Abstract
Candidemia is often a life-threatening infection, with highly variable incidence among countries. We conducted a nationwide study of candidemia in Iceland from 2000 to 2011, in order to determine recent trends in incidence rates, fungal species distribution, antifungal susceptibility patterns, and concurrent antifungal consumption. A total of 208 infection episodes in 199 patients were identified. The average incidence during the 12 years was 5.7 cases/100,000 population/year, which was significantly higher than that from 1990 to 1999 (4.3/100,000/year; P = 0.02). A significant reduction in the use of blood cultures was noted in the last 3 years of the study, coinciding with the economic crisis in the country (P < 0.001). Age-specific incidence rates were highest among patients at the extremes of age, 20.7/100,000 for <1 year of age and 18.1/100,000 for >60 years, and varied by gender. Age-specific incidence among males >80 years old was 28.6/100,000/year, and it was 8.3/100,000/year for females in this age group (P = 0.028). The 30-day survival rate among adult patients remained unchanged compared to that from 1990 to 1999 (70.4% versus 69.5%, P = 0.97). Candida albicans was the predominant species (56%), followed by C. glabrata (16%) and C. tropicalis (13%). The species distribution remained stable compared to that from previous decades. Fluconazole use increased 2.4-fold from 2000 to 2011, with no increase in resistance. In summary, the incidence of candidemia in Iceland has continued to increase but may have reached a steady state, and no increase in antifungal drug resistance has been noted. Decreased use of blood cultures toward the end of the study may have influenced detection rates.
INTRODUCTION
Invasive Candida infections have emerged as a serious threat to hospitalized patients in the past decades, incident to increased prevalence of susceptible hosts (1). Despite the widespread use of antifungals for prophylaxis and treatment of invasive fungal infections, candidemia remains the most frequent life-threatening fungal disease and is associated with prolonged hospital stay, excess cost, and high mortality (2, 3). In recent years, several population-based surveys in the United States, Canada, Europe, and Australia have reported an increasing incidence. In the United States, the incidence of candidemia has ranged from 6.0 to 13.3/100,000 population per year (4–6), whereas, at the same time, the incidence rates in most European countries have been lower, ranging from 1.9 to 4.8/100,000/year (7–12). Exceptionally high incidence of candidemia has recently been reported both in Baltimore, 26.2/100,000/year (4), and Denmark, 8.6/100,000/year (13).
A shift in the epidemiology of hematogenous candidiasis toward greater isolation of non-albicans Candida species has been a global concern in recent years. Although Candida albicans remains the most common Candida species isolated from blood, it currently causes only approximately 50% of candidemia cases (14–18). However, significant geographical differences in species distribution exist. The proportion of candidemia caused by C. albicans ranges from 37% in Latin America up to 70% in the Nordic countries (10, 11, 14). Furthermore, the relative proportions of non-albicans Candida pathogens also vary between the northern and southern parts of the world. C. glabrata is overall the second most common recovered isolate in Europe and North America, while C. parapsilosis and C. tropicalis are more common in Latin America and Asia-Pacific (19). This has implications for the regional choice of antifungal therapy, based on various susceptibilities of different Candida spp. to common antifungal drugs.
A nationwide study in Iceland revealed that the annual incidence of candidemia increased from 1.4 cases per 100,000 population from 1980 to 1984 to 4.9 cases per 100,000 population from 1995 to 1999 (20). Given the significant developments in global epidemiology of invasive candidiasis and antifungal therapy, it is of interest to determine whether this trend has continued. We used a nationwide registry to identify all cases of Candida bloodstream infections in Iceland during the ensuing 12-year period, 2000 to 2011. The aim was to determine recent trends in incidence rates, distribution of fungal species and their antifungal susceptibility, as well as concurrent national antifungal consumption.
MATERIALS AND METHODS
Setting, definitions, and methods for blood culture.
Iceland had a population of 279,049 at the beginning of 2000 and 319,575 at the end of 2011 (http://www.statice.is). During the 12-year study period, there were 2 university hospitals and 12 community hospitals in the country providing inpatient services. Blood cultures from all the hospitals were processed at 2 sites, one of them serving as the national reference laboratory for the country. Two blood culture systems were used: the Difco ESP system (Difco Laboratories, Detroit, MI) until 2001, and the BacT/Alert system (bioMérieux, Marcy l‘Etoile, France) from 2002. An episode of candidemia was defined as at least 1 blood culture positive for Candida species, and episodes were considered to be separate if they occurred at least 30 days apart (11) or were caused by different species. When the incident blood culture was obtained within 48 h of hospital admission, the infection episode was defined as community-onset candidemia. The National Bioethics Committee of Iceland and the Data Protection Authority of Iceland approved the study.
Microbiology.
Information on all blood cultures positive for Candida spp. in Iceland from 1 January 2000 through 31 December 2011 was obtained by search in the reference microbiology department's information system, including information regarding total numbers of bloodstream isolates (BSIs), Candida species, and antifungal susceptibility results. Furthermore, information on the total number of blood culture sets processed at the university hospitals was obtained. Each set consisted of aerobic and anaerobic vials. Species identification was based on colony morphology following culture on chromogenic agar (CHROMagar Co., Paris, France) and analysis of sugar assimilation profiles (API 20C AUX and API ID 32C; bioMérieux). In cases where a definite species identification could not be made by these methods, species identification was achieved by using PCR fingerprinting or ITS sequence analysis, as previously described (21, 22).
Overall, 222 Candida isolates were cultured from blood in Iceland from 2000 to 2011. Susceptibility testing for fluconazole and amphotericin B was performed on all 222 isolates and for itraconazole on 212 (95.5%) isolates. Routine susceptibility testing for voriconazole started in June 2006 and was carried out for all 120 isolates cultured thereafter. Routine testing for caspofungin susceptibility was performed on all 75 isolates cultured after June 2008. Antifungal MICs were determined by using the Etest method (AB bioMérieux, Solna, Sweden) and RPMI 1640 agar medium with 2% glucose (Sigma-Aldrich, St. Louis, MO). Susceptibility results for fluconazole, voriconazole, and the echinocandins were categorized according to recently approved species-specific resistance breakpoints from the Clinical and Laboratory Standards Institute (CLSI) (23–25). Regarding fluconazole, isolates of C. albicans, C. tropicalis, and C. parapsilosis with MICs of ≥8 μg/ml and isolates of C. glabrata with MICs of ≥64 μg/ml were considered resistant. C. krusei was considered intrinsically resistant to fluconazole. For voriconazole, isolates of C. albicans, C. tropicalis, and C. parapsilosis with MICs of ≥1 μg/ml and C. krusei isolates with MICs of ≥2 μg/ml were considered resistant. For caspofungin, isolates of C. albicans, C. tropicalis, and C. krusei with MICs of ≥1 μg/ml, C. parapsilosis isolates with MICs of ≥8 μg/ml, and C. glabrata isolates with MICs of ≥0.5 μg/ml were considered resistant. For C. dubliniensis, the C. albicans breakpoints were used, but for other species, susceptibility results were categorized according to the CLSI M27-S3 guidelines (26). Susceptibility results for itraconazole were categorized according to interpretive breakpoints recommended by the CLSI (26), but in the absence of interpretive breakpoints for amphotericin B, a MIC of >2 μg/ml was considered to be elevated (27).
Antifungal drug consumption.
National sales figures of all antifungal agents in Iceland from 2000 to 2011, expressed as defined daily doses (DDD) per 1,000 inhabitants per day, were obtained from the Icelandic Medicines Agency (http://www.imca.is). The WHO/ATC definition of DDD was applied (http://www.whocc.no). The figures reflect overall antifungal drug use in the country, both in hospitals and primary health care settings.
Statistical analysis.
Information on national demographics on an annual basis, including age distribution and gender, was obtained from the national population registry of Iceland (http://www.statice.is). These data were used to calculate the population-based incidence (cases/100,000 population/year) and age-specific incidence of candidemia in the country. Information on patient days and admissions to medical, surgical, and pediatric wards at the university hospitals was obtained from annual hospital reports, and the incidence rates of candidemia per 10,000 patient days and 1,000 admissions were calculated from these numbers. Trends in incidence were examined with the Poisson regression model. We calculated the case fatality proportion for adult patients (189 cases) within 30 days of the first blood culture positive for Candida spp. by hospital records and the national population registry. For comparison, previously published data from 1990 to 1999 in Iceland (105 cases) was included in a Kaplan-Meier survival analysis (28). The distribution of MICs for fluconazole was compared with the Mann-Whitney test. Proportions were compared by using the Pearson χ2 test and Fisher's exact test, as appropriate. Statistical analyses were performed by IBM SPSS Statistics software (version 20; IBM Corporation, Somers, NY), the Joinpoint statistical software (version 3.5.3; National Cancer Institute [http://surveillance.cancer.gov/joinpoint/]), and the OpenEpi online epidemiologic statistics software. Level of significance was set at P values of <0.05. All comparisons were two-tailed.
RESULTS
Epidemiology.
From 2000 to 2011, we identified 208 episodes of candidemia in 199 patients in Iceland. In total, 191 patients (96.0%) had a single episode of infection. Characteristics of patients with recurrent candidemia have been previously described (29). The average population-based incidence of candidemia during this 12-year period was 5.7 cases/100,000 population/year (Fig. 1A). The rate did not change significantly over the 12-year observation period, whether calculated by population at risk, number of admissions, or 10,000 patient days (data not shown). Compared to the previously published nationwide data on candidemia in Iceland from 1990 to 1999 (20), the average annual incidence of candidemia was significantly higher during the current study period, increasing from 4.3/100,000 from 1990 to 1999 to 5.7 cases/100,000 from 2000 to 2011 (P = 0.02).
Fig 1.
Incidence of candidemia in Iceland, 2000 to 2011. (A) Population-based incidence and the total number of blood cultures at the 2 university hospitals on an annual basis. The solid line represents the population-based incidence, whereas the gray columns depict the absolute number of blood culture sets, unadjusted for population size. (B) Annual age-specific incidence by age and gender.
When the study halves were compared, the use of blood cultures at the two university hospitals increased by 12% in the second half (Fig. 1A), and at the same time the proportion of blood cultures positive for Candida spp. increased significantly (P = 0.031). A notable drop in incidence of candidemia was observed after 2009, with a simultaneous reduction in blood cultures (Fig. 1A). Overall, the annual use of blood cultures decreased significantly during this posteconomic crisis period, with a rate ratio of 0.93 (95% confidence interval [CI], 0.92 to 0.94) for 2009 to 2011 compared to 2000 to 2008 (P < 0.001).
The median age of adults (>16 years of age) was 64 years (range, 17 to 92 years; interquartile range, 48 to 74 years), and that among children was 2 months (range, 1 to 200 months; interquartile range, 1 to 39 months). The proportions of male and female patients were 57% and 43%, respectively (P = 0.077). Age-specific incidence rates are shown in Fig. 1B and were highest among patients at the extremes of the age spectrum (rate, 20.7/100,000 for <1 year of age and 18.1/100,000 for >60 years of age). There was male dominance among candidemic infants <1 year of age and the elderly (>60 years), with the largest age-specific difference observed for patients >80 years old (male rate, 28.6/100,000; female rate, 8.3/100,000; P = 0.028). The majority of episodes were diagnosed in patients who had spent >48 h at the hospital at the time of diagnosis (177/208, 85.1%), whereas community-onset infections were rare (31/208, 14.9%). Most cases were diagnosed in intensive care units (87 [42%] of 208 cases), medical wards (64 [31%] cases), and surgical wards (42 [20%] cases).
Patient outcomes.
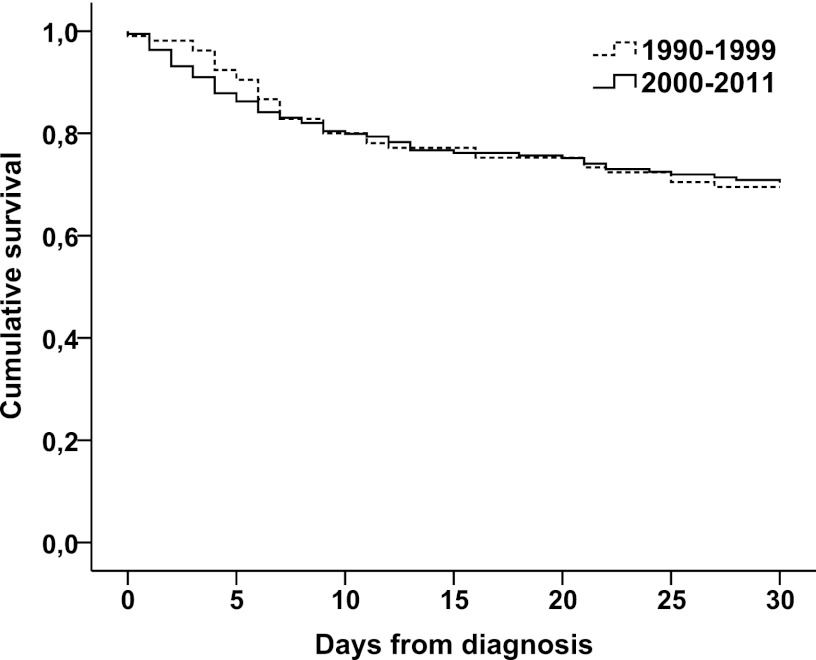
The 30-day survival rate among adult patients was 70.4% (133/189) in the 2000-2011 cohort and remained unchanged compared to that from 1990 to 1999 (69.5% [73/105]; P = 0.97, log-rank test) (Fig. 2).
Fig 2.
Kaplan-Meier survival analysis for patients with candidemia in Iceland, 1990 to 2011 (P = 0.97, log-rank test). Survival analysis was performed for adult patients only (2000 to 2011, 189 cases). For comparison, previously published data from 1990 to 1999 in Iceland (105 cases) was included in the analysis.
Candida species.
An overview of the different Candida species cultured from blood is given in Table 1. Candida albicans was the most frequently isolated species (124 [55.9%] of 222 isolates), followed by C. glabrata (16%), and C. tropicalis (13%). C. dubliniensis and C. parapsilosis were relatively rare (5.4% and 5.0%, respectively). The species distribution remained stable during the 12-year study period (Table 1). Although the proportion of non-albicans Candida species increased slightly from 39% in 2000 to 2003 to 45% in 2008 to 2011, this increase was not significant (P = 0.46). Polyfungal infections were identified in 12 cases (5.8%); 2 different Candida sp. were isolated simultaneously in 10 cases and 3 different Candida sp. in 2 cases. There was a higher proportion of C. glabrata isolates cultured from patients with polyfungemia than from patients infected by a single species (31% [8 of 26] versus 14% [28 of 196]; P = 0.045). The age distribution of patients with polyfungemia and patients infected by one Candida species did not differ significantly.
Table 1.
Species distribution of 222 Candida bloodstream isolates in Iceland, 2000 to 2011
| Species | No. (%) of isolates |
Pa | |||
|---|---|---|---|---|---|
| 2000–2003 | 2004–2007 | 2008–2011 | Total | ||
| C. albicans | 40 (62) | 40 (52) | 44 (55) | 124 (56) | 0.46 |
| C. glabrata | 9 (14) | 12 (16) | 15 (19) | 36 (16) | 0.42 |
| C. tropicalis | 8 (12) | 12 (16) | 8 (10) | 28 (13) | 0.63 |
| C. dubliniensis | 2 (3) | 7 (9) | 3 (4) | 12 (5) | 0.94 |
| C. parapsilosis | 4 (6) | 2 (3) | 5 (6) | 11 (5) | 0.92 |
| Candida spp.b | 2 (3) | 4 (5) | 5 (6) | 11 (5) | 0.39 |
Chi-squared test for trend.
Candida spp. include the indicated number of isolates, as follows: C. lusitaniae, 3; C. krusei, 3; C. kefyr, 2; C. famata, 1; C. guilliemondii, 1; non-albicans Candida sp., 1.
The distribution of Candida BSIs according to species and age of the patients is shown in Table 2. C. albicans was the most common cause in all age groups, but the distribution of other species varied by age. C. parapsilosis showed a predilection toward younger patients (≤20 years old), whereas neither C. glabrata nor C. tropicalis were recovered from this patient group. Conversely, the proportion of infections caused by C. glabrata increased significantly with age (P = 0.001). The species distribution did not vary significantly with gender (data not shown).
Table 2.
Distribution of Candida bloodstream isolates in Iceland, 2000 to 2011, according to species and age group
| Species | No. (%) of isolates for each age (yr) group |
Total no. (%) | |||||
|---|---|---|---|---|---|---|---|
| <1 | 1–20 | 21–40 | 41–60 | 61–80 | >80 | ||
| C. albicans | 10 (91) | 4 (40) | 17 (61) | 34 (55) | 51 (54) | 8 (50) | 124 (56) |
| C. glabrata | 2 (7) | 8 (13) | 21 (22) | 5 (31) | 36 (16) | ||
| C. tropicalis | 5 (18) | 11 (18) | 12 (13) | 28 (13) | |||
| C. dubliniensis | 3 (11) | 3 (5) | 4 (4) | 2 (13) | 12 (5) | ||
| C. parapsilosis | 1 (9) | 4 (40) | 3 (5) | 2 (2) | 1 (6) | 11 (5) | |
| Candida spp.a | 2 (20) | 1 (4) | 3 (5) | 5 (5) | 11 (5) | ||
| Total | 11 | 10 | 28 | 62 | 95 | 16 | 222 |
Candida spp. include the indicated number of isolates, as follows: C. lusitaniae, 3; C. krusei, 3; C. kefyr, 2; C. famata, 1; C. guilliemondii, 1; non-albicans Candida sp., 1.
Antifungal susceptibility.
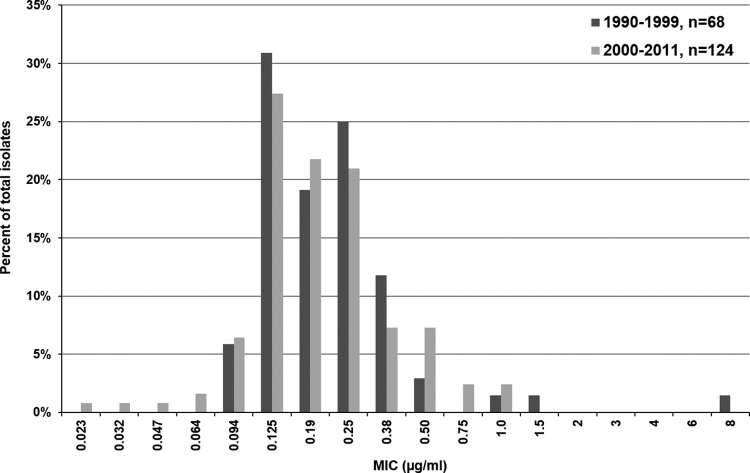
The MICs for amphotericin B, fluconazole, and itraconazole were routinely determined for all available BSIs. A summary of the in vitro antifungal susceptibility test results is given in Table 3. Among the 222 isolates, 216 (97.3%) were susceptible to fluconazole, including all C. albicans, C. parapsilosis, and C. dubliniensis isolates. Fluconazole resistance was due mainly to C. krusei as well as single C. glabrata and C. tropicalis isolates. In addition, an isolate tentatively classified as C. silvicola was fully resistant to fluconazole (MIC = 256 μg/ml). The proportion of isolates highly susceptible to fluconazole (MIC of ≤1 μg/ml) remained stable at 80 to 85% throughout the 12-year study period. Furthermore, no significant change was noted in the distribution of MICs of fluconazole during the current study period (2000 to 2011) compared to previously published data from 1990 to 1999 in Iceland (20), with a median MIC of 0.25 μg/ml during both time periods (P = 0.24). The specific distribution of fluconazole MICs in C. albicans for 1990 to 1999 and 2000 to 2011 is shown in Fig. 3. In addition, 97.8% (89/91) of C. albicans, C. tropicalis, C. parapsilosis, and C. dubliniensis isolates tested were susceptible to voriconazole, and, among other species, 86% (25/29) had a MIC of ≤0.5 μg/ml. Overall, 80.2% (170/212) of isolates tested were susceptible to itraconazole, including all C. albicans BSIs (MIC < 1 μg/ml).
Table 3.
In vitro susceptibilities of Candida bloodstream isolates to five antifungals, Iceland, 2000 to 2011
| Species | Antifungal agent (no. of isolates tested) | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| C. albicans | Amphotericin B (124) | 0.002–0.50 | 0.125 | 0.25 |
| Fluconazole (124) | 0.023–1.0 | 0.19 | 0.5 | |
| Itraconazole (116) | 0.004–0.32 | 0.032 | 0.125 | |
| Voriconazole (64) | 0.002–0.38 | 0.008 | 0.016 | |
| Caspofungin (41) | 0.023–0.38 | 0.19 | 0.25 | |
| C. glabrata | Amphotericin B (36) | 0.002–1.0 | 0.38 | 0.75 |
| Fluconazole (36) | 0.023–48 | 1.5 | 16 | |
| Itraconazole (35) | 0.064–32 | 0.75 | 32 | |
| Voriconazole (22) | 0.002–1.5 | 0.064 | 0.75 | |
| Caspofungin (14) | 0.064–0.38 | 0.19 | 0.38 | |
| C. tropicalis | Amphotericin B (28) | 0.002–0.75 | 0.125 | 0.50 |
| Fluconazole (28) | 0.125–8 | 0.38 | 1.0 | |
| Itraconazole (27) | 0.003–1.5 | 0.016 | 0.064 | |
| Voriconazole (16) | 0.006–0.5 | 0.032 | 0.094 | |
| Caspofungin (7) | 0.064–0.75 | 0.19 | 0.75 | |
| C. dubliniensis | Amphotericin B (12) | 0.012–0.38 | 0.064 | 0.125 |
| Fluconazole (12) | 0.047–2 | 0.125 | 0.38 | |
| Itraconazole (12) | 0.003–0.50 | 0.016 | 0.38 | |
| Voriconazole (6) | 0.002–0.012 | 0.006 | 0.012 | |
| Caspofungin (3) | 0.25–0.50 | 0.38 | 0.50 | |
| C. parapsilosis | Amphotericin B (11) | 0.125–0.38 | 0.19 | 0.25 |
| Fluconazole (11) | 0.047–1.5 | 0.25 | 1.0 | |
| Itraconazole (11) | 0.006–0.094 | 0.023 | 0.064 | |
| Voriconazole (5) | 0.003–0.016 | 0.004 | 0.016 | |
| Caspofungin (5) | 1.0–2 | 1.5 | 2 | |
Fig 3.
Distribution of MICs of fluconazole for 124 Candida albicans bloodstream isolates cultured from 2000 to 2011 compared to 68 C. albicans bloodstream isolates cultured from 1990 to 1999 in Iceland. The MIC distribution did not vary significantly between the two time periods (P = 0.68).
Resistance to caspofungin was not detected; 88% (66/75) of isolates tested were susceptible, whereas the remaining isolates, primarily C. glabrata, had an intermediate susceptibility to the agent. Furthermore, none of the 222 isolates had elevated MIC values of >2 μg/ml for amphotericin B.
National consumption of antifungal agents.
Data on the total nationwide consumption of systemic antifungal agents, 2000 to 2011, are provided in Table 4. As shown, the annual consumption of fluconazole increased by 141%, from 39.5 to 95.1 DDD per 1,000 inhabitants. The annual use of oral formulations increased from 35.9 to 89.7 DDD per 1,000 inhabitants (150%), whereas the annual use of parenteral formulations increased from 3.6 to 5.4 DDD per 1,000 inhabitants (50%). Voriconazole and echinocandins were first imported in Iceland in 2002 and 2003, respectively.
Table 4.
Annual national consumption of systemic antifungal compounds in Iceland, 2000 to 2011, expressed as defined daily doses per 1,000 inhabitants
| Yr | DDD per 1,000 inhabitantsa |
|||||
|---|---|---|---|---|---|---|
| AMB | FLC | ITC | VRC | PSC | ECD | |
| 2000 | 3.2 | 39.5 | 94.8 | |||
| 2001 | 1.4 | 42.9 | 28.1 | |||
| 2002 | 5.6 | 37.7 | 24.1 | 0.10 | ||
| 2003 | 2.7 | 42.0 | 22.9 | 0.86 | 0.06 | |
| 2004 | 0.1 | 48.8 | 25.2 | 0.24 | 0.14 | |
| 2005 | 1.1 | 59.0 | 29.3 | 0.54 | 0.06 | |
| 2006 | 4.4 | 64.1 | 26.4 | 0.47 | 0.18 | |
| 2007 | 5.3 | 68.9 | 25.2 | 0.75 | 0.01 | 0.23 |
| 2008 | 1.4 | 73.2 | 25.9 | 1.72 | 0.02 | 0.30 |
| 2009 | 4.7 | 79.0 | 19.2 | 0.76 | 0.16 | 0.11 |
| 2010 | 2.6 | 78.7 | 16.3 | 3.76 | 0.01 | 0.31 |
| 2011 | 1.7 | 95.1 | 16.0 | 2.49 | 0.05 | 0.32 |
AMB, amphotericin B; FLC, fluconazole; ITC, itraconazole; VRC, voriconazole; PSC, posaconazole; ECD, echinocandins.
DISCUSSION
This nationwide study of candidemia assessed secular trends in incidence and species distribution during a 12-year period, representing data from both university and community hospitals. The annual incidence of candidemia in Iceland is slightly higher than that observed in most other population-based European studies when the incidence for analogous time intervals is compared. The annual incidence in the Nordic countries of Finland from 2004 to 2007 and Norway from 2000 to 2003 was 2.9 cases/100,000 population; identical incidence has been reported from Canada (10, 11, 30). However, Denmark seems to be an outlier, with an annual average incidence of 8.6/100,000 from 2004 to 2009 (13). At the two extremes among western societies, Australia reports a comparatively low rate of 1.8 cases per 100,000 population (31), whereas a recent population-based surveillance study from the United States conducted from 2008 to 2011 showed incidence rates of 13.3 cases/100,000/year in Atlanta and even as high as 26.2 cases/100,000/year in Baltimore (4). Importantly, all the aforementioned studies documented an overall increase in incidence over the course of study. The differences between countries may result from demographic differences, but variations in clinical practice more likely have a larger effect, such as central venous catheter (CVC) use, utilization of blood cultures, antibiotic selection, and use of antifungals for prophylaxis.
The average annual incidence of candidemia increased significantly in 2000 to 2011 compared to the previous decade in Iceland. However, within the current study period, the incidence increased slowly at an average of 2.2% for each year and may be reaching a steady state. Following a peak in 2009 at 7.6, there was a marked fall in incidence rates during the two last years of the study, to 6.6 cases/100,000 population in 2010 and 4.4 cases/100,000 population in 2011. The reasons for this shift in epidemiology of candidemia in the country are unclear. The same blood culture system and other laboratory methods were used throughout the study period. Thus, we propose three potential explanations. First, it might reflect a true decrease in infection rates. This could be explained by increased awareness of hospital staff of the importance of preventive measures and infection control in the hospital environment. Interventions such as hand washing, improved skin disinfections, and removal of unnecessary catheters have been shown to dramatically reduce the rate of catheter-related bloodstream infection in intensive care units (32). Second, the number of susceptible hosts may have dropped due to less aggressive therapeutic practices, such as less frequent use of CVCs. Third, it could be due to underdiagnosis of Candida bloodstream infections. Funding for health care in Iceland is based on a fixed annual budget, determined by the government, and the hospitals have very limited alternative options for revenue. After the economic crisis in Iceland in late 2008, the operation budget at the two university hospitals was slashed by 23%. Thus, the drop in incidence in 2010 and 2011 reported here coincides with a simultaneous reduction in the use of blood cultures at the two university hospitals. Moreover, the proportion of positive blood cultures increased significantly in 2006 to 2011 compared to that in 2000 to 2005, suggesting that more infections may have been left undiagnosed in the second half of the study.
The high incidence of candidemia among patients at the extremes of the age spectrum reported here is consistent with previous reports (10, 11, 13, 30, 31). The average age-specific incidence among infants <1 year of age was, however, surprisingly high at 20.7 cases/100,000 population/year, which was double the incidence found during the 1990s in Iceland (11.2 cases/100,000). This is also two to three times higher than that reported from the other Northern European countries (6.9 to 11.3 cases/100,000 population) (10, 11, 13) but lower than the rates reported from Spain (38.8/100,000 population) (7) and among white neonates in two population-based U.S. studies (37 and 41/100,000 population) (6, 33). By genotyping of Candida blood isolates, we have previously shown that nosocomial clustering of candidemia is particularly prevalent in the neonatal intensive care unit setting (21). Our study, therefore, confirms and emphasizes the risk of candidemia in this vulnerable population and the importance of preventive measures. Of further interest was an increase in age-specific incidence from 10.0 in 1990 to 1999 to 16.8 cases/100,000 in 2000 to 2011 among patients older than 80 years of age. The male dominance in this age category is also of interest, being more than three times higher than among women within the same age range. Similarly, a nationwide study in Denmark from 2004 to 2009 reported a significantly higher incidence among males than females among patients >60 years of age (13), and other studies have documented a higher proportion of males in older patients with candidemia (10, 11). The reasons for this contrast are unclear and need further study but could possibly include gender-related differences in host or risk factors for candidemia, as well as treatment-related singularities.
The all-cause 30-day case fatality rate among adult patients during the 12-year study period was 29.6%, which is in concordance with rates reported from European tertiary care centers, ranging from 26% to 38% (18, 34). Our study therefore supports the notion that the mortality associated with candidemia has not changed substantially in the past 2 decades despite the availability of less toxic and more fungicidal agents.
A shift in the epidemiology of hematogenous candidiasis toward greater isolation of non- albicans Candida species with reduced susceptibility to azole antifungal agents has been a global concern in the past 2 decades, and C. albicans currently causes a little less than 50% of candidemias worldwide (19). In the United States, C. glabrata increased as a cause of invasive candidiasis from 18% of all isolates from 1992 to 2001 to 25% in 2001 to 2007, with a concomitant increase in fluconazole resistance from 9% to 14% (35). A similar development has been reported from the Nordic countries (10, 11, 13). Although C. albicans remained the most frequently isolated species from blood throughout the 12-year study period, there has been a trend toward decreased isolation of C. albicans and C. parapsilosis and a notable increase in isolation of C. tropicalis compared to that in the 1990s (6.7% versus 13%). The relatively stable proportion of C. glabrata bloodstream infections in Iceland in the past 30 years may, in part, be explained by the infrequent use of fluconazole prophylaxis. The increased frequency of C. glabrata bloodstream infections among older patients is in agreement with previous studies (13, 36), and others have noted that elderly patients (>60 years old) may also be at increased risk of dying from candidemia caused by C. glabrata (37). The elderly have higher rates of oropharyngeal colonization with C. glabrata (38), but the relationship to bloodstream infection is not clear. Our results also confirm that candidemia with C. parapsilosis are more commonly encountered in young patients than in adults (39). Surprisingly, this species was not a major pathogen among infants (<1 year of age). Furthermore, we did not observe an increase in C. parapsilosis infections after the introduction of the echinocandins, as reported by Forrest et al. (40). Given the known propensity of C. parapsilosis to adhere to foreign material (41) and a decreasing proportion of C. parapsilosis infections diagnosed in the country in the past 12 years compared to that in the previous decade (20), these results might suggest improved catheter care and infection control procedures in the country.
Overall, 97.3% of the Candida BSIs tested were susceptible to fluconazole, which further confirms the infrequent fluconazole resistance among C. albicans, C. parapsilosis, and C. tropicalis reported in a recent global survey (42). Furthermore, no significant change in distribution of fluconazole MICs was noted during the current study period compared to that in the previous decade in Iceland, despite a more than 2-fold increase in use from 2000 to 2011. Our data on antifungal consumption are directly comparable to national data on fluconazole usage reported from Denmark and Norway. In 2009, the use of fluconazole was 58.8 DDD/1,000 inhabitants/year in Norway, 79.0 DDD/1,000 inhabitants/year in Iceland, and 160 DDD/1,000 inhabitants/year in Denmark (13). This may explain why reduced azole susceptibility in Candida spp. normally susceptible to fluconazole occurs more frequently in Denmark than in Norway and Iceland.
Voriconazole was first registered in Iceland in 2002, and its use increased steadily thereafter. The consumption of other antifungal agents remained stable. Echinocandins are infrequently used in Iceland, and their use has not increased in recent years, which is largely explained by the high cost of treatment. This is of concern, since recent treatment guidelines recommend an echinocandin as a first-line choice for invasive candidiasis, in particular for the critically ill, those with previous azole exposure, and those infected with less susceptible Candida spp., such as C. glabrata and C. krusei (43). Moreover, a recent review of randomized trials for treatment of invasive candidiasis identified removal of the CVC and the use of an echinocandin drug for treatment as the most important factors in improving survival and for patient success (44).
In conclusion, this population-based, nationwide study has shown that the incidence of candidemia in Iceland has increased significantly, albeit more slowly, during the past 12 years after a rapid increase in incidence in the 1980s and 1990s. A drop in incidence rates during the last 2 years of the study period coincided with decreased use of blood cultures and severe hospital budget cuts following the economic crisis in Iceland. The study further confirms the high and increasing incidence of candidemia as well as significant gender differences among patients at the extremes of age. C. albicans remained the most common cause of candidemia througout the study without significant changes in species distribution. Fluconazole use in Iceland more than doubled from 2000 to 2011, but with no apparent increase in resistance to this agent.
ACKNOWLEDGMENTS
We thank Thor Aspelund and Olafur Skuli Indridason for their advice on statistical analysis and the staff at the Department of Clinical Microbiology, Landspitali University Hospital, Reykavik, for assistance in this project.
This work was financially supported by grants from the Eggert V. Briem Memorial Fund and the Landspitali University Hospital Research Fund.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177 [DOI] [PubMed] [Google Scholar]
- 3. Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 26:540–547 [DOI] [PubMed] [Google Scholar]
- 4. Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 55:1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, Pfaller MA. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA, Pfaller MA, Pinner RW, Hajjeh RA. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164–1170 [DOI] [PubMed] [Google Scholar]
- 7. Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NA, Jones BL. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56:1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poikonen E, Lyytikainen O, Anttila VJ, Ruutu P. 2003. Candidemia in Finland, 1995–1999. Emerg. Infect. Dis. 9:985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poikonen E, Lyytikainen O, Anttila VJ, Koivula I, Lumio J, Kotilainen P, Syrjala H, Ruutu P. 2010. Secular trend in candidemia and the use of fluconazole in Finland, 2004–2007. BMC Infect. Dis. 10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandven P, Bevanger L, Digranes A, Haukland HH, Mannsaker T, Gaustad P. 2006. Candidemia in Norway (1991 to 2003): results from a nationwide study. J. Clin. Microbiol. 44:1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, Grillot R. 2006. Candidaemia in Europe: epidemiology and resistance. Int. J. Antimicrob. Agents 27:359–366 [DOI] [PubMed] [Google Scholar]
- 13. Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Moller J, Nielsen L, Rosenvinge FS, Roder B, Schonheyder HC, Thomsen MK, Truberg K. 2011. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 49:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombo AL, Perfect J, DiNubile M, Bartizal K, Motyl M, Hicks P, Lupinacci R, Sable C, Kartsonis N. 2003. Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:470–474 [DOI] [PubMed] [Google Scholar]
- 15. Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 73:45–48 [DOI] [PubMed] [Google Scholar]
- 16. Pfaller MA, Diekema DJ. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl 1):11–23 [DOI] [PubMed] [Google Scholar]
- 17. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Meis JF, Gould IM, Fu W, Colombo AL, Rodriguez-Noriega E. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 45:1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K, Seaton S, Grillot R. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23:317–322 [DOI] [PubMed] [Google Scholar]
- 19. Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 49:396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asmundsdottir LR, Erlendsdottir H, Haraldsson G, Guo H, Xu J, Gottfredsson M. 2008. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin. Infect. Dis. 47:e17–e24 [DOI] [PubMed] [Google Scholar]
- 22. Asmundsdottir LR, Erlendsdottir H, Agnarsson BA, Gottfredsson M. 2009. The importance of strain variation in virulence of Candida dubliniensis and Candida albicans: results of a blinded histopathological study of invasive candidiasis. Clin. Microbiol. Infect. 15:576–585 [DOI] [PubMed] [Google Scholar]
- 23. Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 13:180–195 [DOI] [PubMed] [Google Scholar]
- 24. Pfaller MA, Andes D, Arendrup MC, Diekema DJ, Espinel-Ingroff A, Alexander BD, Brown SD, Chaturvedi V, Fowler CL, Ghannoum MA, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Walsh TJ. 2011. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn. Microbiol. Infect. Dis. 70:330–343 [DOI] [PubMed] [Google Scholar]
- 25. Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 26. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; informational supplement, 3rd ed CLSI document M27-S3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 27. Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 50:2846–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. 2005. Improving survival of patients with candidaemia: analysis of prognostic factors from a long-term, nationwide study in Iceland. Scand. J. Infect. Dis. 37:111–120 [DOI] [PubMed] [Google Scholar]
- 29. Asmundsdottir LR, Erlendsdottir H, Gisladottir AL, Gottfredsson M. 2012. Molecular epidemiology of late recurrent candidaemia—a population-based study in Iceland. Clin. Microbiol. Infect. 18:195–201 [DOI] [PubMed] [Google Scholar]
- 30. Laupland KB, Gregson DB, Church DL, Ross T, Elsayed S. 2005. Invasive Candida species infections: a 5 year population-based assessment. J. Antimicrob. Chemother. 56:532–537 [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, Ellis D, Sorrell T. 2006. Active surveillance for candidemia, Australia. Emerg. Infect. Dis. 12:1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. 2006. An intervention to decrease catheter-related bloodstream infections in the ICU. N. Engl. J. Med. 355:2725–2732 [DOI] [PubMed] [Google Scholar]
- 33. Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, Phelan M, Morgan J, Lee-Yang W, Ciblak MA, Benjamin LE, Sanza LT, Huie S, Yeo SF, Brandt ME, Warnock DW. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kibbler CC, Seaton S, Barnes RA, Gransden WR, Holliman RE, Johnson EM, Perry JD, Sullivan DJ, Wilson JA. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54:18–24 [DOI] [PubMed] [Google Scholar]
- 35. Pfaller MA, Messer SA, Hollis RJ, Boyken L, Tendolkar S, Kroeger J, Diekema DJ. 2009. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J. Clin. Microbiol. 47:3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634–643 [DOI] [PubMed] [Google Scholar]
- 37. Malani A, Hmoud J, Chiu L, Carver PL, Bielaczyc A, Kauffman CA. 2005. Candida glabrata fungemia: experience in a tertiary care center. Clin. Infect. Dis. 41:975–981 [DOI] [PubMed] [Google Scholar]
- 38. Lockhart SR, Joly S, Vargas K, Swails-Wenger J, Enger L, Soll DR. 1999. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J. Dent. Res. 78:857–868 [DOI] [PubMed] [Google Scholar]
- 39. Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. 1998. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin. Infect. Dis. 26:1086–1088 [DOI] [PubMed] [Google Scholar]
- 40. Forrest GN, Weekes E, Johnson JK. 2008. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 56:126–129 [DOI] [PubMed] [Google Scholar]
- 41. Weems JJ., Jr 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756–766 [DOI] [PubMed] [Google Scholar]
- 42. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48:1366–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ. 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54:1110–1122 [DOI] [PubMed] [Google Scholar]