Abstract
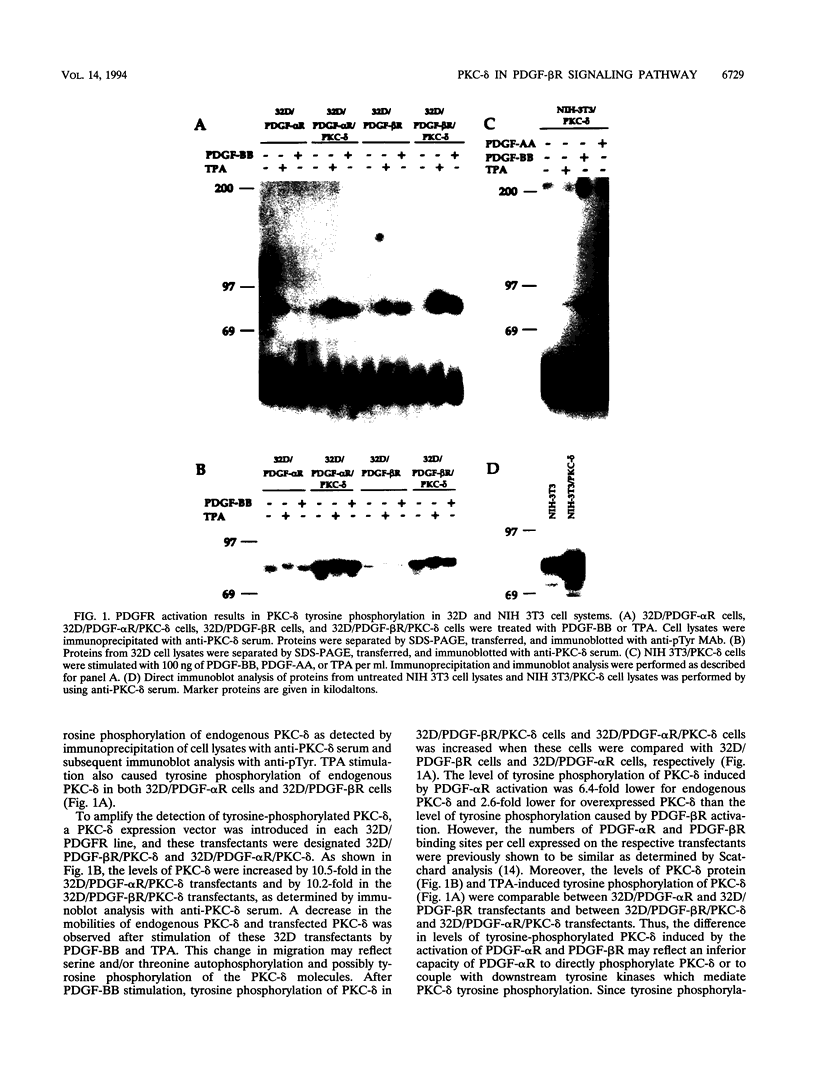
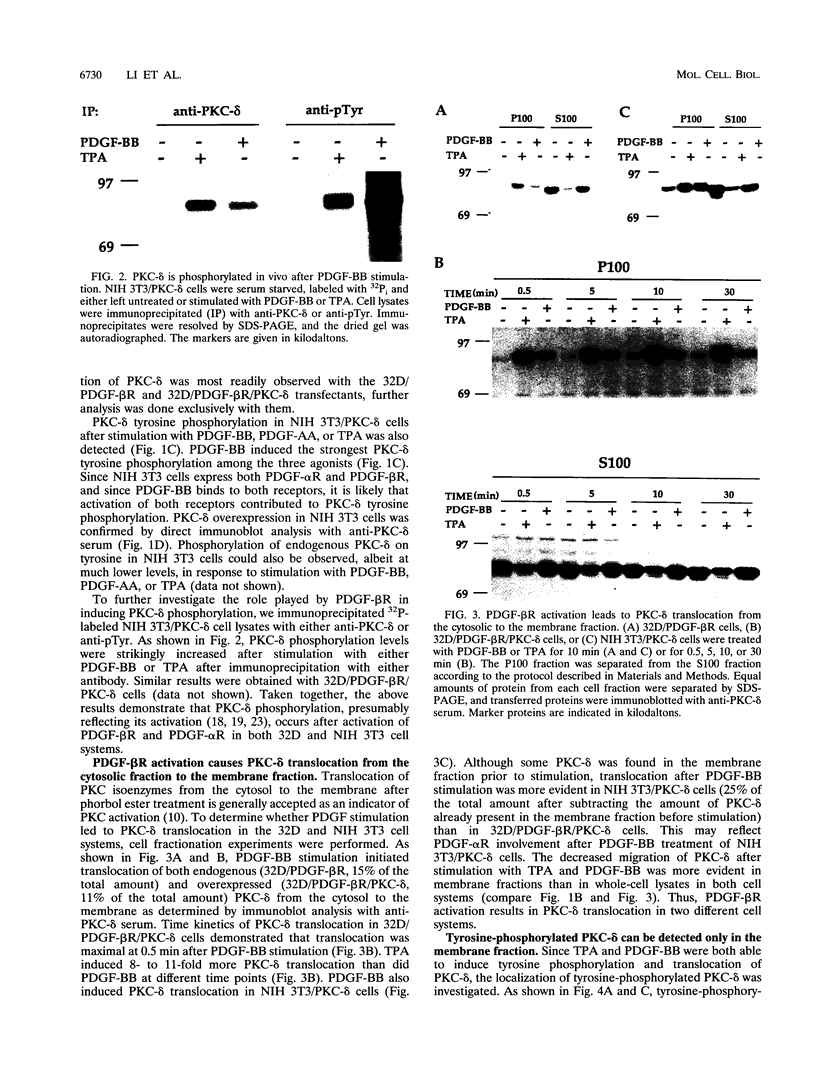
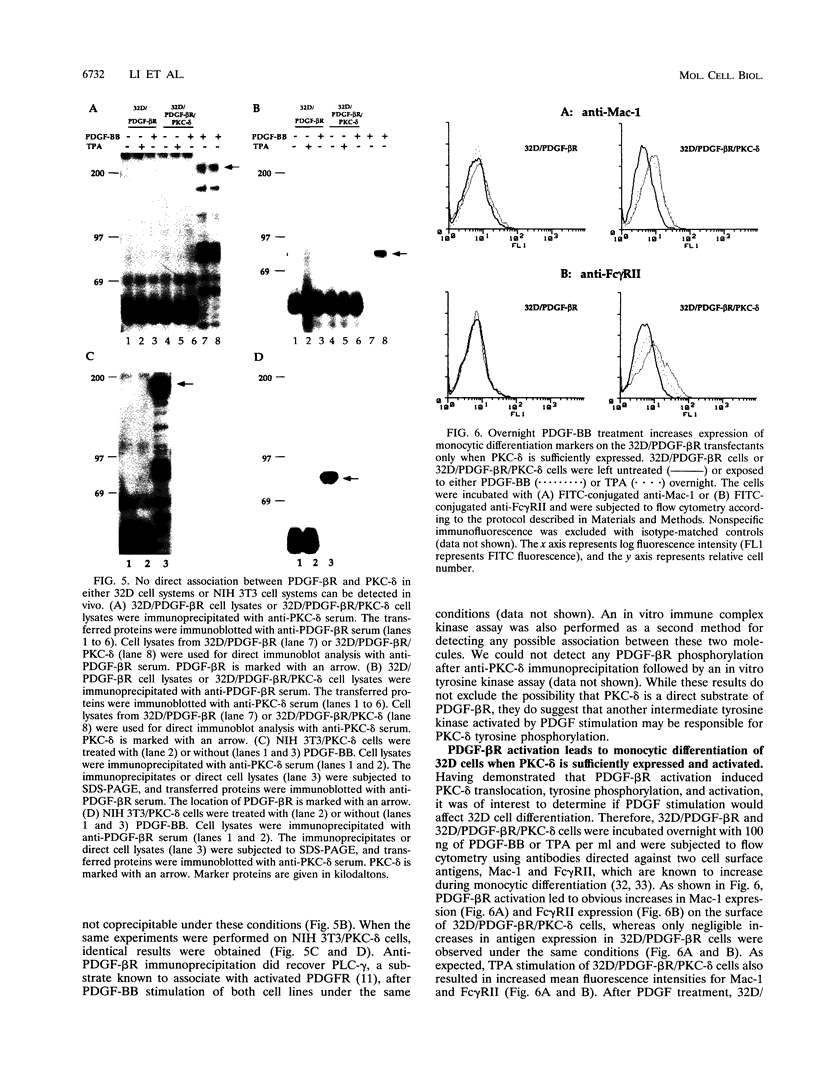
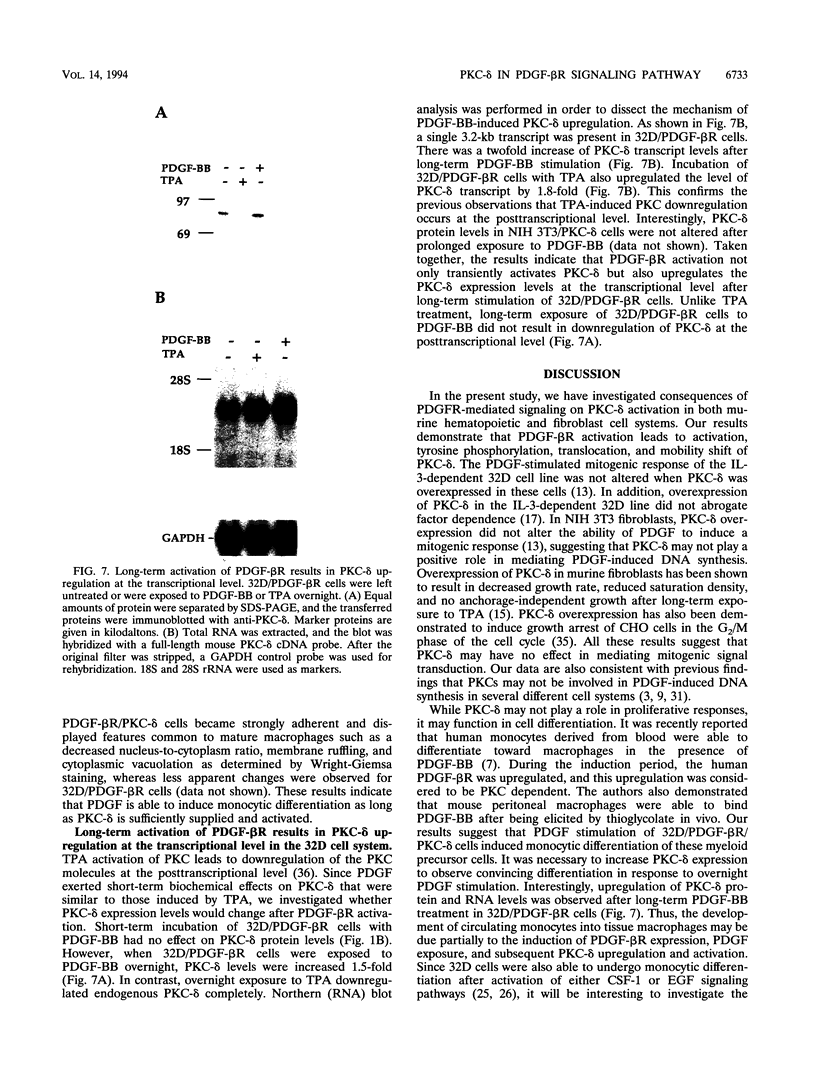
The murine myeloid progenitor cell line 32D was recently shown to undergo monocytic differentiation when protein kinase C-delta (PKC-delta) was overexpressed and activated by 12-O-tetradecanoylphorbol-13-acetate (TPA) (H. Mischak, J.H. Pierce, J. Goodnight, M.G. Kazanietz, P.M. Blumberg, and J.F. Mushinski, J. Biol. Chem. 268:20110-20115, 1993). Tyrosine phosphorylation of PKC-delta occurred when PKC-delta-transfected 32D cells were stimulated by TPA (W. Li, H. Mischak, J.-C. Yu, L.-M. Wang, J.F. Mushinski, M.A. Heidaran, and J.H. Pierce, J. Biol. Chem. 269:2349-2352, 1994). In order to elucidate the role played by PKC-delta in response to activation of a receptor tyrosine kinase, we transfected platelet-derived growth factor beta receptor (PDGF-beta R) alone (32D/PDGF-beta R) or together with PKC-delta (32D/PDGF-beta R/PKC-delta) into 32D cells. NIH 3T3 cells which endogenously express both PDGF-alpha R and PDGF-beta R were also transfected with PKC-delta (NIH 3T3/PKC-delta). Like TPA treatment, PDGF-BB stimulation caused striking phosphorylation of PKC-delta in vivo and translocation of some PKC-delta from the cytosol fraction to the membrane fraction in both cell systems. Some of the phosphorylation induced by PDGF-BB treatment was found to be on a tyrosine residue(s). Tyrosine-phosphorylated PKC-delta was observed only for the membrane fraction after stimulation with PDGF-BB or TPA. The enzymatic activity of PKC-delta in the membrane fraction also increased after stimulation with TPA or PDGF, providing a positive correlation between PKC-delta tyrosine phosphorylation and its activation. Overnight treatment of 32D/PDGF-beta R/PKC-delta cells with PDGF-BB induced monocytic differentiation as judged by an increase in expression of cell surface macrophage differentiation markers. PDGF-BB had much weaker effects on 32D/PDGF-beta R cell differentiation, suggesting that increased PKC-delta expression enhanced monocytic differentiation. These results indicate that PKC-delta is a downstream molecule in the PDGFR signaling pathway and may play a pivotal role in PDGF-beta R-mediated cell differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Beeler J. F., Yu J. C., Ishibashi T., LaRochelle W. J., Pierce J. H., Aaronson S. A. Differences in substrate specificities of alpha and beta platelet-derived growth factor (PDGF) receptors. Correlation with their ability to mediate PDGF transforming functions. J Biol Chem. 1993 May 5;268(13):9287–9295. [PubMed] [Google Scholar]
- Huang K. P. Role of protein kinase C in cellular regulation. Biofactors. 1990 Jul;2(3):171–178. [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Shimano H., Gotoda T., Harada K., Shimada M., Ohsuga J., Watanabe Y., Kawamura M., Yazaki Y., Yamada N. Expression of platelet-derived growth factor beta receptor on human monocyte-derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. J Biol Chem. 1993 Nov 15;268(32):24353–24360. [PubMed] [Google Scholar]
- Johannes F. J., Prestle J., Eis S., Oberhagemann P., Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994 Feb 25;269(8):6140–6148. [PubMed] [Google Scholar]
- Kihara M., Robinson P. J., Buck S. H., Dage R. C. Mitogenesis by serum and PDGF is independent of PI degradation and PKC in VSMC. Am J Physiol. 1989 Apr;256(4 Pt 1):C886–C892. doi: 10.1152/ajpcell.1989.256.4.C886. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Mischak H., Yu J. C., Wang L. M., Mushinski J. F., Heidaran M. A., Pierce J. H. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994 Jan 28;269(4):2349–2352. [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993 Mar 25;268(9):6090–6096. [PubMed] [Google Scholar]
- Mischak H., Kolch W., Goodnight J., Davidson W. F., Rapp U., Rose-John S., Mushinski J. F. Expression of protein kinase C genes in hemopoietic cells is cell-type- and B cell-differentiation stage specific. J Immunol. 1991 Dec 1;147(11):3981–3987. [PubMed] [Google Scholar]
- Mischak H., Pierce J. H., Goodnight J., Kazanietz M. G., Blumberg P. M., Mushinski J. F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem. 1993 Sep 25;268(27):20110–20115. [PubMed] [Google Scholar]
- Mitchell F. E., Marais R. M., Parker P. J. The phosphorylation of protein kinase C as a potential measure of activation. Biochem J. 1989 Jul 1;261(1):131–136. doi: 10.1042/bj2610131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina C. A., Ashendel C. L. Tumor promoter 12-O-tetradecanoylphorbol-13-acetate and sn-1,2-dioctanoylglycerol increase the phosphorylation of protein kinase C in cells. Cancer Res. 1991 Sep 1;51(17):4624–4630. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Hata A., Osada S., Kubo K., Konno Y., Akimoto K., Mizuno K., Saido T., Kuroki T. Structural and functional diversities of a family of signal transducing protein kinases, protein kinase C family; two distinct classes of PKC, conventional cPKC and novel nPKC. Adv Enzyme Regul. 1991;31:287–303. doi: 10.1016/0065-2571(91)90018-h. [DOI] [PubMed] [Google Scholar]
- Ohno S., Konno Y., Akita Y., Yano A., Suzuki K. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J Biol Chem. 1990 Apr 15;265(11):6296–6300. [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Expression and characterization of protein kinase C-delta. Eur J Biochem. 1991 Sep 15;200(3):805–810. doi: 10.1111/j.1432-1033.1991.tb16248.x. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Marco E., Cox G. W., Lombardi D., Ruggiero M., Varesio L., Wang L. M., Choudhury G. G., Sakaguchi A. Y., Di Fiore P. P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ruggiero M., Wang L. M., Pierce J. H. Mitogenic signal transduction in normal and transformed 32D hematopoietic cells. FEBS Lett. 1991 Oct 21;291(2):203–207. doi: 10.1016/0014-5793(91)81284-f. [DOI] [PubMed] [Google Scholar]
- Selbie L. A., Schmitz-Peiffer C., Sheng Y., Biden T. J. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993 Nov 15;268(32):24296–24302. [PubMed] [Google Scholar]
- Sharma R. V., Bhalla R. C. PDGF-induced mitogenic signaling is not mediated through protein kinase C and c-fos pathway in VSM cells. Am J Physiol. 1993 Jan;264(1 Pt 1):C71–C79. doi: 10.1152/ajpcell.1993.264.1.C71. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol. 1987 Jun 1;138(11):3829–3835. [PubMed] [Google Scholar]
- Watanabe T., Ono Y., Taniyama Y., Hazama K., Igarashi K., Ogita K., Kikkawa U., Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]