Abstract
Detection of several pathogens with multiplexed real-time quantitative PCR (qPCR) assays in a one-step setup allows the simultaneous detection of two endemic porcine and four different selected transboundary viruses. Reverse transcription (RT)-qPCR systems for the detection of porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2), two of the most economically important pathogens of swine worldwide, were combined with a screening system for diseases notifiable to the World Organization of Animal Health, namely, classical and African swine fever, foot-and-mouth disease, and Aujeszky's disease. Background screening was implemented using the identical fluorophore for all four different RT-qPCR assays. The novel multiplex RT-qPCR system was validated with a large panel of different body fluids and tissues from pigs and other animal species. Both reference samples and clinical specimens were used for a complete evaluation. It could be demonstrated that a highly sensitive and specific parallel detection of the different viruses was possible. The assays for the notifiable diseases were even not affected by the simultaneous amplification of very high loads of PRRSV- and PCV2-specific sequences. The novel broad-spectrum multiplex assay allows in a unique form the routine investigation for endemic porcine pathogens with exclusion diagnostics of the most important transboundary diseases in samples from pigs with unspecific clinical signs, such as fever or hemorrhages. The new system could significantly improve early detection of the most important notifiable diseases of swine and could lead to a new approach in syndromic surveillance.
INTRODUCTION
Natural coinfection of swine with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) is common in countries with intensive swine production (1, 2). Infection with PRRSV, an enveloped positive-strand RNA virus that belongs to the order Nidovirales, family Arteriviridae (3), is characterized by reproductive failure in pregnant sows and respiratory disease in piglets (4). PRRSV isolates are classified into two distinct genotypes: the European (EU) and the North American (NA) genotypes (5). PCV2, responsible for considerable economic loss in the swine industry worldwide (6), is a nonenveloped single-stranded DNA virus (7). Infection with PCV2 has been associated with the postweaning multisystemic wasting syndrome (PMWS) (8), where typical clinical signs include weight loss, respiratory distress, and jaundice, as well as pathological findings of interstitial pneumonia, generalized, enlarged lymph nodes, hepatitis, and nephritis (9–11). Additionally, PCV2 was suspected to be associated with porcine dermatitis and nephropathy syndrome (PDNS) (12) and reproductive failure (13). The list of differential diagnosis for PMWS includes the respiratory form of PRRS, classical swine fever (CSF), and Aujeszky's disease. Pseudorabies or Aujeszky's disease, caused by Suid herpesvirus 1 (SuHV1), a member of the genus Varicellovirus, subfamily Alphaherpesvirinae, family Herpesviridae, (14), is a notifiable disease in swine of great economic importance. Among the wide range of hosts, including nearly all mammals except humans and higher primates, only members of the Suidae family are able to survive a productive infection and as a consequence serve as a virus reservoir (15). Clinical signs range from central nervous system disorders and death in young piglets to respiratory disease and reproductive failure in older pigs (16).
CSF, caused by classical swine fever virus (CSFV), a positive-sense single-stranded RNA virus, genus Pestivirus, family Flaviviridae (17), and African swine fever (ASF), caused by the large enveloped, double-stranded DNA African swine fever virus (ASFV), assigned to be the only member of the genus Asfivirus, family Asfarviridae (18), cannot be differentiated by either clinical or postmortem examination (19). Clinical symptoms vary from sudden death, fever, and hemorrhages of the skin and internal organs to respiratory signs, stunting of growth, anorexia, and lethargy (20, 21). Similar symptoms were caused by a highly pathogenic strain of PRRSV (HP-PRRSV) since its emergence 2006 in China (22) and subsequent spread to other Asian countries (23, 24).
For the effective control and eradication of foot-and-mouth-disease (FMD), a highly contagious disease of cloven-hoofed animals with a great economic impact (25), a rapid diagnosis is crucial. The causative agent, foot-and-mouth-disease virus (FMDV), is a member of the genus Aphthovirus, family Picornaviridae, and seven serotypes, namely, O, A, C, SAT 1, SAT 2, SAT 3, and Asia 1 (25), are differentiated.
A major problem of most transboundary diseases of pigs is the absence of pathognomonic clinical signs, resulting in a high-risk period of several weeks until an outbreak is detected (26). Therefore, the broad screening of diseased pigs in syndromic surveillance programs could allow earlier detection.
Here, we describe a single-tube multiplex real-time RT-PCR combining the detection of two relevant endemic porcine pathogens with a parallel screening for FMDV as well as SuHV-1, CSFV, and ASFV.
MATERIALS AND METHODS
Viruses and diagnostic samples.
RNA or DNA extracted from different sample materials of animals infected experimentally with CSFV, ASFV, or FMDV, as well as samples containing different SuHV-1 isolates, were kindly provided by the respective German National Reference Laboratories. FMDV samples included strains of serotypes A, O, C, Asia, and SAT1, and SuHV-1 samples comprised strains Bartha (K 61, delta EG 211, and delta EO b212), Kaplan, and 14 different German and Slovak isolates from swine and wild boar. Clinical samples, such as nasal swabs, sera, and lung tissues, were obtained from different pig herds localized in the federal states Lower Saxony and Brandenburg, Germany. The diagnostic samples were stored at −70°C until use. In total, 238 body fluid and tissue samples and 17 virus isolates (cell culture supernatant) were tested. All of them are listed in Table S1 in the supplemental material.
DNA and RNA extraction.
Nucleic acids were extracted using the MagNA Pure LC total nucleic acid isolation kit for automated extraction (Roche Diagnostics Deutschland GmbH, Mannheim, Germany) according to the manufacturer's recommendations.
Primers, probes, and real-time PCR.
The PCV2-specific (27), CSFV-specific (28), and beta-actin-specific (29) real-time quantitative PCR (qPCR) assays have been described previously. PRRSV genome detection was performed as a combination of one assay specific for the EU genotype and another assay detecting NA genotype-specific sequences (30).
The ASFV-specific qPCR assay developed by Zsak and coworkers (31) was slightly modified. Instead of the previously described labeling with 5′-6-carboxyfluorescein (FAM) and a 3′ minor grove binder nonfluorescent quencher, a Texas Red-labeled locked nucleic acid (LNA) probe was used. For the design of the FMDV and SuHV-1 specific systems, all available sequence information (NCBI database) was used.
Sequences of the selected primers and probes are shown in Table S2 in the supplemental material. In each assay, concentrations of primers and probe were optimized in a single-target reverse transcription (RT)-qPCR approach. The multiplex RT-qPCR was performed using reduced concentrations of oligonucleotides (see Table S2). In the single-tube multiplex assay, a total of four different fluorophores were included: (i) FAM for both PRRSV-specific assays, (ii) Cy5 for the PCV2 assay, (iii) Texas Red for the probes specific for CSFV, ASFV, SuHV-1, and FMDV, and (iv) finally, HEX for the β-actin-specific probe of the internal control (IC) system. All oligonucleotides were synthesized by metabion international AG (Planegg-Martinsried, Germany).
The multiplex RT-qPCR was carried out using the AgPath-ID one-step RT-PCR kit (Applied Biosystems, Warrington, Cheshire, United Kingdom). The assay was optimized using a total reaction volume of 25 μl. For a single reaction, 1.0 μl RNase-free water, 12.5 μl 2× RT-PCR buffer, 1.0 μl 25× RT-PCR enzyme mix, and the primers and probes in concentrations given in Table S2 in the supplemental material were merged as a master mix. Finally, 5 μl template was added, and the RT-qPCR was carried out in a Bio-Rad CFX 96 real-time detection system (Bio-Rad, Hercules, CA) using the following thermal profile: reverse transcription at 45°C for 10 min, PCR initial activation at 95°C for 10 min, and 45 cycles of a three-step cycling consisting of the denaturation at 95°C for 15 s, annealing at 57°C for 30 s, and extension at 72°C for 35 s. All samples were tested in duplicate.
Positive standards.
The PRRSV- and CSFV-specific positive standards have been described previously (28, 30). SuHV-1-, FMDV-, and ASF-specific primer and probe sequences were each cloned into a plasmid vector. The plasmid containing FMDV-specific sequences was in vitro transcribed using an SP6/T7 transcription kit (Roche Diagnostics Deutschland GmBH, Mannheim, Germany) according to the manufacturer's instructions. The T7-transcribed standard RNA was subsequently digested with DNase I (Qiagen GmbH, Hilden, Germany) and purified using the RNeasy kit (Qiagen GmbH, Hilden, Germany).
A PCV2-specific standard was designed by extracting DNA from a spleen sample and using PCR amplification with the primers ACCRGYGCACTTCGGBARCKGC and AATACTWACAGCRYACTTCTTTCG. The Platinum Taq DNA polymerase kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer's recommendations for the amplification reaction. The resulting amplicon, with a size of 1,776 bp, was subsequently excised, purified using the Qiaex II gel extraction kit (Qiagen, Hilden, Germany), and cloned into a plasmid vector (PCV2-standard DNA).
The concentration of each positive standard was determined by spectrophotometry, and the exact number of DNA or RNA molecules was calculated with an online software program available at http://www.molbiol.edu.ru/eng/scripts/01_07.html.
RESULTS
Sensitivity and specificity.
Single assays integrated in the newly developed multiplex RT-qPCR have been analyzed previously regarding both sensitivity and specificity (27–31). The sequences of all primers and probes included in the multiplex RT-qPCR were aligned with publically available sequence information (NCBI GenBank) with a special focus on porcine viruses. There was no indication of possible cross-reactions.
Using the newly designed FMDV-specific assay in a single- and multiplex approach, a wide range of sample materials obtained from animals infected experimentally with different strains of serotypes A, O, C, Asia, and SAT1 (see Table S1, sample ID 167 to 172 and 214 to 229, in the supplemental material) were tested and correctly identified. The SuHV-1-specific PCR was validated by testing a variety of isolates as well; each of them tested positive by the SuHV-1 assay included in the multiplex PCR (see Table S1, sample ID 230 and 238 to 255) and confirmed by the conventional PCR system described by Hasebe et al. (32) (data not shown).
The sensitivity of each assay in the multiplex approach was evaluated in comparison to the corresponding single-target system using 10-fold dilutions of genomes of appropriate culture-grown virus strains (PRRSV, CSFV, ASFV, FMDV, and SuHV-1) or a spleen sample (PCV2) (Table 1). In the assays specific for SuHV-1, PCV2, and PRRSV-EU, the last detectable dilution step was equivalent in the multiplex and the single-target RT-qPCR (Table 1).
Table 1.
Comparison of single-target assay with multiplex RT-qPCR assay
| Virus | Dilution | Cq |
||||
|---|---|---|---|---|---|---|
| Single-target RT-qPCR | Multiplex RT-qPCR |
|||||
| PRRSV | PCV-2 | CSFV/ASFV/SuHV-1/FMDV | Beta-actin | |||
| PRRSV EU | 1.00E−02 | 26.3 | 25.9 | No Cq | No Cq | 28.9 |
| 1.00E−03 | 29.9 | 28.9 | No Cq | No Cq | 28.8 | |
| 1.00E−04 | 33.0 | 31.8 | No Cq | No Cq | 29.1 | |
| 1.00E−05 | 36.6 | 35.4 | No Cq | No Cq | 28.8 | |
| 1.00E−06 | 38.3 | 41.0a | No Cq | No Cq | 28.8 | |
| 1.00E−07 | No Cq | No Cq | No Cq | No Cq | 28.7 | |
| 1.00E−08 | No Cq | No Cq | No Cq | No Cq | 29.0 | |
| PRRSV NA | 1.00E−02 | 21.2 | 21.9 | No Cq | No Cq | 27.7 |
| 1.00E−03 | 24.8 | 25.6 | No Cq | No Cq | 28.6 | |
| 1.00E−04 | 27.5 | 28.7 | No Cq | No Cq | 28.7 | |
| 1.00E−05 | 31.1 | 34.3 | No Cq | No Cq | 28.5 | |
| 1.00E−06 | 34.3 | No Cq | No Cq | No Cq | 28.5 | |
| 1.00E−07 | 37.0 | No Cq | No Cq | No Cq | 28.9 | |
| 1.00E−08 | No Cq | No Cq | No Cq | No Cq | 29.0 | |
| PCV2 | 1.00E−02 | 19.2 | No Cq | 19.0 | No Cq | 28.7 |
| 1.00E−03 | 22.3 | No Cq | 22.2 | No Cq | 28.5 | |
| 1.00E−04 | 26.1 | No Cq | 25.9 | No Cq | 29.0 | |
| 1.00E−05 | 28.9 | No Cq | 28.6 | No Cq | 28.8 | |
| 1.00E−06 | 31.3 | No Cq | 31.5 | No Cq | 29.0 | |
| 1.00E−07 | 35.7 | No Cq | 37.9 | No Cq | 29.1 | |
| 1.00E−08 | No Cq | No Cq | No Cq | No Cq | 29.2 | |
| CSFV | 1.00E−03 | 22.1 | No Cq | No Cq | 23.3 | 28.7 |
| 1.00E−04 | 26.0 | No Cq | No Cq | 26.2 | 28.9 | |
| 1.00E−05 | 29.8 | No Cq | No Cq | 30.1 | 29.0 | |
| 1.00E−06 | 33.1 | No Cq | No Cq | 33.9 | 28.9 | |
| 1.00E−07 | 36.6 | No Cq | No Cq | 44.4a | 29.0 | |
| 1.00E−08 | 39.2 | No Cq | No Cq | No Cq | 29.0 | |
| 1.00E−09 | No Cq | No Cq | No Cq | No Cq | 29.1 | |
| ASFV | 1.00E−01 | 23.5 | No Cq | No Cq | 23.9 | 28.2 |
| 1.00E−02 | 26.8 | No Cq | No Cq | 27.3 | 28.5 | |
| 1.00E−03 | 30.2 | No Cq | No Cq | 30.6 | 28.8 | |
| 1.00E−04 | 34.1 | No Cq | No Cq | 34.3 | 28.6 | |
| 1.00E−05 | 36.7 | No Cq | No Cq | 38.5 | 28.6 | |
| 1.00E−06 | 38.8 | No Cq | No Cq | No Cq | 28.9 | |
| 1.00E−07 | No Cq | No Cq | No Cq | No Cq | 29.0 | |
| SuHV-1 | 1.00E−03 | 19.6 | No Cq | No Cq | 19.8 | 28.3 |
| 1.00E−04 | 22.9 | No Cq | No Cq | 23.0 | 28.4 | |
| 1.00E−05 | 26.2 | No Cq | No Cq | 26.5 | 29.0 | |
| 1.00E−06 | 29.4 | No Cq | No Cq | 29.6 | 28.9 | |
| 1.00E−07 | 33.2 | No Cq | No Cq | 33.1 | 28.8 | |
| 1.00E−08 | 36.0 | No Cq | No Cq | 36.6 | 29.0 | |
| 1.00E−09 | 39.3 | No Cq | No Cq | 42.6 | 29.1 | |
| FMDV | 1.00E−04 | 25.5 | No Cq | No Cq | 28.7 | 28.5 |
| 1.00E−05 | 28.5 | No Cq | No Cq | 31.3 | 28.5 | |
| 1.00E−06 | 31.4 | No Cq | No Cq | 35.3 | 28.4 | |
| 1.00E−07 | 34.4 | No Cq | No Cq | No Cq | 28.3 | |
| 1.00E−08 | No Cq | No Cq | No Cq | No Cq | 28.3 | |
Only one of the duplicates scored positive.
In the PCR systems for CSFV, ASFV, and FMDV, only the last dilution step positive in the single-target assay scored negative in the multiplex assay, and the PRRSV-NA system gave a negative result in the last two dilution steps that was positive in the corresponding single-target assay (Table 1).
In addition, the functionality of the four transboundary disease assays, namely, CSFV, ASFV, SuHV-1, and FMDV, was validated in the presence of very high loads of PRRSV and/or PCV2 genomes. Interestingly, no further decrease in sensitivity was caused by the multiplexing itself in any of the tested virus combinations (Table 2).
Table 2.
Comparison of single-target assay with multiplex RT-qPCR assay using samples with high genome loads of PCV2 and PRRSV and decreasing concentrations of CSFV, ASFV, SuHV-1, and FMDV
| Virus and/or combination | Dilution | Cq |
||||
|---|---|---|---|---|---|---|
| Single-target RT-qPCR, CSFV/ASFV/SuHV-1/FMDV | Multiplex RT-qPCR |
|||||
| CSFV/ASFV/SuHV-1/FMDV | PRRSV | pCV-2 | Beta-actin | |||
| CSFV | 1.00E−04 | 26.4 | 26.8 | No Cq | 14.3 | 28.9 |
| +PCV2 | 1.00E−05 | 30.0 | 28.8 | No Cq | 13.9 | 29.2 |
| 1.00E−06 | 33.2 | 34.8 | No Cq | 14.1 | 28.9 | |
| 1.00E−07 | 36.7 | 43.1a | No Cq | 14.1 | 28.8 | |
| +PRRSV | 1.00E−04 | 26.6 | 27.3 | 20.4 | No Cq | 27.5 |
| 1.00E−05 | 29.5 | 30.2 | 20.5 | No Cq | 27.9 | |
| 1.00E−06 | 33.7 | 35.7 | 20.3 | No Cq | 28.2 | |
| 1.00E−07 | 37.2 | No Cq | 20.3 | No Cq | 28.0 | |
| +PCV2 | 1.00E−04 | 27.3 | 27.8 | 20.3 | 13.7 | 28.7 |
| +PRRSV | 1.00E−05 | 30.5 | 32.0 | 20.2 | 13.6 | 28.7 |
| 1.00E−06 | 34.1 | 40.1 | 20.2 | 13.6 | 28.9 | |
| 1.00E−07 | 37.0 | No Cq | 20.2 | 13.6 | 28.6 | |
| ASFV | 1.00E−03 | 29.5 | 30.1 | No Cq | 14.3 | 28.6 |
| +PCV2 | 1.00E−04 | 31.8 | 33.0 | No Cq | 14.2 | 28.7 |
| 1.00E−05 | 36.0 | 36.2 | No Cq | 14.1 | 29.0 | |
| 1.00E−06 | 38.6a | No Cq | No Cq | 14.1 | 28.8 | |
| +PRRSV | 1.00E−03 | 30.0 | 30.6 | 20.2 | No Cq | 27.7 |
| 1.00E−04 | 33.1 | 33.8 | 20.2 | No Cq | 27.9 | |
| 1.00E−05 | 36.3 | 38.9 | 20.3 | No Cq | 27.9 | |
| 1.00E−06 | No Cq | No Cq | 20.2 | No Cq | 27.9 | |
| +PCV2 | 1.00E−03 | 29.7 | 31.0 | 20.9 | 13.6 | 29.0 |
| +PRRSV | 1.00E−04 | 33.2 | 34.6 | 20.2 | 13.8 | 28.6 |
| 1.00E−05 | 36.2 | 39.7 | 20.1 | 13.7 | 28.7 | |
| 1.00E−06 | No Cq | No Cq | 20.2 | 13.6 | 28.6 | |
| SuHV-1 | 1.00E−06 | 27.1 | 26.7 | No Cq | 14.2 | 29.0 |
| +PCV2 | 1.00E−07 | 29.6 | 29.7 | No Cq | 14.1 | 29.6 |
| 1.00E−08 | 34.3 | 34.3 | No Cq | 14.2 | 29.0 | |
| 1.00E−09 | No Cq | No Cq | No Cq | 13.9 | 29.3 | |
| +PRRSV | 1.00E−06 | 26.6 | 26.7 | 20.4 | No Cq | 28.1 |
| 1.00E−07 | 30.2 | 30.0 | 20.4 | No Cq | 28.2 | |
| 1.00E−08 | 33.9 | 36.1 | 20.6 | No Cq | 28.3 | |
| 1.00E−09 | No Cq | No Cq | 20.3 | No Cq | 28.1 | |
| +PCV2 | 1.00E−06 | 27.7 | 27.3 | 19.9 | 13.9 | 27.8 |
| +PRRSV | 1.00E−07 | 31.3 | 31.3 | 19.9 | 13.7 | 28.2 |
| 1.00E−08 | 35.6 | 35.7 | 20.0 | 13.8 | 27.9 | |
| 1.00E−09 | No Cq | No Cq | 19.8 | 13.8 | 28.6 | |
| FMDV | 1.00E−04 | 25.5 | 27.6 | No Cq | 13.6 | 28.6 |
| +PCV2 | 1.00E−05 | 29.3 | 31.3 | No Cq | 12.9 | 28.7 |
| 1.00E−06 | 32.4 | 35.4 | No Cq | 13.5 | 29.1 | |
| 1.00E−07 | 34.8 | 39.7a | No Cq | 14.4 | 29.0 | |
| +PRRSV | 1.00E−04 | 25.8 | 28.6 | 20.2 | No Cq | 27.7 |
| 1.00E−05 | 28.8 | 31.9 | 20.4 | No Cq | 28.1 | |
| 1.00E−06 | 31.7 | 35.6 | 20.7 | No Cq | 27.8 | |
| 1.00E−07 | 35.5 | No Cq | 20.8 | No Cq | 27.8 | |
| +PCV2 | 1.00E−04 | 26.0 | 28.5 | 19.6 | 13.8 | 28.5 |
| +PRRSV | 1.00E−05 | 28.5 | 32.5 | 19.7 | 13.9 | 28.6 |
| 1.00E−06 | 31.7 | 37.2 | 19.6 | 13.9 | 28.6 | |
| 1.00E−07 | 36.9 | No Cq | 19.7 | 13.9 | 28.6 | |
Only one of the duplicates scored positive.
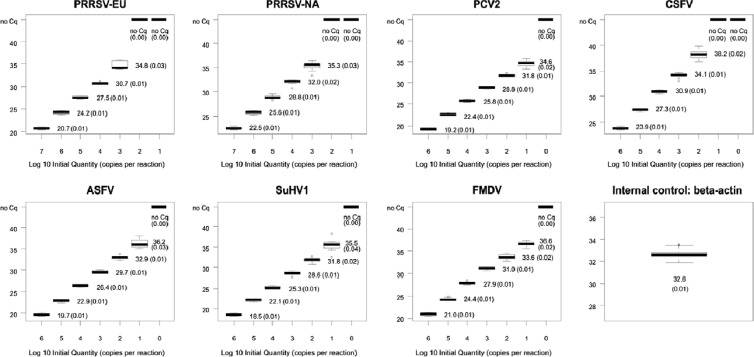
The analytical sensitivity of the complete multiplex RT-qPCR system was determined using series of 10-fold dilutions of the individual positive standards (diluted in RNA safe buffer [33]) in three replicates per run on three different days. RNA extracted from porcine EDTA-blood was included in the master mixture to test for the influence of porcine DNA and RNA. In the multiplex approach, 1.00E+01 copies of the PCV2, ASFV, SuHV-1, and FMDV standards, 1.00E+02 copies of CSFV, and 1.00E+03 PRRSV-EU and -NA copies per reaction were reliably detected. The mean quantification cycle (Cq) (34) value of the IC was 32.6, with a coefficient of variation of 0.1 (Fig. 1).
Fig 1.
Analytical sensitivities of the assays included in the multiplex RT-qPCR based on 10-fold dilution series of positive standard RNA. Mean Cq values of the nine replicates of each dilution step are indicated alongside each box plot; coefficients of variation are depicted in parentheses. Box plots were designed by using the R software package (55).
Finally, to exclude unspecific reactions, nucleic acids extracted from sera, pools of sera, EDTA-blood samples, nasal swabs, saliva samples, vesicles, and different tissue homogenates collected from healthy pigs were tested. All samples scored negative in any of the assays included in the multiplex RT-qPCR (see Table S1, sample ID 01-52, in the supplemental material).
Diagnostic samples.
The diagnostic sensitivity of the multiplex assay was evaluated by testing a number of different sample types, including sera, pools of sera, EDTA-blood samples, tonsils, saliva, cerebrum tissues, nasal swabs, and lung tissues (see Table S1). In total, 255 individual samples were tested by the multiplex RT-qPCR and in some instances in the respective singleplex assays. Overall, a high accordance could be observed between the multiplex system and each single-target PCR assay for the clinical samples. However, only one serum sample tested positive in the singleplex PRRSV-NA assay, with a Cq value of 35.0, and in a nasal swab very weakly positive for PCV2 (Cq of 39.6), the corresponding assays within the multiplex approach failed (see Table S1, sample ID 87 and 213, in the supplemental material).
Serum samples obtained from animals experimentally coinfected with a CSFV isolate and a PRRSV-EU isolate (35) were investigated using the multiplex RT-qPCR system. Fifteen pigs were assigned to three groups. Five animals (PRRS/CSF group) were inoculated with PRRSV and infected 3 days later with CSFV (see Table S1, sample ID 88 to 114, in the supplemental material), the second group of five animals (PRRS group, sample ID 115 to 129) received PRRSV, while the third group (CSF group, sample ID 130 to 158) was inoculated solely with CSFV. In a serum sample of one animal out of the PRRS/CSF group, the PCV2 genome was additionally detected at the day of CSFV infection, followed by increasing genome loads until the end of the study (see Table S1, sample ID 105 to 109). In two animals out of the PRRSV/CSF and CSF groups, respectively, the CSFV genome was first detected at 7 days postinfection (dpi) (see Table S1, sample ID 107, 112, 139, and 157), while the remaining three individuals of both groups showed positive results at 4 dpi for the first time (see Table S1, sample ID 90, 95, 100, 132, 144, and 150). Using the multiplex RT-qPCR, the PRRSV genome was detected in samples from every individual swine 3 days after PRRSV inoculation (see Table S1, sample ID 116, 119, 122, 125, and 128). In serum samples of these animals, CSFV RNA was not detected at any time.
Different sample materials from cattle, sheep, and goats were also tested using the multiplex system. The PRRSV or PCV2 genome was not detected; solitary exceptions were two PCV2-positive bovine lingual vesicles from one animal (see Table S1, sample ID 214 and 215, in the supplemental material) experimentally infected with FMDV. Nasal swabs and saliva collected on the same day as the lingual vesicles, and the serum (see Table S1, sample ID 228, 229, 220, 221, and 172) from the same animal were negative in the PCV2-specific assay.
Using the multiplex RT-qPCR assay, PCV2 genomes were detected in serum samples and tonsils collected from wild boar (see Table S1, sample ID 161, 231, and 233 to 236), with one boar showing a Cq value below 10.
By testing numerous negative sample materials, unspecific reactions could not be observed. In addition, with the exception of two serum samples, the tested panel of body fluids and tissues was positive in the β-actin-specific internal control assay.
DISCUSSION
Based on clinical and postmortem examination, several viral infections of swine are hard to distinguish from each other, which emphasizes the necessity of adequate laboratory diagnostics. In addition, due to increasing animal transport, human traveling, and global trade, the emergence of transboundary diseases in free countries is a serious risk (19).
To prevent the spread of transboundary diseases into large geographic areas, which thereby affects animal populations of high density, especially after sudden reemergence in free countries, the rapid diagnosis of the causative agent is of utmost importance (36). By using multiplex PCR systems, several infectious agents can be detected and differentiated simultaneously in a single reaction, reducing costs and efforts as well as the amount of sample material and time required (37, 38). A variety of conventional multiplex PCR assays for the detection and differentiation of different swine viruses based on amplicon size have been developed during recent years (39–46). However, qPCR assays have several advantages compared to conventional PCR, combining a reduced risk of cross-contamination with a high sensitivity and the possibility of semiquantitative analysis. Oligonucleotide probes labeled with different fluorophores permit multiplexing in a qPCR format (47), enabling the detection of different target sequences as well as the coamplification of internal controls.
Here, an RT-qPCR assay for the simultaneous detection of PRRSV and PCV2, combined with an early detection system for diseases notifiable to the World Organization for Animal Health, namely, those caused by CSFV, ASFV, SuHV-1, and FMDV, was developed and validated. In order to verify efficient nucleic acid extraction and to confirm the absence of PCR-inhibiting factors, an internal control based on the β-actin gene was included, completing the four-color multiplex RT-qPCR.
The probes specific for genome detection of the four notifiable viruses were labeled with the same fluorophore (Texas Red), resulting in a background screening for those diseases and a Texas Red signal in case of a positive genome detection for one of the transboundary diseases. The Texas Red signal would subsequently induce the immediate testing of the sample in the respective national or international reference institution. The multiplex approach could be also very valuable in situations where, after positive diagnosis of an endemic viral infection within a single target assay, potential coinfections with a notifiable disease remain initially undetected. Another advantage of the single-color multiplex screening for transboundary diseases is the possibility of including a detection system for further pathogens. Viruses causing vesicular lesions in epithelial tissues (vesicular stomatitis virus, swine vesicular disease virus, and vesicular exanthema of swine virus) may be included, for instance, to complete the assay. However, considerable validation of such complex assays, including international interlaboratory studies, will be necessary.
The newly developed multiplex RT-qPCR assay offers a rapid, convenient, and reliable screening system for FMDV, CSFV, ASFV, and SuHV-1, even in the presence of large amounts of PCV2 and PRRSV genomes. In serum samples obtained from animals infected experimentally with CSFV, the multiplex RT-qPCR gave a positive result at 4 or 7 dpi, and in case of a PRRSV/CSFV coinfection, the CSFV genome was first detected at 4dpi. Since CSFV could be isolated from sample materials on 7 or 12 dpi in the CSF group and on 4 or 7 dpi in the PRRS/CSF group (35), sensitivity for CSFV in the multiplex approach is equal to or even greater than the sensitivity of virus isolation, emphasizing the benefits of the qPCR, especially in the early stages of an infection. Due to the labeling of the probes specific for the notifiable diseases with the identical fluorophore, a fluorescence signal in this channel requires further differentiation using the PCR systems in single-target approaches to receive the final diagnosis.
One of the main problems caused by a large number of oligonucleotides in the same reaction tube is a possible interaction of those molecules with each other, resulting in inhibition of the amplification reactions and a subsequent reduced sensitivity (38, 48, 49). In our assays, despite the minor decrease in sensitivity, 1,000 genome copies per reaction or less were reliably detected by each of the assays included in the multiplex RT-qPCR. With the exception of only two samples, which were very weakly positive for PRRSV-NA or PCV2 in the single-target assays, for each of the large number of diagnostic samples, similar results in both the single and multiplex approaches were achieved.
Developed primarily for viral genome detection from serum or blood samples from diseased swine and wild boar, the newly developed multiplex RT-qPCR has been shown to also be applicable to additional sample materials and animal species, such as cattle, sheep, or goats. When samples from cattle infected with FMDV were investigated, one animal scored positive for PCV2. Although nasal swabs and saliva collected on the same day and serum from the animal scored negative in the PCV2-specific assay, lingual vesicles were positive both in the single-target assay and in the multiplex system. Interestingly, the presence of PCV2-like isolates has been found in cattle previously (50–52), though this observation was not confirmed by other authors (53, 54). Recently, PCV2 has been detected in German calves affected with hemorrhagic disease syndrome (HDS) (50). The example shows the versatile use of the novel PCR system, which is not only for porcine sample materials.
In conclusion, the newly developed RT-qPCR allows the simultaneous detection of PRRSV and PCV2 combined in a single-tube assay with a rapid, convenient, and reliable screening system for FMDV, CSFV, ASFV, and SuHV-1. The novel broad-spectrum multiplex assay allows, in a unique form, the routine investigation for endemic porcine pathogens with exclusion diagnostics of the most important transboundary diseases. The new system could therefore significantly improve the early detection of the most important notifiable diseases of swine and could lead to a new approach in syndromic surveillance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christian Korthase and Patrick Zitzow for excellent technical assistance and Donata Kalthoff for critically reading the manuscript.
This work was supported by SAB, Sächsische Aufbaubank-Förderbank.
Footnotes
Published ahead of print 9 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02947-12.
REFERENCES
- 1. Allan GM, Ellis JA. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 12:3–14 [DOI] [PubMed] [Google Scholar]
- 2. Segales J, Calsamiglia M, Rosell C, Soler M, Maldonado J, Martin M, Domingo M. 2002. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Vet. Microbiol. 85:23–30 [DOI] [PubMed] [Google Scholar]
- 3. Cavanagh D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629–633 [PubMed] [Google Scholar]
- 4. Rossow KD. 1998. Porcine reproductive and respiratory syndrome. Vet. Pathol. 35:1–20 [DOI] [PubMed] [Google Scholar]
- 5. Nelsen CJ, Murtaugh MP, Faaberg KS. 1999. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 73:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Segales J, Allan GM, Domingo M. 2005. Porcine circovirus diseases. Anim. Health Res. Rev. 6:119–142 [DOI] [PubMed] [Google Scholar]
- 7. Tischer I, Gelderblom H, Vettermann W, Koch MA. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64–66 [DOI] [PubMed] [Google Scholar]
- 8. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. 1999. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 121:1–11 [DOI] [PubMed] [Google Scholar]
- 9. Harding JCS, Clark ETG, Strokappe JH, Willson PI, Ellis JH. 1998. Postweaning multisystemic wasting syndrome: epidemiology and clinical presentation. Swine Health Prod. 6:249–254 [Google Scholar]
- 10. Ladekjaer-Mikkelsen AS, Nielsen J, Stadejek T, Storgaard T, Krakowka S, Ellis J, McNeilly F, Allan G, Botner A. 2002. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and non-immunostimulated 3-week-old piglets experimentally infected with porcine circovirus type 2 (PCV2). Vet. Microbiol. 89:97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segales J, Domingo M. 2002. Postweaning multisystemic wasting syndrome (PMWS) in pigs. A review. Vet. Q. 24:109–124 [DOI] [PubMed] [Google Scholar]
- 12. Wellenberg GJ, Stockhofe-Zurwieden N, de Jong MF, Boersma WJ, Elbers AR. 2004. Excessive porcine circovirus type 2 antibody titres may trigger the development of porcine dermatitis and nephropathy syndrome: a case-control study. Vet. Microbiol. 99:203–214 [DOI] [PubMed] [Google Scholar]
- 13. Sanchez RE, Jr, Nauwynck HJ, McNeilly F, Allan GM, Pensaert MB. 2001. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Vet. Microbiol. 83:169–176 [DOI] [PubMed] [Google Scholar]
- 14. Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69:462–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kluge JP, Mare CJ. 1974. Swine pseudorabies: abortion, clinical disease, and lesions in pregnant gilts infected with pseudorabies virus (Aujeszky's disease). Am. J. Vet. Res. 35:991–995 [PubMed] [Google Scholar]
- 16. Zuckermann FA. 2000. Aujeszky's disease virus: opportunities and challenges. Vet. Res. 31:121–131 [DOI] [PubMed] [Google Scholar]
- 17. Thiel H-J, Collett MS, Gould EA, Heinz FX, Houghton M, Meyers G, Purcell RH, Rice CM. 2005. Family Flaviviridae, p 979–996 In Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (ed), Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 18.Murphy FA, Gibbs EP, Horzinek MC, Studdert MJ. 1999. Asfarviridae and Iridoviridae, p 293–300 In Murphy FA, Gibbs EP, Horzinek MC, Studdert MJ. (ed), Veterinary virology. Academic Press, London, United Kingdom [Google Scholar]
- 19. Kleiboeker SB. 2002. Swine fever: classical swine fever and African swine fever. Vet. Clin. North Am. Food Anim. Pract. 18:431–451 [DOI] [PubMed] [Google Scholar]
- 20. Mebus CA. 1988. African swine fever. Adv. Virus Res. 35:251–269 [DOI] [PubMed] [Google Scholar]
- 21. Moennig V, Floegel-Niesmann G, Greiser-Wilke I. 2003. Clinical signs and epidemiology of classical swine fever: a review of new knowledge. Vet. J. 165:11–20 [DOI] [PubMed] [Google Scholar]
- 22. Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2:e526 doi:10.1371/journal.pone.0000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng Y, Zhao T, Nguyen T, Inui K, Ma Y, Nguyen TH, Nguyen VC, Liu D, Bui QA, To LT, Wang C, Tian K, Gao GF. 2008. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg. Infect. Dis. 14:1774–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Normile D. 2007. China, Vietnam grapple with ‘rapidly evolving’ pig virus. Science 317:1017. [DOI] [PubMed] [Google Scholar]
- 25. Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scudamore JM, Harris DM. 2002. Control of foot and mouth disease: lessons from the experience of the outbreak in Great Britain in 2001. Rev. Sci. Tech. 21:699–710 [DOI] [PubMed] [Google Scholar]
- 27. Brunborg IM, Moldal T, Jonassen CM. 2004. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods 122:171–178 [DOI] [PubMed] [Google Scholar]
- 28. Leifer I, Blome S, Beer M, Hoffmann B. 2011. Development of a highly sensitive real-time RT-PCR protocol for the detection of classical swine fever virus independent of the 5′ untranslated region. J. Virol. Methods 171:314–317 [DOI] [PubMed] [Google Scholar]
- 29. Toussaint JF, Sailleau C, Breard E, Zientara S, De Clercq K. 2007. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 140:115–123 [DOI] [PubMed] [Google Scholar]
- 30. Wernike K, Hoffmann B, Dauber M, Lange E, Schirrmeier H, Beer M. 2012. Detection and typing of highly pathogenic porcine reproductive and respiratory syndrome virus by multiplex real-time rt-PCR. PLoS One 7:e38251 doi:10.1371/journal.pone.0038251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zsak L, Borca MV, Risatti GR, Zsak A, French RA, Lu Z, Kutish GF, Neilan JG, Callahan JD, Nelson WM, Rock DL. 2005. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 43:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hasebe H, Wheeler JG, Osorio FA. 1993. Gene specific assay to differentiate strains of pseudorabies virus. Vet. Microbiol. 34:221–231 [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann B, Depner K, Schirrmeier H, Beer M. 2006. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 136:200–209 [DOI] [PubMed] [Google Scholar]
- 34. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 35. Depner KR, Lange E, Pontrakulpipat S, Fichtner D. 1999. Does porcine reproductive and respiratory syndrome virus potentiate classical swine fever virus infection in weaner pigs? Zentralbl. Veterinarmed. B 46:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belak S. 2007. Molecular diagnosis of viral diseases, present trends and future aspects: a view from the OIE Collaborating Centre for the Application of Polymerase Chain Reaction Methods for Diagnosis of Viral Diseases in Veterinary Medicine. Vaccine 25:5444–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards MC, Gibbs RA. 1994. Multiplex PCR: advantages, development, and applications. PCR Methods Appl. 3:S65–S75 [DOI] [PubMed] [Google Scholar]
- 38. Markoulatos P, Siafakas N, Moncany M. 2002. Multiplex polymerase chain reaction: a practical approach. J. Clin. Lab. Anal. 16:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aguero M, Fernandez J, Romero LJ, Zamora MJ, Sanchez C, Belak S, Arias M, Sanchez-Vizcaino JM. 2004. A highly sensitive and specific gel-based multiplex RT-PCR assay for the simultaneous and differential diagnosis of African swine fever and classical swine fever in clinical samples. Vet. Res. 35:551–563 [DOI] [PubMed] [Google Scholar]
- 40. Diaz de Arce H, Perez LJ, Frias MT, Rosell R, Tarradas J, Nunez JI, Ganges L. 2009. A multiplex RT-PCR assay for the rapid and differential diagnosis of classical swine fever and other pestivirus infections. Vet. Microbiol. 139:245–252 [DOI] [PubMed] [Google Scholar]
- 41. Fernandez J, Aguero M, Romero L, Sanchez C, Belak S, Arias M, Sanchez-Vizcaino JM. 2008. Rapid and differential diagnosis of foot-and-mouth disease, swine vesicular disease, and vesicular stomatitis by a new multiplex RT-PCR assay. J. Virol. Methods 147:301–311 [DOI] [PubMed] [Google Scholar]
- 42. Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. 2008. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J. Vet. Diagn. Invest. 20:545–558 [DOI] [PubMed] [Google Scholar]
- 43. Jiang Y, Shang H, Xu H, Zhu L, Chen W, Zhao L, Fang L. 2010. Simultaneous detection of porcine circovirus type 2, classical swine fever virus, porcine parvovirus and porcine reproductive and respiratory syndrome virus in pigs by multiplex polymerase chain reaction. Vet. J. 183:172–175 [DOI] [PubMed] [Google Scholar]
- 44. Liu S, Zhao Y, Hu Q, Lv C, Zhang C, Zhao R, Hu F, Lin W, Cui S. 2011. A multiplex RT-PCR for rapid and simultaneous detection of porcine teschovirus, classical swine fever virus, and porcine reproductive and respiratory syndrome virus in clinical specimens. J. Virol. Methods 172:88–92 [DOI] [PubMed] [Google Scholar]
- 45. Ogawa H, Taira O, Hirai T, Takeuchi H, Nagao A, Ishikawa Y, Tuchiya K, Nunoya T, Ueda S. 2009. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J. Virol. Methods 160:210–214 [DOI] [PubMed] [Google Scholar]
- 46. Yue F, Cui S, Zhang C, Yoon KJ. 2009. A multiplex PCR for rapid and simultaneous detection of porcine circovirus type 2, porcine parvovirus, porcine pseudorabies virus, and porcine reproductive and respiratory syndrome virus in clinical specimens. Virus Genes 38:392–397 [DOI] [PubMed] [Google Scholar]
- 47. Wittwer CT, Herrmann MG, Gundry CN, Elenitoba-Johnson KS. 2001. Real-time multiplex PCR assays. Methods 25:430–442 [DOI] [PubMed] [Google Scholar]
- 48. Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. 2000. Multiplex PCR: optimization and application in diagnostic virology. Clin. Microbiol. Rev. 13:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH. 1997. Multiplex PCR: critical parameters and step-by-step protocol. Biotechniques 23:504–511 [DOI] [PubMed] [Google Scholar]
- 50. Kappe EC, Halami MY, Schade B, Alex M, Hoffmann D, Gangl A, Meyer K, Dekant W, Schwarz BA, Johne R, Buitkamp J, Bottcher J, Muller H. 2010. Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl. Munch. Tierarztl. Wochenschr. 123:31–41 [PubMed] [Google Scholar]
- 51. Li L, Shan T, Soji OB, Alam MM, Kunz TH, Zaidi SZ, Delwart E. 2011. Possible cross-species transmission of circoviruses and cycloviruses among farm animals. J. Gen. Virol. 92:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nayar GP, Hamel AL, Lin L, Sachvie C, Grudeski E, Spearman G. 1999. Evidence for circovirus in cattle with respiratory disease and from aborted bovine fetuses. Can. Vet. J. 40:277–278 [PMC free article] [PubMed] [Google Scholar]
- 53. Ellis JA, Konoby C, West KH, Allan GM, Krakowka S, McNeilly F, Meehan B, Walker I. 2001. Lack of antibodies to porcine circovirus type 2 virus in beef and dairy cattle and horses in western Canada. Can. Vet. J. 42:461–464 [PMC free article] [PubMed] [Google Scholar]
- 54. Rodriguez-Arrioja GM, Segales J, Domingo M, Plana-Duran J. 2003. Lack of PCV-2 infection in non-porcine species in Spain. Vet. Rec. 153:371–372 [PubMed] [Google Scholar]
- 55.Development Core Team R 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.