Abstract
Glycopeptide-resistant Staphylococcus epidermidis (GRSE) strains are of increasing concern in bone and joint infections (BJIs). Using multilocus sequence typing and multilocus variable-number tandem repeat analysis, we show that BJI-associated GRSE strains are genetically diverse but arise from related, multiresistant hospital sequence types (STs), mostly ST2, ST5, and ST23.
TEXT
Staphylococcus epidermidis is a major nosocomial pathogen causing a variety of device-related infections in humans (1, 2). S. epidermidis can develop antibiotic resistance and also form biofilm on implanted medical devices (3, 4). In most countries, 75 to 90% of all hospital S. epidermidis isolates are resistant to methicillin (1, 5). Resistance to fluoroquinolones, erythromycin, clindamycin, gentamicin, rifampin, tetracycline, and chloramphenicol is also frequent (1, 6–8). Multilocus sequence typing (MLST) analysis suggests that multidrug resistance in S. epidermidis is associated with a small number of related clones, mostly belonging to ST2 and related sequence types (STs) (9–11).
Recent studies report the recovery of increasing numbers of glycopeptide-resistant S. epidermidis (GRSE) strains around the world (3, 4, 12–16). Glycopeptide resistance has been detected in a large number of S. epidermidis isolates from patients with bone and joint infections (BJIs) and is a matter of particular concern in orthopedic surgery (3). Most of the GRSE isolates recovered are resistant to methicillin, and many are also resistant to other antibiotics widely used to treat staphylococcal BJIs, including rifampin or clindamycin (3). However, nothing is known about the genetic background of the GRSE strains involved in BJIs and the epidemicity of these strains.
We used both MLST (17–19) and multilocus variable-number tandem repeat analysis (MLVA) (20–22) to study S. epidermidis strains from BJIs. We found that the shift of these strains toward greater resistance to glycopeptides is a widespread phenomenon occurring in many STs rather than the result of the spread of a small number of GRSE strains. However, most BJI GRSE strains emerge from nosocomial, multiresistant STs (e.g., ST2, ST5, ST23), making them a serious problem in orthopedics.
The study was conducted in the Orthopedic Department of the Groupe Hospitalier Raymond Poincaré—Ambroise Paré (France), a reference center for the management of BJIs in the greater Paris area. We included all infecting S. epidermidis strains recovered from BJI cases between January 2003 and December 2005. An infecting strain was defined as a single strain (i.e., isolates having the same colony morphology, antibiotic susceptibility patterns, and sodA sequences) isolated from ≥2 independent intraoperative samples following a single surgical procedure. Patients could be included several times as cases if these criteria were fulfilled and if at least 2 months elapsed between surgical procedures.
All isolates were identified to the species level by partial sodA sequencing (23). A disk diffusion method was used for antibiotic susceptibility testing according to the EUCAST 2012 recommendations (http://www.eucast.org/antimicrobial_susceptibility_testing/); strains with inconclusive results for resistance to methicillin were further studied by testing for the mecA gene (24). The MICs of vancomycin and teicoplanin were determined with the 2-fold agar dilution method (3), and values were interpreted according to EUCAST criteria (25). A GRSE strain was defined as any strain resistant to teicoplanin (MIC value of >4 mg/liter) and/or vancomycin (MIC value of >4 mg/liter). A glycopeptide-sensitive S. epidermidis (GSSE) strain was defined as any strain susceptible to both teicoplanin (MIC value of ≤4 mg/liter) and vancomycin (MIC value of ≤4 mg/liter). A GRSE case was defined as any case from which at least one GRSE strain was isolated. A GSSE case was defined as any case from which only GSSE was isolated.
MLST was performed using the scheme developed by Thomas et al. (17). Sequences were compared with the sequences of known alleles for each locus in the MLST database (http://www.mlst.net), and the resulting seven-digit profiles, defining STs, were used to interrogate the database for matches. Clonal relationships were analyzed with the BURST clustering algorithm (26, 27), using eBURST version 3 (http://eburst.mlst.net), and included all available STs from the MLST database. MLVA was performed using the published primers Se1, Se2, Se3, and Se4 (22), and the following three additional primers were chosen for enhanced discriminatory power (this study), with sequences selected from the Microorganism Tandem Repeats Database (http://www.minisatellites.u-psud.fr) (28): Se6APR (forward/reverse, AGACATTTTAGCATTTTACCGATTG/CATTTGGAGCATCACCCTTT), Se7APR (TGGTTTCAGTGGGGCATAAG/CACGAATGAGTCTGGGACAA), and Se8APR (TGAAGCACCACAGATGTCTT/GGGCTTCTGAAAATTGTGTT). START2 software was used for lineage assignment (26). Minimum spanning trees were constructed with the MLST Data Analysis Web tool provided online at the PubMLST website (http://www.pubmlst.org/analysis).
Fisher's exact test was used to evaluate the significance of 2-by-2 contingency tables. Wilcoxon's signed-rank test was used for quantitative data. Correlations between vancomycin and teicoplanin MICs were assessed with Spearman's signed-rank test. P values of <0.05 were considered to be significant.
We included 75 BJI cases (70 patients) with the isolation of at least one infecting S. epidermidis strain (Table 1); 52 cases had one S. epidermidis strain, 21 had two, and two had three. Thus, 100 different strains were isolated and included in the analysis. Thirty-eight (50.7%) cases involved at least one GRSE strain (GRSE cases) and 37 (49.3%) only GSSE (GSSE cases). GRSE cases tended to be older than GSSE cases (median age, 60.5 compared to 49 years), but the difference was not statistically significant (P = 0.122). The other characteristics studied, as follows, did not differ between the two groups (Table 1): the sex ratio, the nature of the BJI, a coinfection with other bacterial species, the time elapsed since the first procedure, and the number of previous interventions at the same location.
Table 1.
Characteristics of cases
| Characteristic | Value |
Pd | ||
|---|---|---|---|---|
| All cases (n = 75) | GSSE cases (n = 37) | GRSE cases (n = 38) | ||
| Median (IQR) age, in yrs | 55 (39.5–72.5) | 49 (39–66) | 60.5 (42–74.5) | 0.122 |
| Sex ratio, male to female (95% confidence interval) | 2.6 (1.6–4.6) | 2.7 (1.4–6.9) | 2.5 (1.3–5.9) | 1 |
| Prosthesis-associated BJI | 42 (56.0)a | 22 (59.5) | 20 (52.6) | 0.644 |
| No. of cases (%) with coinfection with other species | 20 (26.7) | 9 (24.3) | 11 (28.9) | 0.795 |
| Median (IQR) time since the first procedure, in mob | 24 (6–72) | 24 (2.5–52) | 17 (6.25–150) | 0.641 |
| (n = 71) | (n = 35) | (n = 36) | ||
| Median (IQR) no. of previous proceduresb,c | 2 (1–3) | 2 (1–3) | 2 (1–3.75) | 0.734 |
Twenty-nine hip and 13 knee implants; other BJIs: fracture nonunions (n = 2), contiguous osteitis (n = 31).
At the same location.
Including the first procedure.
GRSE compared to GSSE cases.
Teicoplanin and vancomycin MIC values were determined for all infecting strains (n = 100) recovered from the 75 BJI cases (Fig. 1), and 47% (47/100) of the strains were GRSE. All GRSE strains were resistant to teicoplanin, with most (37/47, 78.7%) showing a teicoplanin MIC value of 8 mg/liter (Fig. 1), but all GRSE strains except one (vancomycin MIC value of 8 mg/liter) were susceptible to vancomycin. However, for all strains included, the MIC values for vancomycin and teicoplanin were positively correlated (Spearman's signed-rank test, P < 10−3).
Fig 1.
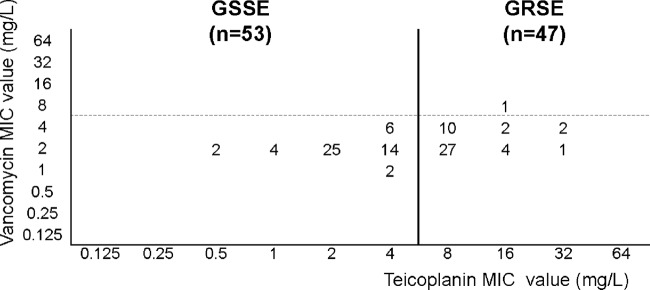
Distribution of teicoplanin and vancomycin MIC values in the S. epidermidis strains studied. Threshold values for resistance to teicoplanin (continuous line) and to vancomycin (broken line) are indicated. All GRSE strains were resistant to teicoplanin and all but one (vancomycin MIC value = 8 mg/liter) were susceptible to vancomycin. The MICs of vancomycin and teicoplanin were positively correlated (Spearman's signed-rank test, P < 10−3).
GRSE strains were significantly more likely than GSSE strains to be resistant to multiple antimicrobial agents, with a median (interquartile range [IQR]) of 9 (4.5 to 10.5) compared to 4 (2 to 9) associated resistance markers, respectively (P = 0.0012). Resistance markers significantly associated with glycopeptide resistance were methicillin (GRSE compared to GSSE, 93.6% compared to 47.2%; P < 10−6), ofloxacin (76.6% compared to 47.2%; P = 0.004), erythromycin (61.7% compared to 35.8%; P = 0.016), kanamycin (68.1% compared to 45.3%; P = 0.027), tobramycin (63.8% compared to 41.5%; P = 0.029), gentamicin (55.3% compared to 32.1%; P = 0.026), and tetracycline (29.8% compared to 11.3%; P = 0.026). Resistance to penicillin, rifampin (44.6% compared to 35.8%; P = 0.4), lincomycin, pristinamycin, fosfomycin, fucidic acid, and trimethoprim-sulfamethoxazole was not significantly associated with glycopeptide resistance.
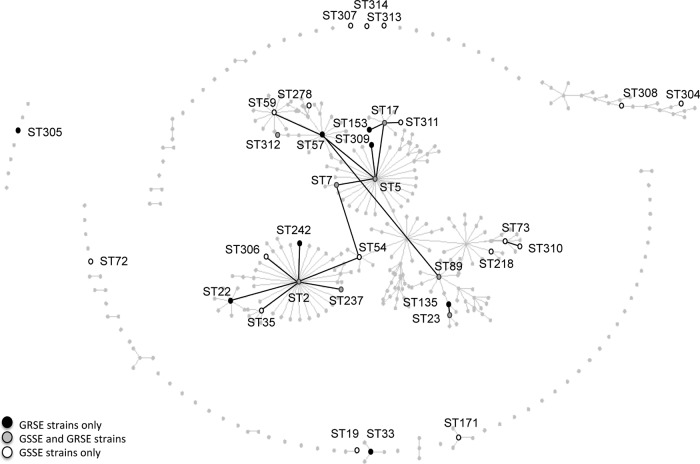
Ninety-seven strains were successfully typed by MLST and clustered into 33 STs (Fig. 2): GRSE strains (n = 46) belonged to 16 STs, mostly ST5 (n = 14), ST23 (n = 10), and ST2 (n = 6); GSSE strains (n = 51) belonged to 25 STs, mostly ST2 (n = 12), ST5 (n = 6), and ST23 (n = 7). Overall, 44 of the 46 GRSE strains (compared to 42 of the 51 GSSE strains; P = 0.054) belonged to the main clonal complex, with a particularly high proportion of GRSE in ST23 (58.8%) and ST5 (70%). The number of resistance markers was significantly higher for GRSE than GSSE subsets within the main clonal complex (median [IQR], 9 [5.75 to 11] and 5.5 [2.25 to 9.75] resistance markers, respectively; P = 0.015).
Fig 2.
eBURST analysis of the strains typed by MLST. ST-numbered circles represent the 33 STs identified among the 97 typeable strains. The size of the circle indicates the number of GRSE (black) and GSSE (white) strains. STs that are directly connected differ at only one single locus. STs previously reported elsewhere and not found in the present study are shown as light-gray dots (population snapshot, http://sepidermidis.mlst.net/). The asterisk indicates the GRSE strain resistant to vancomycin (MIC value = 8 mg/liter).
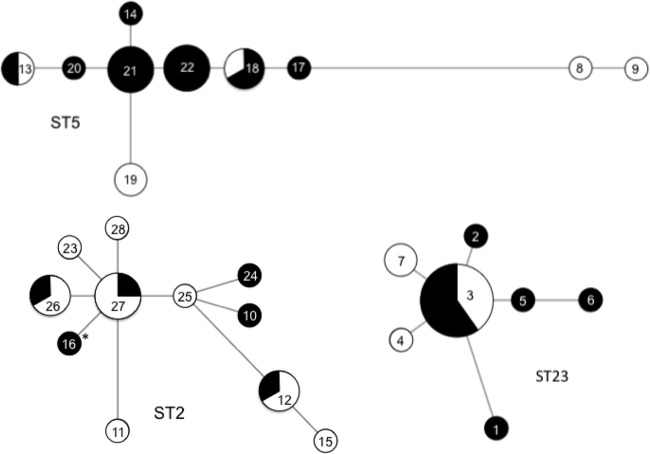
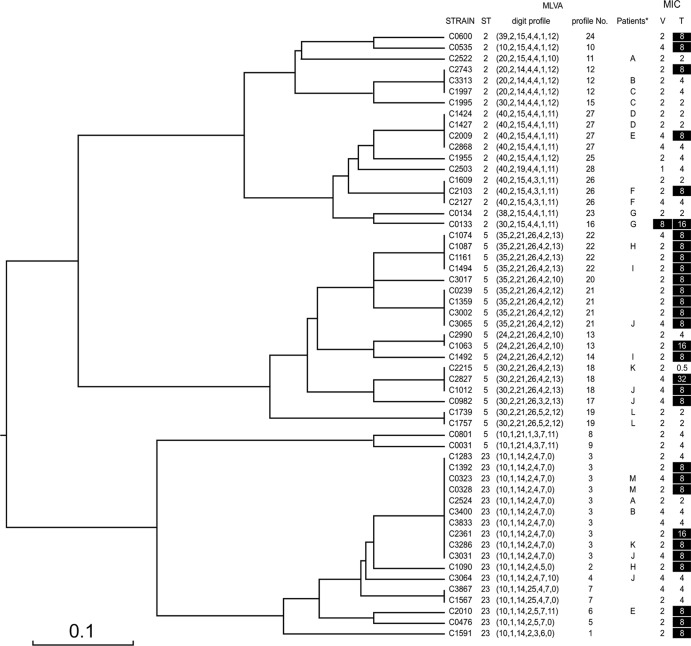
The strains of ST2, ST5, and ST23 (GRSE, n = 30; GSSE, n = 25) were subtyped by MLVA (Fig. 3). This led to the identification of 28 profiles forming three major clusters, consistent with the MLST data (ST2, 11; ST5, 10; ST23, 7). Twelve profiles were associated with GRSE strains only, six with both GRSE and GSSE strains, and 10 with GSSE strains only (Fig. 2). GRSE strains thus displayed 18 distinct profiles and GSSE strains 16. Only three MLVA profiles were shared by more than two GRSE strains: profile 3 (ST23, 6 strains), profile 21 (ST5, 4 strains), and profile 22 (ST5, 4 strains). GRSE strains of profiles 3 and 21 displayed various antibiotic resistance patterns, whereas the four GRSE strains of profile 22 were undistinguishable from each other (same MIC values for glycopeptides and same antibiotic resistance pattern or differing by only one marker). Thus, four of the 38 GRSE cases (4 patients) were infected with the same ST5 strain, with profile 22 (Fig. 4).
Fig 3.
Minimum spanning tree analysis of ST2, ST5, and ST23 strains typed by MLVA. Each circle denotes a particular MLVA profile, and the size of the circle indicates the number of GRSE (black) and GSSE (white) strains displaying that particular profile. The lines denote connections between MLVA profiles. The asterisk indicates the GRSE strain resistant to vancomycin (MIC value = 8 mg/liter).
Fig 4.
MLVA subtyping of ST2, ST5, and ST23 strains. Dendrogram built with START2 software, using the unweighted-pair group method using average linkages (UPGMA) method. For each strain belonging to STs 2, 5, and 23, ST, MLVA digit, MLVA number, patient identifier (anonymized by single-letter coding) for patients with multiple ST2, ST5, and ST23 strains isolated and analyzed, vancomycin MIC, and teicoplanin MIC are shown. Resistant strains according to EUCAST 2012 are shown in white against a black background. *, only patients with more than two S. epidermidis isolates are shown; V, vancomycin; T, teicoplanin.
These findings show that the GRSE strains isolated from BJIs in the Paris area are representative of the populations of multidrug-resistant strains circulating in hospitals worldwide (10, 11, 29–33). Almost 95% of these strains belong to the STs of the main clonal complex, principally ST2, ST5, and ST23, and most of these strains are resistant to numerous antibiotics, such as oxacillin, macrolides, quinolones, and fucidic acid. By combining MLST and MLVA, we show that this population of strains displayed substantial genetic diversity. Indeed, they belonged to 17 different STs, and the subtyping of ST2, ST5, and ST23 strains by MLVA identified 20 different profiles. Phenotypic profiles of resistance to antibiotics, including glycopeptides, were often heterogeneous, further evidence of the diversity of the strains. In most cases, resistance to glycopeptides appeared to be an individual phenomenon repeatedly emerging in the main clonal complex, rather than the result of the spread of a small number of clones. These findings are consistent with those of previous coagulase-negative Staphylococcus typing studies, which reported a decrease in susceptibility to teicoplanin in isolates from various clinical settings with a broad strain diversity (34, 35).
Our findings and published results, together with the positive correlation between MIC values for vancomycin and teicoplanin, suggest that the most likely scenario is that of GRSE strain emergence as a consequence of the increasingly widespread use of glycopeptides (35, 36). Sieradzki et al. have shown that strains of S. epidermidis isolated before the introduction of antibiotics can express heteroresistance to teicoplanin (15, 37) and that, in laboratory conditions, vancomycin can select bacteria with higher MICs for teicoplanin (37). Schwalbe et al. showed that there is a relationship between the cumulative dose of glycopeptides administered to a patient and the emergence of resistance to these antibiotics (38). A number of phenotypic traits have been reported in GRSE strains, including cell wall thickening and a tendency of bacterial cells to form cellular aggregates (16, 37, 39), but no specific genetic determinant of resistance to glycopeptides has yet been identified. GRSE strains thus appear to be variants that emerge under glycopeptide selection pressure, from populations circulating in the hospital environment.
However, a small number of patients in our series were infected with GRSE strains from ST5 that could not be distinguished by MLVA and had identical antibiotic resistance profiles. This may reflect the limitations in terms of discrimination power of MLVA for hyperclonal populations. Alternatively, GRSE may circulate among patients, as recently reported for strains of S. epidermidis resistant to linezolid (40). Although this phenomenon appears to make only a minor contribution to the overall emergence of glycopeptide resistance in S. epidermidis, it nevertheless warrants keeping GRSE populations under surveillance in orthopedic wards and in hospitals more generally. Either through transmission of resistant clones or through acquisition of resistance in previously susceptible strains, the emergence of glycopeptide-resistant strains in S. epidermidis is yet another motive to promote strict antibiotic stewardship practices to preserve the activity of glycopeptides.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Otto M. 2009. Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat. Rev. Microbiol. 7:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Eiff C, Peters G, Heilmann C. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677–685 [DOI] [PubMed] [Google Scholar]
- 3. Cremniter J, Slassi A, Quincampoix JC, Sivadon-Tardy V, Bauer T, Porcher R, Lortat-Jacob A, Piriou P, Judet T, Herrmann JL, Gaillard JL, Rottman M. 2010. Decreased susceptibility to teicoplanin and vancomycin in coagulase-negative staphylococci isolated from orthopedic-device-associated infections. J. Clin. Microbiol. 48:1428–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Natoli S, Fontana C, Favaro M, Bergamini A, Testore GP, Minelli S, Bossa MC, Casapulla M, Broglio G, Beltrame A, Cudillo L, Cerretti R, Leonardis F. 2009. Characterization of coagulase-negative staphylococcal isolates from blood with reduced susceptibility to glycopeptides and therapeutic options. BMC Infect. Dis. 9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lo WT, Lin WJ, Chiueh TS, Lee SY, Wang CC, Lu JJ. 2011. Changing trends in antimicrobial resistance of major bacterial pathogens, 1985–2005: a study from a medical center in northern Taiwan. J. Microbiol. Immunol. Infect. 44:131–138 [DOI] [PubMed] [Google Scholar]
- 6. Miragaia M, Couto I, Pereira SF, Kristinsson KG, Westh H, Jarlov JO, Carrico J, Almeida J, Santos-Sanches I, de Lencastre H. 2002. Molecular characterization of methicillin-resistant Staphylococcus epidermidis clones: evidence of geographic dissemination. J. Clin. Microbiol. 40:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Widerstrom M, Monsen T, Karlsson C, Wistrom J. 2006. Molecular epidemiology of meticillin-resistant coagulase-negative staphylococci in a Swedish county hospital: evidence of intra- and interhospital clonal spread. J. Hosp. Infect. 64:177–183 [DOI] [PubMed] [Google Scholar]
- 8. Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 41:109–119 [DOI] [PubMed] [Google Scholar]
- 9. Ibrahem S, Salmenlinna S, Lyytikainen O, Vaara M, Vuopio-Varkila J. 2008. Molecular characterization of methicillin-resistant Staphylococcus epidermidis strains from bacteraemic patients. Clin. Microbiol. Infect. 14:1020–1027 [DOI] [PubMed] [Google Scholar]
- 10. Li M, Wang X, Gao Q, Lu Y. 2009. Molecular characterization of Staphylococcus epidermidis strains isolated from a teaching hospital in Shanghai, China. J. Med. Microbiol. 58:456–461 [DOI] [PubMed] [Google Scholar]
- 11. Widerstrom M, Monsen T, Karlsson C, Edebro H, Johansson A, Wistrom J. 2009. Clonality among multidrug-resistant hospital-associated Staphylococcus epidermidis in northern Europe. Scand. J. Infect. Dis. 41:642–649 [DOI] [PubMed] [Google Scholar]
- 12. Chiew YF, Charles M, Johnstone MC, Thompson KM, Parnell KD, Penno EC. 2007. Detection of vancomycin heteroresistant Staphylococcus haemolyticus and vancomycin intermediate resistant Staphylococcus epidermidis by means of vancomycin screening agar. Pathology 39:375–377 [DOI] [PubMed] [Google Scholar]
- 13. Maniati M, Petinaki E, Kontos Maniatis AN, Spiliopoulou I, Petropoulou-Mylona D, Malamou-Lada H, Spaliara L, Koutsia-Carouzou C. 2005. Rapid increase in numbers of Staphylococcus epidermidis strains with reduced susceptibility to teicoplanin in Greece. Int. J. Antimicrob. Agents 25:346–348 [DOI] [PubMed] [Google Scholar]
- 14. Trueba F, Garrabe E, Hadef R, Fabre R, Cavallo JD, Tsvetkova K, Chesneau O. 2006. High prevalence of teicoplanin resistance among Staphylococcus epidermidis strains in a 5-year retrospective study. J. Clin. Microbiol. 44:1922–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tacconelli E, Tumbarello M, Donati KG, Bettio M, Spanu T, Leone F, Sechi LA, Zanetti S, Fadda G, Cauda R. 2001. Glycopeptide resistance among coagulase-negative staphylococci that cause bacteremia: epidemiological and clinical findings from a case-control study. Clin. Infect. Dis. 33:1628–1635 [DOI] [PubMed] [Google Scholar]
- 16. Nunes AP, Teixeira LM, Iorio NL, Bastos CC, de Sousa Fonseca L, Souto-Padron T, dos Santos KR. 2006. Heterogeneous resistance to vancomycin in Staphylococcus epidermidis, Staphylococcus haemolyticus and Staphylococcus warneri clinical strains: characterisation of glycopeptide susceptibility profiles and cell wall thickening. Int. J. Antimicrob. Agents. 27:307–315 [DOI] [PubMed] [Google Scholar]
- 17. Thomas JC, Vargas MR, Miragaia M, Peacock SJ, Archer GL, Enright MC. 2007. Improved multilocus sequence typing scheme for Staphylococcus epidermidis. J. Clin. Microbiol. 45:616–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang XM, Noble L, Kreiswirth BN, Eisner W, McClements W, Jansen KU, Anderson AS. 2003. Evaluation of a multilocus sequence typing system for Staphylococcus epidermidis. J. Med. Microbiol. 52:989–998 [DOI] [PubMed] [Google Scholar]
- 19. Sivadon V, Rottman M, Quincampoix JC, Prunier E, de Mazancourt P, Bernard L, Lortat-Jacob A, Piriou P, Judet T, Gaillard JL. 2006. Polymorphism of the cell wall-anchoring domain of the autolysin-adhesin AtlE and its relationship to sequence type, as revealed by multilocus sequence typing of invasive and commensal Staphylococcus epidermidis strains. J. Clin. Microbiol. 44:1839–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbier F, Ruppe E, Hernandez D, Lebeaux D, Francois P, Felix B, Desprez A, Maiga A, Woerther PL, Gaillard K, Jeanrot C, Wolff M, Schrenzel J, Andremont A, Ruimy R. 2010. Methicillin-resistant coagulase-negative staphylococci in the community: high homology of SCCmec IVa between Staphylococcus epidermidis and major clones of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 202:270–281 [DOI] [PubMed] [Google Scholar]
- 21. Francois P, Hochmann A, Huyghe A, Bonetti EJ, Renzi G, Harbarth S, Klingenberg C, Pittet D, Schrenzel J. 2008. Rapid and high-throughput genotyping of Staphylococcus epidermidis isolates by automated multilocus variable-number of tandem repeats: a tool for real-time epidemiology. J. Microbiol. Methods 72:296–305 [DOI] [PubMed] [Google Scholar]
- 22. Johansson A, Koskiniemi S, Gottfridsson P, Wistrom J, Monsen T. 2006. Multiple-locus variable-number tandem repeat analysis for typing of Staphylococcus epidermidis. J. Clin. Microbiol. 44:260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sivadon V, Rottman M, Quincampoix JC, Avettand V, Chaverot S, de Mazancourt P, Trieu-Cuot P, Gaillard JL. 2004. Use of sodA sequencing for the identification of clinical isolates of coagulase-negative staphylococci. Clin. Microbiol. Infect. 10:939–942 [DOI] [PubMed] [Google Scholar]
- 24. Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J. Clin. Microbiol. 29:2240–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. EUCAST 2009. Breakpoint tables for interpretation of MICs and zone diameters version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.0_20091221.pdf
- 26. Jolley KA, Feil EJ, Chan MS, Maiden MC. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230–1231 [DOI] [PubMed] [Google Scholar]
- 27. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denoeud F, Vergnaud G. 2004. Identification of polymorphic tandem repeats by direct comparison of genome sequence from different bacterial strains: a Web-based resource. BMC Bioinformatics 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziebuhr W, Hennig S, Eckart M, Kranzler H, Batzilla C, Kozitskaya S. 2006. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int. J. Antimicrob. Agents 28(Suppl 1):S14–S20 [DOI] [PubMed] [Google Scholar]
- 30. Kozitskaya S, Olson ME, Fey PD, Witte W, Ohlsen K, Ziebuhr W. 2005. Clonal analysis of Staphylococcus epidermidis isolates carrying or lacking biofilm-mediating genes by multilocus sequence typing. J. Clin. Microbiol. 43:4751–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wisplinghoff H, Rosato AE, Enright MC, Noto M, Craig W, Archer GL. 2003. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob. Agents Chemother. 47:3574–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miragaia M, Thomas JC, Couto I, Enright MC, de Lencastre H. 2007. Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J. Bacteriol. 189:2540–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Widerstrom M, McCullough CA, Coombs GW, Monsen T, Christiansen KJ. 2012. A multidrug-resistant Staphylococcus epidermidis clone (ST2) is an ongoing cause of hospital-acquired infection in a Western Australian hospital. J. Clin. Microbiol. 50:2147–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlegel L, Saliba F, Mangeney N, Mathieu D. 2001. Pulsed field gel electrophoresis typing of coagulase-negative staphylococci with decreased susceptibility to teicoplanin isolated from an intensive care unit. J. Hosp. Infect. 49:62–68 [DOI] [PubMed] [Google Scholar]
- 35. Boisson K, Thouverez M, Talon D, Bertrand X. 2002. Characterisation of coagulase-negative staphylococci isolated from blood infections: incidence, susceptibility to glycopeptides, and molecular epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 21:660–665 [DOI] [PubMed] [Google Scholar]
- 36. Bertin M, Muller A, Bertrand X, Cornette C, Thouverez M, Talon D. 2004. Relationship between glycopeptide use and decreased susceptibility to teicoplanin in isolates of coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 23:375–379 [DOI] [PubMed] [Google Scholar]
- 37. Sieradzki K, Villari P, Tomasz A. 1998. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 42:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwalbe RS, Stapleton JT, Gilligan PH. 1987. Emergence of vancomycin resistance in coagulase-negative staphylococci. N. Engl. J. Med. 316:927–931 [DOI] [PubMed] [Google Scholar]
- 39. Gazzola S, Cocconcelli PS. 2008. Vancomycin heteroresistance and biofilm formation in Staphylococcus epidermidis from food. Microbiology 154:3224–3231 [DOI] [PubMed] [Google Scholar]
- 40. Seral C, Saenz Y, Algarate S, Duran E, Luque P, Torres C, Castillo FJ. 2011. Nosocomial outbreak of methicillin- and linezolid-resistant Staphylococcus epidermidis associated with catheter-related infections in intensive care unit patients. Int. J. Med. Microbiol. 301:354–358 [DOI] [PubMed] [Google Scholar]