with components such as Edc3 and Ste13. Although Pdc1 is not required for the interaction between Dcp1 and Dcp2, our data suggest that Pdc1 acts as a functional homologue of Edc4, a third component of the decapping enzymes that is thought to be absent from fungi. Together, these results highlight the diverse P-body protein compositions between different species and might help to provide insight into their evolutionary paths.
INTRODUCTION
Cytoplasmic processing bodies (P bodies) are dynamic RNA protein aggregates that play critical roles in mRNA degradation, nonsense-mediated mRNA decay, translational repression, and RNA-mediated gene silencing (reviewed in reference 1). Because several components of P bodies, such as the decapping enzyme and its coactivators, are found in both yeast and mammalian cells, it is believed that P bodies are evolutionarily conserved among eukaryotes. However, a detailed study of P bodies in the fission yeast has not yet been described.
The decapping of mRNAs, which occurs inside P bodies, represents a critical step in mRNA turnover and is therefore tightly regulated. The decapping enzyme Dcp2 requires additional proteins for full activity and/or stability. These proteins are generally termed decapping coactivators (or enhancers of decapping [EDCs]) although they may activate decapping by different mechanisms (2). In Saccharomyces cerevisiae, proteins that activate decapping include Dcp1, enhancer of decapping 1 to 3 (Edc1-3), the heptameric Lsm1-7 complex, DEXH/D-box RNA helicase 1 (Dhh1; RCK/p54 in mammals), and Pat1 (3). Dcp1 interacts directly with Dcp2 and is required for decapping in vivo (4). All of these proteins colocalize to P bodies.
In human cells, the interaction between Dcp1 and Dcp2 appears to be mediated by the decapping activator Edc4 (also known as Ge-1 or Hedls, for human enhancer of decapping large subunit) (5, 6). Edc4, Dcp1, and Dcp2 are part of a multimeric protein complex, which also includes Edc3 and RCK/p54 (5). Similarly, Arabidopsis thaliana Dcp1 and Dcp2 interact with Varicose (the A. thaliana Edc4 ortholog) (7), suggesting that the role of Edc4 as a physical bridge between Dcp1 and Dcp2 is conserved in multicellular organisms.
Because of the important role of P bodies in diverse cellular functions, the assembly of these structures has been investigated in several model systems, and studies in S. cerevisiae have been crucial in unraveling P-body biology. In yeast, aggregation of mRNPs into P bodies has been shown to be primarily dependent on a self-interaction domain (Yjef-N) in the Edc3 protein and in a glutamine/asparagine (Q/N)-rich prion-like domain in the Lsm4 C terminus (8). However, Edc4, which has no obvious homologue in yeast, appears to be the central component of decapping complex in high eukaryotes because small interfering RNA (siRNA) knockdown of Edc4 resulted in P-body disassembly (6), and depletion of Edc3 does not affect P-body integrity in Drosophila cells (9).
Schizosaccharomyces pombe, the fission yeast, has long been a crucial model for the study of the eukaryote cell cycle, for many stages of which S. pombe is widely accepted as a better model than S. cerevisiae and is more closely related to high eukaryotes in some aspect. To gain more insight into the protein compositions of P bodies between different species and how these protein complexes evolved, here we describe the characterization of P bodies in S. pombe. Our data have shown that P bodies from the fission yeast through mammals contain a conserved core of proteins. However, even though species share similar protein compositions, the underlying molecular mechanisms for the formation of P bodies can be distinct. We found that, unlike in S. cerevisiae, Edc3 was dispensable for P-body formation in S. pombe. Pdc1, a novel partner of the fission yeast decapping enzyme required for the assembly of P bodies, was identified in this study. Although sequence comparisons reveal limited similarity, our data suggest that Pdc1 might act as a functional homologue of Edc4, a third component of the Dcp1-Dcp2 decapping enzyme that is thought to be absent from fungi. The protein composition and structural organization of fission yeast P bodies, which also lack the yeast-specific components of Edc1 and Edc2, are therefore more closely related to high eukaryotes than to of S. cerevisiae. The implication of these results is discussed.
MATERIALS AND METHODS
Fission yeast strains and methods.
Conditions for growth, maintenance, and genetic manipulation of fission yeast were as described previously (10). A complete list of the strains used in this study is given in Table S1 in the supplemental material. One-step gene disruption or modification via homologous recombination was performed following PCR-mediated generation of ura4+ or kanMX-selectable cassettes flanked by 80-bp segments from appropriate regions of the genes of interest using oligonucleotides described in Table S2 in the supplemental material. Except where otherwise stated, strains were grown at 30°C in yeast extract (YE) or Edinburgh minimal medium (EMM2) with appropriate supplements. Where necessary, gene expression from the nmt1 promoter was repressed by the addition of 5 μM thiamine to the culture medium. To enhance P-body formation, cells were incubated in water or after deprivation of glucose or exposure to 1 M KCl for 15 min.
Plasmids.
Plasmids for overexpression or truncation of Dcp2 and Pdc1 were constructed by PCR amplification of the corresponding open reading frame from S. pombe cDNA, using the primer pairs described in Table S2 in the supplemental material, and subsequently ligated as NdeI and NotI fragments into plasmids derived from pREP1 and pREP41 encoding a triple hemagglutinin (HA) or green fluorescent protein (GFP) tag at the C terminus after the NotI site.
Microscopy.
Visualization of mCherry and green fluorescent protein (GFP)-tagged proteins in living cells was performed at room temperature. Images were acquired using a Leica DM RA2 microscope equipped with a Leica DC 350F camera and were assembled using Adobe PhotoShop. Image quantitation was done as described previously (11).
Antibodies and immunoblotting.
Yeast extracts prepared by alkaline extraction as described previously (10) were resolved by SDS-PAGE before being subjected to immunoblotting using anti-GFP antibody (Abcam) to reveal GFP fusion proteins. Anti-HA-peroxidase high-affinity (3F10) antibody was from Roche. Antibody against α-tubulin (Sigma-Aldrich) was used as a control.
Tandem affinity purification (TAP) and immunoprecipitation.
The two-step immunoglobulin G (IgG)-Sepharose and calmodulin resin affinity purification was carried out as described previously (11). The purified proteins were eluted with 20 mM EGTA to be precipitated with trichloroacetic acid (TCA) at a final concentration of 20%, separated on SDS-PAGE gels, and visualized by Sypro Ruby. Protein bands were excised from gels, treated with trypsin, and subjected to concerted matrix-assisted laser desorption ionization (MALDI) peptide mass fingerprinting (PMF) and collision-induced dissociation (CID) tandem mass spectrometry (MS/MS) analysis for protein identification using a dedicated quadrupole time of flight (Q-TOF) Ultima MALDI instrument (Micromass, Manchester, United Kingdom) by the Core Facilities for Proteomics and Glycomics located at the Institute of Biological Chemistry, Academia Sinica. The instrument was operated using the MassLynx, version 4.0, program, and raw MS data were processed for database searching using the ProteinLynx Global Server, version 2.0, against the SwissProt database with the Mascot program.
For immunoprecipitation, 2 × 108 cells were lysed in 200 μl of NP-40 buffer (6 mM Na2HPO4, 4 mM NaH2PO4, 1% NP-40, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, 1 mM PMSF, 1 mM DTT, Complete protease inhibitor cocktail) by vortexing with acid-washed glass beads. The lysate was clarified by centrifugation, and GFP fusion proteins were retrieved using GFP-Trap-coupled agarose beads (Chromotek) according to the manufacturer's instructions.
Cell culture and immunofluorescence.
HeLa cells were cultured in Dulbecco's modified Eagle medium (12100-046; Invitrogen) supplemented with 10% fetal calf serum. For transfection, OptiMEM I medium (31985-070; Invitrogen) was used. siRNAs for Edc3, Edc4, and a control obtained from Ambion, Inc. (Edc3, catalog item 140933; Edc4, catalog item 21122; and control, catalog item 4605), were transfected at a final concentration of 10 nM using Lipofectamine 2000 (11668-019; Invitrogen). To induce P-body formation, 48 h after transfection, HeLa cells were treated with 500 μM NaAsO2 for 30 min.
Whole-cell lysates were prepared using lysis buffer (10 mM Tris-HCl, pH 7.0, 150 mM NaCl, 0.5% NP-40, Complete protease inhibitor cocktail, phosphoStop), and subjected to SDS-PAGE and immunoblotting using anti-Edc4 (ab72408; Abcam) and anti-Edc3 (ab57780; Abcam) antibodies. Anti-Dcp1a (H00055802-M06) was from Abnova. Antibody against α-tubulin (Sigma-Aldrich) was used as a control.
For immunofluorescence, HeLa cells grown on coverslips were fixed with 3.7% formaldehyde and stained with mouse anti-Dcp1a antibodies diluted 1:150 in phosphate-buffered saline (PBS) containing 1% bovine serum albumin, 0.02% saponin, and 0.05% sodium azide. Alexa Fluor 488-coupled goat secondary antibody (A11001; Invitrogen) was used in a dilution of 1:150. Cells were mounted using 50% glycerol in PBS. Images were acquired using a Leica TCS SP5 confocal microscope with Z-series compilations of 10 images per stack.
Splinted ligation reverse transcription-PCR (RT-PCR).
Splinted ligation of RNA was performed as described previously (12). Briefly, an RNA anchor was ligated to the free hydroxyl of a decapped rps23 mRNA facilitated by a gene-specific DNA splint. Ligation reactions were performed overnight at 16°C. After removal of the splint by DNase I (Roche) treatment, an rps23 gene-specific reverse transcriptase primer was used for reverse transcription to synthesize cDNA using SuperScript III (Invitrogen). cDNA served as the template for PCR amplification using a primer complementary to the RNA anchor and an rps23-specific reverse primer. For the total mRNA level, an rps23-specific forward primer was used. PCR products were analyzed by gel electrophoresis. Images were quantitated using VisionWork LS software (UVP) to determine the ratio of the level of rps23 decapped to total mRNA. For tobacco acid pyrophosphatase treatment, 10 μg of total RNA was decapped by treatment with 20 U of RNase Inhibitor Plus (Promega) and 5 U of tobacco acid pyrophosphatase (Epicentre Biosystems) in the supplied 1× TAP buffer for 30 min at 37°C before splinted ligation of RNA.
RESULTS
Characterization of fission yeast P bodies.
P bodies have emerged as important subcellular structures that are involved in mRNA metabolism. Although components of P bodies have been described in S. pombe, to date, a detailed description of P bodies is still lacking. To this end, we have initiated a study to characterize the fission yeast P bodies. For this purpose, in addition to the decapping enzyme Dcp2-tdTomato (the signature constituent of P bodies) (10), targeted recombination was used to generate GFP fusion versions of the core components of P bodies including Dcp1 (13), the exonuclease Xrn1 (Exo2 in S. pombe) (14), the activator of decapping Edc3 (15), and Dhh1/RCK/p54 (Ste13 in S. pombe) (16) (Table 1). Sequence comparison failed to identify any significant hits of the yeast-specific decapping activators Edc1 and Edc2. For simplicity, fission yeast nomenclature will be used throughout the study.
Table 1.
List of fission yeast P-body core components in this study
| Function | Component ina: |
|||
|---|---|---|---|---|
| S. cerevisiae | S. pombe | H. sapiens | A. thaliana | |
| Exonuclease | Xrn1 | Exo2 | Xrn1 | Xrn2 |
| Decapping enzyme | Dcp1 | Dcp1 | Dcp1 | Dcp1 |
| Dcp2 | Dcp2 | Dcp2 | Dcp2 | |
| Enhancers | ||||
| WD40-like | — | Pdc1* | Edc4/Ge1/Hedls | Varicose |
| RNA helicase | Dhh1 | Ste13 | RCK/p54 | AT3G61240 |
| YjeF domain | Edc3 | Edc3 | Edc3 | — |
| Yeast specific | Edc1 | — | — | — |
| Edc2 | — | — | — | |
Asterisk, novel component identified in this study; —, absent in the species.
The GFP fusion proteins are integrated at the relevant genomic locus under the control of the endogenous promoter, including the full-length protein C-terminally tagged with GFP. These proteins appeared to be functional, as judged by cell viability (in the case of Dcp1 and Dcp2) and the lack of sensitivity to drugs tested (see Fig. S1 in the supplemental material). Examination of living cells by fluorescence microscopy reveals accumulation of these proteins in cytoplasmic foci (Fig. 1A). We refer to these foci as fission yeast P bodies and note that, unlike in S. cerevisiae, multiple foci can be readily detected in unstressed cells. As the previously described characteristic feature of P bodies (17), these foci can be further enhanced by various stresses (after a 15-min incubation in water or after deprivation of glucose or exposure to 1 M KCl for 15 min), with an increase in size and intensity that colocalized well with the decapping enzyme Dcp2 (Fig. 1B). This provides an ideal condition to identify lesions that reduce or compromise P-body assembly. Together, these results demonstrated that P bodies from fission yeast through mammals contain a conserved core of proteins.
Fig 1.
Fission yeast P bodies share conserved core components among eukaryotes. (A) Fluorescence micrographs of strains expressing Dcp2-tdTomato or GFP fusion proteins of various P-body components grown at 30°C (control) or after a 15-min incubation in water (H2O), glucose deprivation, or exposure to 1 M KCl for 15 min are shown. Scale bar, 5 μm. (B) Merged images of fluorescence micrographs showing localization of GFP fusion proteins of various P-body components (green) and Dcp2-tdTomato (red) in living cells after deprivation of glucose for 15 min.
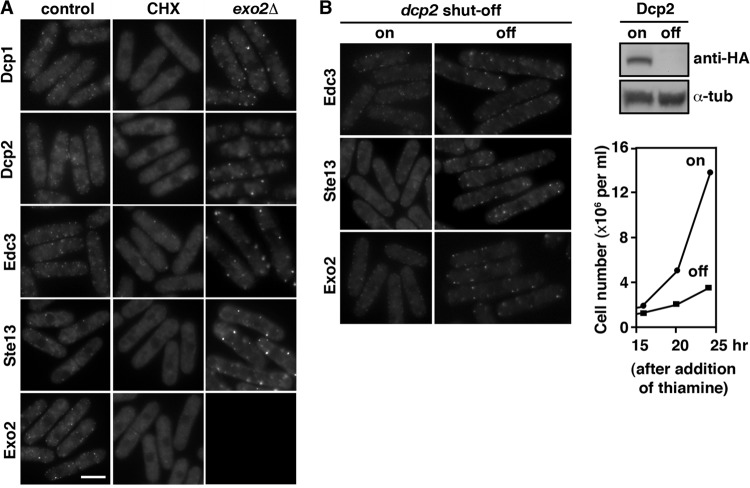
In keeping with a function in RNA turnover, we found that inhibition of translation elongation by trapping the mRNA in polysomes with cycloheximide (CHX) leads to a decrease of these foci (Fig. 2A), and blocks in the catalytic steps of decapping by depletion of Dcp2 (Fig. 2B) or 5′-to-3′ degradation in the exo2 mutants enhanced P-body formation (Fig. 2A). In S. cerevisiae, a function of Dcp2 in the assembly of P bodies has been described previously (18). However, depletion of Dcp2 does not affect P-body integrity in mammalian cells (6). Together with the data presented here, this suggests that the function of Dcp2 in P-body formation is not conserved throughout evolution and that species diversity might have developed.
Fig 2.
Inhibition of translation disrupts P-body formation, and blocks in the catalytic steps of decapping or 5′-to-3′ degradation lead to increased P-body formation. (A) Fluorescence micrographs of strains expressing Dcp2-tdTomato or GFP fusion proteins of various P-body components grown at 30°C (control) or after exposure to 100 μg/ml cycloheximide (CHX) for 15 min or in the exo2 mutant genetic background are shown. Scale bar, 5 μm. (B) Fluorescence micrographs of dcp2 shutoff strains expressing GFP fusion proteins of various P-body components under derepression conditions (nmt41 promoter on) or after depletion of Dcp2 by addition of thiamine to the medium for 16 h are shown (left panel). Total cell lysates separated by SDS-PAGE were subjected to Western blotting using anti-HA to reveal Dcp2 protein levels (upper right panel). Antibodies against α-tubulin were used as a loading control. Growth curves of dcp2 shutoff strains after depletion of Dcp2 by the addition of thiamine to medium are shown (lower right panel).
Fission yeast Edc3 is dispensable for P-body formation.
Most information on the property and function of P bodies stems from studies in S. cerevisiae. In yeast, Edc3 has been identified as a scaffolding protein important for P-body assembly (19). To test the role of Edc3 in fission yeast P-body assembly, we examined the effect of Edc3 when overexpressed on P-body formation. PCR-mediated gene integration was used to replace the promoter of the edc3+ gene in its normal chromosomal context with the thiamine-repressible nmt1+ promoter, at the same time introducing an HA epitope tag sequence fused in frame to the 5′ end of the edc3+ open reading frame. Removal of thiamine from the medium led to readily detectable levels of HA-Edc3 after 18 h growth at 30°C (Fig. 3A). Comparison with levels of Edc3 expressed from the edc3+ promoter in its normal chromosomal context indicated that the nmt1+ promoter-driven gene was overexpressed at least 10-fold in comparison with endogenous edc3+. Even at this level of overexpression, the formation of P bodies was only moderately enhanced with an increase in intensity as judged by four different proteins (Fig. 3A). Next, we examined the ability of edc3 mutants to form P bodies. As shown in Fig. 3B, we observed that P bodies could still form in edc3 mutants under different growth conditions. In fact, strains lacking Edc3 accumulated Dcp1-2, Ste13, and Exo2 in P bodies even in mid-log-phase cultures, probably due to the defects in RNA degradation (see Fig. 7). The large P bodies observed in edc3 mutants did not increase significantly when cells were subjected to glucose deprivation. Taking these observations together, we concluded that, unlike in S. cerevisiae, fission yeast Edc3 is dispensable for P-body formation.
Fig 3.
Fission yeast Edc3 is dispensable for P-body formation. (A) Localization of Dcp2-tdTomato or GFP fusion proteins of various P-body components in strains expressing the Edc3-HA fusion proteins under the control of its own promoter (Pedc3) or overexpressed by the nmt1 promoter (Pnmt1). Total cell lysates separated by SDS-PAGE were subjected to Western blotting using anti-HA to reveal Edc3 protein levels. Antibodies against α-tubulin were used as a loading control. Scale bar, 5 μm. (B) Fluorescence micrographs of wild-type strains or edc3 mutants expressing Dcp2-tdTomato or GFP fusion proteins of various P-body components grown at 30°C (control) or after deprivation of glucose for 15 min are shown.
Fig 7.
Pdc1 is required for decapping in vivo. (A) The splinted ligation assay for detecting decapped mRNA. (B) Splinted ligation RT-PCR was used to detect endogenous decapped rps23 mRNA present in 10 μg of total RNA isolated from strains in which pdc1 was deleted or overexpressed by the nmt1 promoter (pdc1-op) as described in Materials and Methods. As a positive control, total RNA was treated with tobacco acid pyrophosphatase (+TAP treatment) to remove the 5′ cap, as indicated on the right. (C) Relative amounts of decapped rps23 mRNA as a percentage of the total mRNA in the strains indicated are shown (average of two independent experiments).
Identification of Pdc1 as a novel component of the fission yeast P body.
The results described above indicate that although species share similar protein compositions, the underlying molecular mechanisms for the assembly of P bodies can be distinct between species. To gain more insight into the molecular mechanism of P-body assembly in S. pombe, tandem affinity purification (TAP) was performed to identify proteins associated with Dcp2. The two-step immunoglobulin G (IgG)-Sepharose and calmodulin resin affinity purification was carried out as described previously (11) on 10-liter cultures of the Dcp2-TAP strain. The protein composition of the Dcp2-TAP complex was examined directly by silver staining. As shown in Fig. 4A, multiple polypeptides were detected, and their identities were determined by MALDI MS/MS analysis. In addition to Dcp1 (identified in a different set of experiments for low-molecular-weight peptides [data not shown]), the exonuclease Exo2 and Hsp75, probably acting as a chaperon protein, were identified in the complex. In addition, a previously uncharacterized, nonessential protein encoded by the chromosomal locus SPAC20G4.08 was identified. We refer to this S. pombe gene as pdc1+ (for partner of decapping enzyme protein 1). ProfileScan analysis of the sequence of the corresponding predicted 1,076 amino acid residues revealed a WD40 repeat-like domain at its N terminus (Fig. 4D). A C-terminal coiled-coil region with limited similarity (only 60 amino acids over the total of 1,076) to Arabidopsis thaliana Edc4, but not to other orthologues, was also identified (Fig. 4C). To determine whether or not Pdc1 is a component of fission yeast P bodies, targeted recombination was used to generate GFP fusion proteins under the control of the endogenous promoter in its normal chromosomal context, expressing the full-length protein C-terminally tagged with GFP. Examination of living cells by fluorescence microscopy reveals the accumulation of Pdc1-GFP fusion proteins in cytoplasmic foci that can be further enhanced by stresses and that overlap the decapping enzyme Dcp2 (Fig. 4B).
Fig 4.
Pdc1 as a novel component of fission yeast P bodies. (A) Tandem affinity purification (TAP) tag pulldown of Dcp2 proteins resolved by SDS-PAGE was visualized by silver staining. The identities of constituent proteins (identified by MALDI MS/MS analysis of individual bands) are indicated on the right. (B) Merged images of fluorescence micrographs showing localization of the fusion proteins Pdc1-GFP (green) and Dcp2-tdTomato (red) in living cells grown at 30°C (control) or after a 15-min incubation in water, deprivation of glucose, or exposure to 1 M KCl for 15 min are shown. Scale bar, 5 μm. (C) An alignment of the amino acid sequences of Arabidopsis Edc4 and the fission yeast Pdc1 described here. Only the region of significant similarity (approximately 65 amino acid residues) is shown in each case. White text on a black background indicates amino acid identity; black text on a shaded background indicates a conservative amino acid substitution. (D) Schematic representation of the domain structures of Pdc1 and Dcp2 (N, N-terminal regulatory plus catalytic domains; C, C-terminal extension; M, the region in between N and C domains). Arrow, interaction domains between Pdc1 and Dcp2. (E) Coimmunoprecipitation was performed with extracts prepared from Pdc1-GFP tagging strains (upper panel) or deletion strains transformed with plasmids expressing various truncated proteins as well as a GFP control and Dcp2-HA proteins (lower panel). GFP-Trap affinity resin was used to pull down Pdc1-GFP fusion proteins. Immunoprecipitates were then analyzed by Western blotting using antibodies against GFP and HA. a.a., amino acids; IP, immunoprecipitation. (F) Total cell lysates prepared from Pdc1-GFP tagging strains coexpressing different versions of Dcp2-HA proteins from plasmids were subjected to immunoprecipitation using GFP-Trap affinity resin. Immunoprecipitates were then analyzed by Western blotting using antibodies against GFP and HA.
The results described above demonstrated Pdc1 as a novel component of the fission yeast P body that interacts with Dcp2. The interaction was further confirmed by coimmunoprecipitation, and the C-terminal region residues 789 to 1076 of Pdc1 responsible for the interaction were identified (Fig. 4E). To determine the portion of Dcp2 that mediates interaction with Pdc1, plasmids encoding Dcp2-HA proteins harboring a series of deletions in the C terminus were expressed with Pdc1-GFP in yeast cells. As shown in Fig. 4F, amino acids 1 to 243 of Dcp2 at the N terminus were sufficient for interaction with Pdc1. Given the close interaction between Pdc1 and Dcp2, the roles of Pdc1 in P-body assembly were determined further.
Requirement of Pdc1 in fission yeast P-body assembly.
First, we examined the effect of Pdc1 overexpression on P-body formation. As shown in Fig. 5A, overexpression of Pdc1 leads to formation of aberrant P-body structures with multiple enlarged foci revealed by various components, which is in sharp contrast to the moderate effect of Edc3 overexpression, indicating that Pdc1 affects the formation of P bodies. Next, we examined the ability of pdc1 mutants to form P bodies. In pdc1 mutants, the small P bodies normally observed in mid-log-phase cultures were no longer seen (Fig. 5B). Moreover, during glucose starvation, where P bodies are large, we observed that pdc1 mutants showed a strong reduction in the accumulation of multiple proteins into microscopically visible P bodies although some P bodies could still form. The strong reduction in P bodies, as judged by five different proteins and under different growth conditions, including glucose deprivation, suggests that Pdc1 plays a vital role in the formation of P bodies.
Fig 5.
Pdc1 is important for P-body assembly. (A) Localization of Dcp2-tdTomato or GFP fusion proteins of various P-body components in strains expressing the Pdc1-HA fusion protein under the control of its own promoter (Ppdc1) or overexpressed by the nmt1 promoter (Pnmt1). Scale bar, 5 μm. (B) Fluorescence micrographs of wild-type strains or pdc1 mutants expressing Dcp2-tdTomato or GFP fusion proteins of various P-body components grown at 30°C (control) or after deprivation of glucose for 15 min are shown. Numbers indicate protein foci enhanced after deprivation of glucose (at least 200 cells were counted for each strain).
One important aspect of P-body assembly is the protein-protein interaction domain present on numerous proteins (2). In addition, proteins required for the assembly of P bodies often contain self-interaction domains such as the YjeF domain of Edc3 (8) and the ARM/HEAT repeat in Edc4 (20). By analogy, Pdc1 contains a C-terminal coiled-coil region, which often serves as a dimerization domain. To test this hypothesis, we examined the ability of Pdc1 for self-interaction. Plasmids encoding Pdc1-GFP proteins harboring a series of deletions in the C terminus were coexpressed with Pdc1-HA in yeast cells. We found that Pdc1-GFP specifically pulled down Pdc1-HA and that the coiled-coil region of Pdc1 is necessary for the interaction (Fig. 6A). In line with these results, when Pdc1 lacking the coiled-coil region was overexpressed, it failed to accumulate in cytoplasmic foci and affect their morphology (Fig. 6B). When expressed in its normal chromosomal context under the control of the endogenous promoter, the truncated Pdc1 lacking the coiled-coil region distributed diffusely throughout the cytoplasm, and the assembly of P bodies was impaired in these cells (Fig. 6C). These results are consistent with the idea that self-interaction mediated by the coiled-coil region is required for Pdc1-mediated assembly of P bodies. Additional protein-protein interactions can be demonstrated by coimmunoprecipitation between Pdc1 and other components such as Edc3 and Ste13 (Fig. 6D). Together, these results are in line with the model that Pdc1 acts as a scaffold protein that provides sites for protein interactions interconnecting components of P bodies for assembly (Fig. 6E) and is functionally analogous to Edc4 in high eukaryotes.
Fig 6.
The coiled-coil region is required for Pdc1 to function in P-body assembly. (A) Coimmunoprecipitation was performed with extracts prepared from Pdc1-HA tagging strains coexpressing different versions of Pdc1-GFP proteins as well as a GFP control. GFP-Trap affinity resin was used to pull down Pdc1-GFP fusion proteins. Immunoprecipitates (IP) were then analyzed by Western blotting using antibodies against GFP and HA. (B) Localization of different versions of Pdc1-GFP proteins overexpressed by the nmt1 promoter. (C) Localization of Edc3-mCherry (mC) in strains expressing different versions of Pdc1-GFP fusion proteins after deprivation of glucose for 15 min. Scale bar, 5 μm. (D) Total cell lysates prepared from strains expressing proteins as indicated were subjected to immunoprecipitation using GFP-Trap affinity resin. Immunoprecipitates were then analyzed by Western blotting using antibodies against GFP and HA. (E) Diagram showing the determined interaction.
To test if Pdc1, like Edc4, plays a role in decapping, we used splinted ligation followed by RT-PCR (12) to detect products of the decapping reactions that are short-lived in wild-type cells (Fig. 7). The rps23 mRNA from S. pombe was used because the 5′ end is mapped, and the mRNA is abundant. In this procedure, an RNA adaptor is ligated to the 5′ end of the decapped mRNA assisted by a DNA splint (no products when mRNA is capped). The intermediate is used as a template for cDNA synthesis and amplification of DNA products by PCR (Fig. 7A). As shown in Fig. 7B, decapped products of rps23 mRNA confirmed by sequencing were detected in wild-type cells that were reduced in edc3 mutants, where mRNA decapping is affected. As a positive control, removal of the 5′ cap in vitro by tobacco acid pyrophosphatase resulted in detection of RT-PCR products both in wild-type and edc3Δ samples (Fig. 7B, +TAP treatment). Consistent with a function in mRNA decapping, Pdc1 strongly enhanced decapping activity when overexpressed, and the decapped products were not detected when pdc1 was deleted, as revealed by tobacco acid pyrophosphatase treatment (Fig. 7B and C). We conclude that Pdc1 is an enhancer of decapping.
Depletion of Edc4, but not Edc3, leads to a decrease in P bodies in human cells.
The results described above suggest that although species share similar protein compositions, the molecular mechanisms for the assembly of P bodies could be distinct between species. Although the YjeF domain of Edc3 is conserved among different species, Edc3 is dispensable for P-body assembly in S. pombe (Fig. 3), and the formation of fission yeast P bodies largely depends on Pdc1 (Fig. 5). Similarly, depletion of Edc4 leads to a decrease in P bodies in Drosophila cells, and depletion of Edc3 does not affect P-body integrity (9). To test whether these findings also apply to human cells, we determined the effect of depletion of Edc3 on P-body formation in HeLa cells. To test the presence of P bodies, endogenous Dcp1a was used as a marker to visualize P bodies. As shown in Fig. 8A, arsenite treatment enhanced the formation of P bodies in cells transfected with nonspecific siRNA. Dcp1a foci became more evident and could be readily detected in the cells. When Edc4 was depleted, Dcp1a distributed more diffusely throughout the cytoplasm, and arsenite treatment failed to induce P-body formation. In contrast, depletion of Edc3 failed to suppress arsenite-induced P-body formation although the proteins were depleted efficiently (Fig. 8B). In fact, as seen in S. pombe cells, P-body formation was enhanced when Edc3 was depleted, with enlarged Dcp1a foci accumulated in these cells (Fig. 8A). Together, these results are consistent with the idea that, as in S. pombe, Edc3 is not absolutely required for the assembly of P bodies in Metazoa, indicating that although these proteins might have evolved together, species difference developed.
Fig 8.

Depletion of Edc4 but not Edc3 leads to decrease of P bodies in human HeLa cells. (A) Confocal fluorescence micrographs of HeLa cells from which the indicated proteins were depleted. To induce P-body formation, 48 h after transfection, HeLa cells were treated with 500 μM NaAsO2 for 30 min. Cells were stained with anti-Dcp1a antibody to reveal P bodies. Numbers indicate the fraction of cells with enhanced Dcp1a foci identical to that shown in the representative panel in two independent knockdowns performed per protein (at least 200 cells were counted per knockdown). Scale bar, 25 μm. (B) The efficiency of Edc3 or Edc4 depletion was assessed by Western blotting. Total cell lysates from which the indicated proteins were depleted were separated by SDS-PAGE and subjected to Western blotting using anti-Edc3 and -Edc4 antibodies to reveal the protein levels. Antibodies against α-tubulin were used as a loading control.
DISCUSSION
mRNA degradation plays an important role in the control of gene expression. The basic set of proteins that regulate mRNA degradation is conserved from yeast to humans (Table 1). However, in higher eukaryotes, additional proteins such as Edc4 are required for Dcp1-Dcp2 catalytic activity (5), indicating that differences in the regulation of mRNA decapping may have evolved. A homologue of Edc4 is not present in S. cerevisiae. Here, we described the identification of fission yeast Pdc1 as a functional homologue of Edc4 that might provide insight into the evolutionary path of these protein complexes. We have shown that in S. pombe, Pdc1 but not Edc3 is a scaffolding protein bridging different components of P bodies for assembly (Fig. 3 to 6). Our results indicate that the coiled-coil region of Pdc1 is required for it to be able to contribute to P-body aggregation (Fig. 6). Because the coiled-coil region interacts with itself, its function in P-body aggregation is most probably to provide direct interaction between different Pdc1 molecules and is analogous to the ARM/HEAT repeat in Edc4. Similar to Edc4, protein-protein interactions can be demonstrated between Pdc1 and other components such as Edc3 and Ste13 (RCK/p54 in mammals) in addition to Dcp2 (Fig. 6). Although sequence comparison failed to identify significant similarity between Pdc1 and Edc4, an N-terminal WD40-like domain can be found in Pdc1 (Fig. 4), indicating that Pdc1 has significantly diverged from Edc4 from other species. To expand the scope of this phylogeny, possible historical relationships between S. pombe Dcp2 and homologues from different species were analyzed. Phylogenetic analysis revealed that S. pombe Dcp2 was clustered within a monophyletic branch with Dcp2 sequence from other eukaryotes, including A. thaliana, Drosophila melanogaster, and Homo sapiens, with S. cerevisiae Dcp2 more distantly related to the others (see Fig. S2 in the supplemental material). Similar results were obtained for Dcp1, indicating that these proteins might have evolved together and that species differences developed. For example, S. pombe Dcp2 retains its interaction with Dcp1 (4). In high eukaryotes, Edc4 bridges the interaction (5). Its biological function changes, too, as the function of Dcp2 in P-body formation (18) is not conserved in S. pombe (Fig. 2B) and high eukaryotes (6). Similarly, changes in the roles of decapping coactivators developed. From fission yeast through mammals, the essential function of Edc3 in the assembly of P bodies was replaced by Edc4-like proteins (Fig. 8). Intriguingly, no obvious Edc3 homologue can be found in A. thaliana (21). Although further studies are required, it is tempting to speculate that in A. thaliana, the role of Edc3 might have been completely replaced by Edc4. In other species, its role as a decapping coactivator remains (Fig. 7). Cross-species studies of this sort will continue to point the way toward understanding the evolutionary path of these protein complexes and provide new insight into their molecular mechanisms. The more closely related structural organization of fission yeast P bodies to that of high eukaryotes makes S. pombe an ideal model for studying P-body biology. A deeper understanding of these RNA granules in these unicellular eukaryotes may reveal principles that are applicable to other granules in general.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Health Research Institute, Taiwan (MG-101-PP-10), and National Science Council (NSC 99-2311-B-400-001-MY3). Proteomic mass spectrometry analyses were performed by the Core Facilities for Proteomics and Glycomics located at the Institute of Biological Chemistry, Academia Sinica, supported by a National Science Council grant (NSC 98-3112-P-001-023) and the Academia Sinica.
We thank Gerald Smith and the Yeast Genetic Resource Center Japan for yeast strains.
Footnotes
Published ahead of print 14 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01583-12.
REFERENCES
- 1.Eulalio A, Behm-Ansmant I, Izaurralde E. 2007. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8:9–22 [DOI] [PubMed] [Google Scholar]
- 2.Franks TM, Lykke-Andersen J. 2008. The control of mRNA decapping and P-body formation. Mol. Cell 32:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissan T, Rajyaguru P, She M, Song H, Parker R. 2010. Decapping activators in Saccharomyces cerevisiae act by multiple mechanisms. Mol. Cell 39:773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.She M, Decker CJ, Chen N, Tumati S, Parker R, Song H. 2006. Crystal structure and functional analysis of Dcp2p from Schizosaccharomyces pombe. Nat. Struct. Mol. Biol. 13:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J. 2005. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20:905–915 [DOI] [PubMed] [Google Scholar]
- 6.Yu JH, Yang WH, Gulick T, Bloch KD, Bloch DB. 2005. Ge-1 is a central component of the mammalian cytoplasmic mRNA processing body. RNA 11:1795–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Yang JY, Niu QW, Chua NH. 2006. Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18:3386–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker CJ, Teixeira D, Parker R. 2007. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 179:437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. 2007. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27:3970–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen WL, Stevenson AL, Wang CY, Chen HJ, Kearsey SE, Norbury CJ, Watt S, Bahler J, Wang SW. 2010. Vgl1, a multi-KH domain protein, is a novel component of the fission yeast stress granules required for cell survival under thermal stress. Nucleic Acids Res. 38:6555–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CY, Wen WL, Nilsson D, Sunnerhagen P, Chang TH, Wang SW. 2012. Analysis of stress granule assembly in Schizosaccharomyces pombe. RNA 18:694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Sweet TJ, Chamnongpol S, Baker KE, Coller J. 2009. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature 461:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuno T, Araki Y, Ohya Y, Kofuji S, Takahashi S, Hoshino S, Katada T. 2004. Decapping reaction of mRNA requires Dcp1 in fission yeast: its characterization in different species from yeast to human. J. Biochem. 136:805–812 [DOI] [PubMed] [Google Scholar]
- 14.Szankasi P, Smith GR. 1996. Requirement of S pombe exonuclease II, a homologue of S. cerevisiae Sep1, for normal mitotic growth and viability. Curr. Genet. 30:284–293 [DOI] [PubMed] [Google Scholar]
- 15.Fromm SA, Truffault V, Kamenz J, Braun JE, Hoffmann NA, Izaurralde E, Sprangers R. 2012. The structural basis of Edc3- and Scd6-mediated activation of the Dcp1:Dcp2 mRNA decapping complex. EMBO J. 31:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa H, Nakagawa T, Uno Y, Kitamura K, Shimoda C. 1994. The ste13+ gene encoding a putative RNA helicase is essential for nitrogen starvation-induced G1 arrest and initiation of sexual development in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 244:456–464 [DOI] [PubMed] [Google Scholar]
- 17.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. 2005. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira D, Parker R. 2007. Analysis of P-body assembly in Saccharomyces cerevisiae. Mol. Biol. Cell 18:2274–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan JR, Muhlrad D, Parker R. 2008. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 183:441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinek M, Eulalio A, Lingel A, Helms S, Conti E, Izaurralde E. 2008. The C-terminal region of Ge-1 presents conserved structural features required for P-body localization. RNA. 14:1991–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anantharaman V, Aravind L. 2004. Novel conserved domains in proteins with predicted roles in eukaryotic cell-cycle regulation, decapping and RNA stability. BMC Genomics 5:45 doi:10.1186/1471-2164-5-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.