Abstract
The introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in September 2006 has markedly reduced the burden of invasive pneumococcal disease (IPD) including meningitis in England and Wales. This study examined changes in the molecular epidemiology of pneumococcal isolates causing meningitis from July 2004 to June 2009. The Health Protection Agency conducts enhanced pneumococcal surveillance in England and Wales. In addition to serotyping, pneumococcal isolates causing meningitis were genotyped by multilocus sequence typing (MLST). A total of 1,030 isolates were both serotyped and genotyped over the 5-year period. Fifty-two serotypes, 238 sequence types (STs), and 87 clonal complexes were identified, with no significant difference in the yearly Simpson's diversity index values (range, 0.974 to 0.984). STs commonly associated with PCV7 serotypes declined following PCV implementation, with a proportionally greater decline in ST124 (commonly associated with serotype 14). No other ST showed significant changes in distribution, even within individual serotypes. Replacement disease following PCV7 introduction was mainly due to serotypes 1, 3, 7F, 19A, 22F, and 33F through clonal expansion. A single instance of possible capsule switching was identified where one ST4327 clone expressed a serotype 14 capsule in 2005 and a serotype 28A capsule in 2009. In 2008 to 2009, ST191 (7F) became the most prevalent clone causing meningitis (10.3%). Case fatality (145 fatalities/1,030 cases; 14.1%) was high across all age groups and serotype groups. Thus, the introduction of PCV7 resulted in an increase in non-PCV7 serotypes, including some not covered by the 13-valent vaccine, such as serotypes 22F and 33F, emphasizing the importance of long-term epidemiological and molecular surveillance.
INTRODUCTION
Streptococcus pneumoniae is the leading cause of acute bacterial meningitis in developed countries (1). Compared with other pathogens, pneumococci causing meningitis have the highest case fatality rates (CFRs), and up to 30% of survivors develop significant long-term sequelae, including sensorineural deafness and cerebral palsy (2). In England and Wales, the overall annual incidence of pneumococcal meningitis in 2003 to 2005 was 1 case/100,000 population, with the highest incidence in the first year of life, with a rate as high as 15/100,000 among 4- to 11-month-old children (3). The serotypes included in the 7-valent pneumococcal conjugate vaccine (PCV7) were responsible for 72% of pneumococcal meningitis cases in children of <2 years of age and for 57% of cases across all age groups (3).
In September 2006, the United Kingdom, unlike other countries, introduced PCV7 into the childhood immunization schedule at a reduced 2 + 1 schedule (immunization at 2, 4, and 13 months) with a 12-month catch-up campaign offering the vaccine to all children less than 2 years old (4). The vaccine was highly effective in rapidly reducing the incidence of invasive pneumococcal disease (IPD) across all age groups through a combination of direct and indirect (herd) protection (5). In addition to an overall 34% reduction in overall IPD in 2008 to 2010 compared with 2004 to 2006, PCV7-type pneumococcal meningitis cases decreased by 95% in children of <5 years of age (6). Moreover, because of indirect protection through carriage reduction among vaccinated children, PCV7-type pneumococcal meningitis cases also declined by 67% among 5- to 64-year-olds and by 70% among ≥65-year-olds (6). However, this reduction was offset by an increase in meningitis caused by non-PCV7 serotypes (77%, 54%, and 19% in the three age groups, respectively), such that the overall reduction in pneumococcal meningitis was only significant in the <5-year-old group (44% reduction; 95% confidence interval [CI], 11 to 64%) (6).
In the United States, where PCV7 was introduced in 2000, the overall incidence of pneumococcal meningitis declined by 30% from 1.13/100,000 population in 1998 to 1999 to 0.79/100,000 population in 2004 to 2005, with a 73% (0.66 to 0.18/100,000 population) decline in PCV7 serotype meningitis and a 61% (0.32 to 0.51/100,000 population) increase in non-PCV7 serotype meningitis over the same period (7). The greatest decline in overall pneumococcal meningitis was observed in children of <2 years of age (64%) followed by ≥65-year-olds (54%) (7).
Despite wide geographical variation in pneumococcal serotypes causing IPD, most countries with established PCV7 immunization programs have observed serotype replacement of various magnitudes in the age groups targeted for vaccination as well as in groups of older unvaccinated individuals (8). In the United States, there was a marked increase in IPD (including meningitis) due to serotype 19A, not only through expansion of the major pneumococcal lineage (sequence type 199 [ST199]) usually associated with this serotype but also because of emergence of “escape” strains that have switched their capsule type from a PCV7 serotype to non-PCV7 serotype (9, 10). This phenomenon, termed capsular switching (CS), is due to transfer of the capsular gene cluster via horizontal recombination and presumably occurs during carriage when strains expressing distinct capsule types cocolonize the nasopharynx (11).
Surveillance of IPD and characterization of pneumococcal strains causing invasive disease are essential for monitoring the beneficial effects of PCV7 implementation and its potential impact on the structure of the pneumococcal population. In anticipation of the introduction of PCV7 into the national childhood immunization program, the Health Protection Agency (HPA) implemented enhanced epidemiological and molecular surveillance of IPD in England and Wales in 2004 (5, 6). This prospective study investigated changes in the serotype and genotype distribution of pneumococci causing meningitis—the most severe clinical presentation of IPD, with the highest case fatality—in England and Wales prior to and during the first 3 years after the introduction of PCV7.
MATERIALS AND METHODS
The HPA conducts enhanced national surveillance of IPD in England and Wales, as described previously (6). Briefly, the HPA routinely collects computerized hospital laboratory reports of invasive pneumococcal isolates and provides a free national service for confirmation and serotyping of invasive pneumococcal isolates. A single IPD data set is maintained through regular reconciliation of laboratory reports with serotype information, patient demographics (http://www.connectingforhealth.nhs.uk/systemsandservices/demographics/pds) and death registrations obtained from the United Kingdom Office for National Statistics (www.statistics.gov.uk). In children eligible for PCV7, vaccination status was obtained from the child's general practitioner (GP). For this study, pneumococcal isolates submitted to the HPA from meningitis cases of patients of any age diagnosed between July 2004 and June 2009 were analyzed. Meningitis isolates were selected on the basis that they were cultured from cerebrospinal fluid (CSF) or from blood cultures with documented evidence of meningitis. Serotyping was performed using a pneumococcal antigen assay (12) and conventional methods, including slide agglutination using a full panel (including specific 6C antiserum) of pneumococcal typing antisera (Statens Serum Institut, Copenhagen, Denmark) or Quellung reaction. Repeat samples of the same serotype from the same patient within a 30-day period were considered to represent the same episode and were therefore excluded from subsequent analyses. Multilocus sequence typing (MLST) was carried out using a method described previously, and sequence types (STs) were allocated using the online MLST database (www.mlst.net) (13). Sequence data were processed using the Bionumerics pipeline (14). Genetic analysis was performed using Bionumerics software, version 6.1 (Applied Maths, Belgium) and the eBURST, version 3, program (15).
Data analysis.
Data were analyzed using Microsoft Excel and Stata, version 11.0 (Statcorp, TX). The introduction of PCV7 has resulted in large decreases in PCV7 serotype meningitis as well as increases in non-PCV7 serotype meningitis. Therefore, changes in individual serotype and ST distribution before (July 2004 to June 2006) and after (July 2007 to June 2009) PCV7 introduction were compared separately among PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F), the extra six PCV13 serotypes (1, 3, 5, 6A, 7F, and 19A), and the remaining PCV13 serotypes; prior to analysis, STs were grouped by the serotype they are usually associated with. The epidemiological year of 2006 to 2007 was considered a transition period and was excluded from comparison analyses. Because many of the non-PCV13 serotypes had too few isolates for analysis, those with less than eight isolates across the 5-year period were grouped together. STs were similarly grouped as PCV7 type, extra six PCV13 type, or non-PCV13 type after identification of the serotype they most commonly corresponded to. As well as assessing changes in the ST distribution within these serotype groups, changes were also assessed within individual serotypes. Because of the small number of isolates when serotypes and STs were subgrouped by age and epidemiological year, changes within age groups were analyzed only if a significant overall effect was observed. Proportions were compared using a chi-square test or Fisher's exact test as appropriate. Logistic regression was use to adjust for age. For the assessment of ST distribution within serotypes as well as CFR by serotype and ST, we report only changes with a P value of <0.01 because of the large number of comparisons.
RESULTS
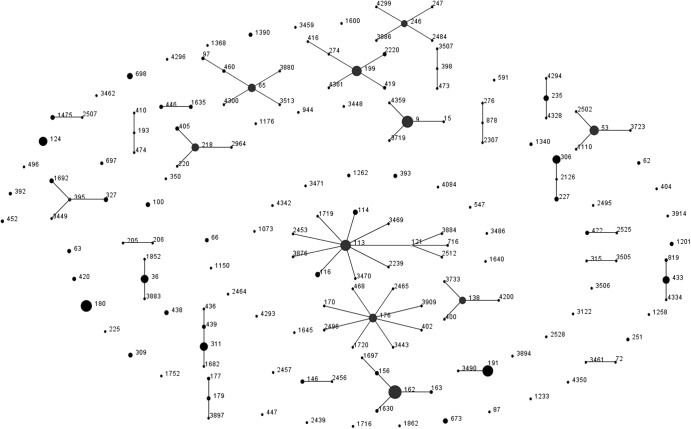
Between July 2004 and June 2009, 1,506 cases of meningitis across all age groups were identified, including 270 (17.9%) that were diagnosed by PCR alone and 206 (13.7%) that either did not have an isolate submitted to the HPA or for which the isolate could not be resuscitated for sequence typing. Therefore, 1,030 isolates were both serotyped and genotyped over the 5-year period, and they included 52 different serotypes and 238 distinct STs. The number of distinct STs detected per year ranged from 75 to 89. Overall, there was no significant difference in the yearly Simpson's diversity index (DI) values during the study period (DI range, 0.974 to 0.984). The genetic characteristics of the pneumococci changed over time, with several STs being detected during only a single epidemiological year over the 5-year period (25 STs detected in epidemiological year 2004 to 2005, 44 in 2005 to 2006, 34 in 2006 to 2007, 30 in 2007 to 2008, and 27 in 2008 to 2009). eBURST analyses clustered pneumococci into 87 clonal complexes (CC), defined as a group of STs sharing six out of seven loci, including 14 singletons (Fig. 1) (STs that are not genetically related to other STs and, therefore, not part of a clonal complex). Molecular analysis of the pneumococcal isolates revealed one potential vaccine escape strain due to possible capsular switching. One isolate of ST4327 was associated with serotype 14 in 2005, and one isolate of the same ST was serotyped as 28A in 2009. No previous report of ST4327 was found in the MLST database.
Fig 1.
eBURST diagram displaying genetic relatedness of pneumococcal sequence types (STs) causing meningitis across all age groups in England and Wales (2004 to 2009).
PCV7 serotypes.
Prior to PCV7 introduction (2004 to 2006), the four most common serotypes causing meningitis were serotypes 14 (13.0%), 19F (11.3%), 18C (9.1%), and 6B (8.6%). These serotypes are all included in PCV7 and accounted for 40% of all meningitis cases (Table 1). The next most common serotype, serotype 8, which is not contained in PCV7, accounted for 6.4% of all cases. Among PCV7 serotypes causing meningitis, the five most common clones in decreasing order were ST162, ST9, ST113, ST191, and ST124, with each ST accounting for between 3.1% and 5.1%. By 2007 to 2009, PCV7 serotypes accounted for a significantly smaller proportion of meningitis cases compared with 2004 to 2006 (P < 0.001) (Fig. 2). In 2008 to 2009, only nine meningitis cases were caused by PCV7 serotypes in children of <5 years of age, mainly due to serotype 6B (five cases, each with a distinct ST) and one case each of serotypes 4, 14, 19F, and 23F. Three cases had received at least two PCV7 doses, and only one child had significant comorbidity (cochlear implant) at the time of the infection). Over the study period, there were 11 vaccine failures: six cases were caused by 6B (each with a different ST), two each by serotypes 9V (ST163) and 23F (ST36) and one by serotype 4 (ST246).
Table 1.
Changes in pneumococcal serotypes causing meningitis across all age groups in England and Wales between July 2004 and June 2009
| Serotype group and individual serotypes | No. of isolates by epidemiological yeara |
|||||
|---|---|---|---|---|---|---|
| 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | Total | |
| All serotypes | 174 (72) | 234 (108) | 191 (90) | 199 (66) | 232 (92) | 1,030 (428) |
| PCV7 | ||||||
| 4 | 8 (3) | 13 (3) | 6 (1) | 0 (0) | 5 (1) | 32 (8) |
| 6B | 17 (12) | 18 (15) | 10 (8) | 20 (9) | 8 (5) | 73 (49) |
| 9V | 8 (3) | 7 (2) | 9 (4) | 9 (0) | 2 (0) | 35 (9) |
| 14 | 21 (11) | 32 (25) | 18 (12) | 6 (1) | 2 (1) | 79 (50) |
| 18C | 10 (4) | 27 (13) | 18 (10) | 8 (3) | 10 (0) | 73 (30) |
| 19F | 20 (13) | 26 (17) | 16 (10) | 8 (2) | 6 (1) | 76 (43) |
| 23F | 13 (5) | 11 (7) | 13 (4) | 6 (2) | 9 (1) | 52 (19) |
| Total for group | 97 (51) | 134 (82) | 90 (49) | 57 (17) | 42 (9) | 420 (208) |
| Extra PCV13 | ||||||
| 1 | 4 (1) | 11 (4) | 7 (5) | 12 (4) | 12 (6) | 46 (20) |
| 3 | 6 (1) | 11 1) | 8 (0) | 17 (3) | 15 (3) | 57 (8) |
| 5 | 0 (0) | 0 (0) | 1 (0) | 1 (1) | 2 (2) | 4 (3) |
| 6A | 9 (6) | 8 (4) | 8 (4) | 10 (2) | 8 (2) | 43 (18) |
| 7F | 7 (1) | 14 (6) | 15 (7) | 16 (9) | 24 (16) | 76 (39) |
| 19A | 8 (3) | 5 (4) | 5 (3) | 7 (5) | 16 (12) | 41 (27) |
| Total for group | 34 (12) | 49 (19) | 44 (19) | 63 (24) | 77 (41) | 267 (115) |
| Non-PCV13 | ||||||
| 6C | 3 (2) | 0 (0) | 6 (3) | 5 (0) | 5 (1) | 19 (6) |
| 8 | 12 (3) | 14 (2) | 13 (2) | 6 (1) | 13 (6) | 58 (14) |
| 9N | 1 (0) | 4 (0) | 3 (0) | 5 (1) | 2 (0) | 15 (1) |
| 10A | 1 (0) | 4 (0) | 0 (0) | 2 (1) | 4 (2) | 11 (3) |
| 11A | 0 (0) | 2 (0) | 2 (0) | 2 (0) | 2 (1) | 8 (1) |
| 12F | 4 (1) | 10 (0) | 5 (3) | 9 (2) | 8 (2) | 36 (8) |
| 15B | 1 (0) | 0 (0) | 4 (4) | 4 (2) | 4 (0) | 13 (6) |
| 15C | 1 (0) | 1 (0) | 3 (2) | 2 (1) | 8 (4) | 15 (7) |
| 20 | 1 (0) | 2 (0) | 2 (1) | 3 (1) | 5 (0) | 13 (2) |
| 22F | 3 (1) | 2 (0) | 1 (1) | 14 (4) | 21 (8) | 41 (14) |
| 23A | 2 (0) | 2 (0) | 1 (0) | 3 (0) | 4 (1) | 12 (1) |
| 23B | 0 (0) | 1 (0) | 0 (0) | 3 (1) | 6 (3) | 10 (4) |
| 27 | 1 (1) | 1 (1) | 2 (1) | 0 (0) | 5 (5) | 9 (8) |
| 33F | 3 (1) | 0 (0) | 2 (1) | 7 (5) | 11 (5) | 23 (12) |
| 35F | 2 (0) | 4 (2) | 2 (1) | 2 (1) | 3 (1) | 13 (5) |
| 38 | 1 (0) | 0 (0) | 2 (1) | 3 (2) | 2 (1) | 8 (4) |
| Otherb | 7 (0) | 4 (2) | 9 (2) | 9 (3) | 10 (2) | 39 (9) |
| Total for group | 43 (9) | 51 (7) | 57 (22) | 79 (25) | 113 (42) | 343 (105) |
Values in parenthesis represent isolates from pneumococcal meningitis cases in children of <5 years of age.
Non-PCV13 serotypes with fewer than eight isolates over the 5-year period.
Fig 2.
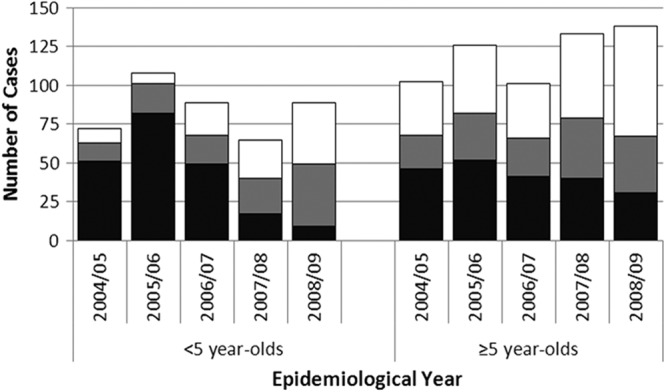
Distribution of PCV7 (black), the extra PCV13 (gray), and the remaining (white) pneumococcal serotypes causing meningitis in England and Wales (2004 to 2009).
Comparison of STs most commonly associated with PCV7 serotypes showed that the incidence of only ST124 was proportionally lower in 2007 to 2009 (1 of 100 PCV7 serotypes, 1.0%) than in 2004 to 2006 (19/231, 8.2%; P = 0.01) (Table 2). ST124 was associated only with serotype 14 and occurred mainly in children of <5 years of age, where most of the reduction was observed. No other ST associated with PCV7 showed significant changes in distribution, even within individual serotypes, between 2004 and 2006 and between 2007 and 2009. The largest change was observed in 18C, where ST113 decreased proportionally from 59% (22/37) to 28% (5/18) (P = 0.027).
Table 2.
STs with at least eight isolates over the 5-year period grouped by associated PCV7, extra PCV13, or non-PCV13 serotypes
| Serotype group and ST [most common serotype(s)]a | No. of isolates by epidemiological year |
|||||
|---|---|---|---|---|---|---|
| 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | Total | |
| PCV7-related STs | ||||||
| 9 (14) | 14 | 15 | 9 | 4 | 1 | 43 |
| 36 (23F) | 6 | 2 | 6 | 3 | 5 | 22 |
| 113 (18C) | 8 | 14 | 8 | 2 | 3 | 35 |
| 124 (14) | 6 | 13 | 5 | 1 | 25 | |
| 138 (6B) | 5 | 4 | 3 | 3 | 15 | |
| 162 (9V, 19F) | 13 | 18 | 10 | 11 | 2 | 54 |
| 176 (6B) | 5 | 8 | 2 | 8 | 2 | 25 |
| 246 (4) | 5 | 3 | 3 | 3 | 14 | |
| 311 (23F) | 4 | 7 | 6 | 1 | 4 | 22 |
| Other | 31 | 50 | 40 | 24 | 20 | 165 |
| Total for group | 97 | 134 | 89 | 57 | 43 | 420 |
| Extra PCV13-related STs | ||||||
| 65 (6A) | 4 | 2 | 5 | 5 | 5 | 21 |
| 180 (3) | 6 | 10 | 8 | 16 | 15 | 55 |
| 191 (7F) | 7 | 14 | 11 | 15 | 24 | 71 |
| 199 (19A)b | 6 | 1 | 2 | 4 | 11 | 24 |
| 306 (1) | 2 | 5 | 4 | 11 | 9 | 31 |
| Other | 9 | 17 | 14 | 13 | 12 | 65 |
| Total for group | 34 | 49 | 44 | 64 | 76 | 267 |
| Non-PCV13-related STs | ||||||
| 53 (8) | 7 | 10 | 11 | 3 | 10 | 41 |
| 199 (15B, 15C)b | 2 | 1 | 6 | 2 | 3 | 14 |
| 218 (12B, 12F) | 4 | 9 | 4 | 8 | 9 | 34 |
| 235 (20) | 1 | 2 | 2 | 2 | 3 | 10 |
| 393 (25A, 38) | 2 | 2 | 3 | 2 | 9 | |
| 405 (9N) | 1 | 4 | 3 | 8 | ||
| 433 (22F) | 1 | 2 | 9 | 11 | 23 | |
| 439 (23B) | 1 | 3 | 6 | 10 | ||
| 673 (33F) | 2 | 4 | 5 | 11 | ||
| 698 (22F) | 1 | 3 | 9 | 13 | ||
| 1475 (27) | 1 | 2 | 5 | 8 | ||
| Other | 22 | 21 | 31 | 38 | 50 | 162 |
| Total for group | 43 | 51 | 58 | 78 | 113 | 343 |
STs were grouped by the serotype they are usually associated with.
ST199 was associated with serotype 19A, which is included in PCV13, as well as serotypes 15B and 15C, which are not included in any of the pneumococcal conjugate vaccines.
Extra PCV13 serotypes.
Among the six extra PCV13 serotypes causing meningitis, there were no significant changes in serotype distribution overall or within individual serotypes between 2004 and 2006 and between 2007 and 2009 (Tables 1 and 2). The genetic structure of serotypes 1, 3, and 7F was clonal. Over the 5-year study period, ST306 became the main clone associated with serotype 1, representing 83% of serotype 1 meningitis cases, and gradually replaced ST227, a closely related clone. ST227 was observed only once in 2008 to 2009. ST180 accounted for 97% of serotype 3 and caused meningitis predominantly in older children and adults (8 cases in patients <5 years old, 35 among 5- to 64-year-olds, and 14 among ≥65-year-olds). Two pneumococcal lineages (CC191 and CC218) were associated with serotype 7F. ST191 (95% of 7F pneumococci) became the most important clone in 2008 to 2009, accounting for 9.6% (24/232) of meningitis isolates, and caused meningitis in both children of <5 years of age (39 cases) and older children and adults (37 cases). The other isolates of serotype 1 were genetically closely related, and all belonged to CC191 (single-locus variant of ST191). Serotype 5 was rare throughout the study period, accounting for only four cases (three in children), all of which occurred after PCV7 implementation and belonged to ST289.
Serotypes 6A and 19A were genetically more diverse. Serotype 6A was associated with 18 distinct STs clustered into nine clonal complexes and ST65 (CC460) was the major ST (49%). The distribution of ST65 did not change over the study period and accounted only for 2.2% (5/232) of the total cases in 2008 to 2009. Other lineages carrying 6A were CC176, CC395, CC396, CC439, CC473, CC490, ST1752, and ST2528. Within serotype 19A, isolates were clustered into five clonal complexes, among which CC199 accounted for 89%. ST199 contributed to 2.5% (10/398) of meningitis cases from 2004 to 2006 and to 4.6% (20/431) from 2007 to 2009 (P = 0.088) and caused meningitis in both children of <5 years old (21 cases) and older children and adults (17 cases).
Non-PCV13 serotypes.
Among non-PCV13 serotypes, there were also no significant changes in ST distributions overall or within individual serotypes between 2004 and 2006 and between 2007 and 2009 (Table 2). A total of 36 other serotypes and 97 STs clustered into 50 distinct clonal complexes were detected among 343 meningitis isolates (Tables 1 and 2; see also Table S1 in the supplemental material). Genetic diversity of isolates associated with non-PCV7 serotypes increased during the post-PCV7 period, from 25 STs in 2005 to 2006 to 48 in 2008 to 2009. The increase in serotype 22F meningitis cases was due mainly to the expansion of two distinct lineages, ST433 and ST698. Serotype 6C (19 isolates over 5 years) was associated with 11 STs, particularly ST1692 (7/19 [37%] of 6C isolates). Serotypes 8 and 12F both had a rather clonal structure, with ST53 (serotype 8) and ST218 (12F) being the most important. All 10 23B isolates belonged to ST439.
CFR.
The case fatality ratio (CFR) within 30 days of infection was 14.1% (145 fatalities/1,030 cases) and was similar before and after PCV7 introduction (61/408 [15.0%] during 2004 to 2006 versus 60/431 [13.9%] during 2007 to 2009; P = 0.67). The CFR was also not significantly different for PCV7 (58/420, 13.8%), the extra PCV13 (43/267, 16.1%), or the remaining (44/343, 12.8%) serotypes (P = 0.50; P = 0.90 after adjusting for age). One PCV7 vaccine failure case (1/11, 9.1%) with severe developmental delay and a ventriculo-peritoneal shunt in situ died of pneumococcal meningitis. The CFR decreased with age from 17.1% (73/428) among <5-year-olds to 12.8% (57/447) in 5- to 64-year-olds and 9.7% (15/155) and among ≥65-year-olds (P value for the trend [Ptrend] of 0.013). Analysis by serotype and ST identified only ST199 to have a significantly higher CFR (12/38 [31.6%] versus 133/992 [13.4%]; P = 0.002), even after adjusting for age (adjusted odds ratio of 2.9; 95% CI of 1.4 to 5.9; P = 0.004). ST199 was associated mainly with serotype 19A (24/38, 63.2%) followed by 15B (7/38, 18.4%) and 15C (7/38, 18.4%). The CFR for serotype 19A meningitis was 26.8% (11/41 cases) compared with 13.6% (134/989 cases) for the other serotypes (P = 0.017). Within the 41 serotype 19A cases, however, there was no significant difference in the CFR for ST199 compared with CFRs of other STs (6/24 [25.0%] versus 5/17 [29.4%]; P = 0.75).
DISCUSSION
We have already reported the impact of PCV7 on IPD in England and Wales (6) and demonstrated significant declines in the incidence of overall and PCV7-type IPD (including meningitis) across all age groups. The introduction of PCV7 into the childhood vaccination program has also had a marked effect on the structure of the pneumococcal population causing meningitis. Over the 5-year period, we genotyped over 1,000 pneumococcal isolates causing meningitis, and although this represented only 68% of the total reported cases, more than half of the cases where the serotype was not known had been diagnosed by PCR only and, therefore, were not amenable to molecular analysis. Given the rapidity with which S. pneumoniae can cause meningitis, it is not uncommon practice for empirical intravenous antibiotics to be administered early and delay a lumbar puncture until the patient is clinically stable. In such cases, the CSF is often sterile but may remain PCR positive and, therefore, help confirm the diagnosis.
Meningitis caused by PCV7 serotypes.
Our results confirm the substantial decline in PCV7-type meningitis, particularly in young children (the age group targeted for pneumococcal conjugate vaccination) but also among older individuals who benefited from indirect (herd) protection through reduction in carriage among vaccinated children (16). Among PCV7 serotypes causing meningitis, only the reduction in ST124 (associated with serotype 14) was proportionally greater following PCV7 implementation. This is likely to have occurred because serotype-specific vaccine effectiveness for PCV7 was high for serotype 14 (5, 6), resulting in a 99% reduction in meningitis caused by this serotype among <5-year-olds, 90% for 5- to 64-year-olds, and 84% for ≥65-year-olds.
Molecular analysis of pneumococcal isolates causing meningitis before and after PCV7 introduction identified one potential vaccine escape strain caused by a possible capsular switching from a PCV7 (serotype 14) to a non-PCV7 (serotype 28A) serotype. In the United States, emergence of escape strains was observed 3 years after the introduction of PCV7, probably in response to selection pressure (9, 17). Since then, there have been more reports of vaccine escape strains in the United States (18, 19). In particular, capsular switch variants of a serotype 4 clone have contributed to an increase in serotype 19A IPD in the United States, where serotype 19A IPD increased from 0.8 to 2.5/100,000 population between 1998 and 2005 and was associated with an increase in penicillin resistance rates from 6.7% to 35% over the same period (19). In Italy, too, capsular switching among two serotype 19A (ST695) isolates was recently reported 7 years after PCV7 implementation (20). In England and Wales, there is currently no evidence of any increase in serotype 28A IPD following PCV7 introduction (6, 21), but this will require careful monitoring in the coming years.
Meningitis caused by non-PCV7 serotypes.
Although the overall incidence of pneumococcal meningitis declined, cases due to non-PCV7 serotypes increased following PCV7 introduction. There was, however, no evidence of emergence of any particular serotype or ST causing meningitis. Serotypes 7F, 19A, 3, and 1 among the extra PCV13 serotypes and serotypes 22F, 8, and 33F among the remaining serotypes have become the main causes of pneumococcal meningitis although these serotypes were also reported to cause meningitis prior to PCV7 implementation. The increase in serotype 7F meningitis following PCV7 introduction is concerning since this serotype has been associated with more severe disease in children, with increased complications and higher case fatality than with other serotypes (22). Pneumococcal carriage studies in England before and after PCV7 introduction revealed an increase in carriage for a number of serotypes, including four of those listed above (3, 7F, 19A, and 33F), while others such as serotypes 8 and 22F emerged in disease but had very low carriage prevalence (23). Most of emerging serotypes have undergone clonal expansion, suggesting that a limited number of genotypes are replacing PCV7 genotypes. Changes in serotype 1 IPD as well as carriage are likely to be due to natural/secular variations (6, 23). Interestingly, opposite dynamics were observed for the two major clones—ST227 and ST306—commonly associated with serotype 1 in the United Kingdom. ST227 has nearly disappeared not only among pneumococci causing meningitis but also among other clinical presentations (data not shown) and may partially explain the overall reduction of serotype 1 IPD incidence since 2005 to 2006 (6). It is not known why ST306 increased during this period, but it is possible that this ST may have gained genetic elements that improved its fitness/virulence over other STs.
In keeping with our surveillance of all IPD cases (6), serotype 3 (which was mainly associated with ST180) was more likely to cause meningitis in older adults. Compared with other serotypes, serotype 3 has a particularly high case-to-carrier ratio (CCR), suggesting that individuals exposed to serotype 3 were more likely to develop IPD rather than becoming asymptomatic carriers (23). Serotype 3 carriage (23, 24) as well as invasive disease (6) is uncommon in children and appears to be more adapted to circulate within the older population. This serotype will require careful monitoring across all age groups because of the limited protection afforded against this serotype by both PCV13 and the 23-valent pneumococcal polysaccharide vaccine (PPV23) (25, 26).
While many pneumococcal serotypes causing meningitis increased following PCV7 implementation, cases due to some serotypes decreased. These reductions may result from secular fluctuation (e.g., serotype 8), while for serotypes within a serogroup that is included in PCV7, the reduction may also be due to cross-protection. Reduction in 6A IPD, for example, may be a result of cross-protection from antibodies induced by the 6B polysaccharide component of PCV7 (27, 28), as observed with the reduction of 6A IPD among <5-year-olds following PCV7 implementation in the United Kingdom (6). On the other hand, there is no evidence to support cross-protection from 19F toward 19A (6).
Case fatality.
The CFR following pneumococcal meningitis remains unacceptably high across the age groups as well as serotype groups. Analysis of individual serotypes and STs identified a higher CFR for ST199 only although this might be a chance finding due to multiple comparisons. ST199 is commonly associated with serotype 19A (18). Within this serotype, however, ST1999 was not associated with an increased risk of death compared with other STs. Given that sequence type is inferred from housekeeping genes within S. pneumoniae, it is more likely that other genetic factors modulate the virulence potential of different strains in addition to the polysaccharide capsule (29, 30). The complex interplay between the host and pathogen and the contribution of serotypes and STs to clinical disease and outcome certainly merit further investigation.
Conclusions.
With changes in serotype and ST distributions following PCV7 implementation, the genetic composition of the pneumococcal population causing meningitis has changed. A limited number of major clones, however, predominate alongside minor satellites clones, which are diverse and have a fluctuating genetic composition such that new clones are being continually identified. This suggests that the pneumococcal genetic pool is vast and that a large number of pneumococcal clones (some of them yet to be characterized) may have the potential to cause meningitis. Selective pressure following pneumococcal conjugate vaccination may lead to the emergence or reemergence of pneumococci with similar pathogenic potential. In April 2010, PCV7 was replaced by PCV13 in the national childhood immunization program (31). By July 2011, IPD incidence caused by the extra PCV13 serotypes was halved in children of <2 years of age (21). As seen following PCV7 implementation, it is likely that at least some non-PCV13 serotypes such as 22F and 33F will increase and that others yet may emerge because of selection pressure although it is difficult to predict the extent of subsequent disease replacement by such serotypes. Vaccines with broader coverage are, therefore, urgently required to prevent IPD in the future. Protein vaccines such as those targeting pneumococcal surface protein A (PspA)/C (PspC), pneumolysin (Ply) or caseinolytic protease (ClpP) are currently being investigated although the high recombination frequency among such surface proteins suggests that these loci are under constant selective pressure from human immune responses, which may, therefore, reduce their overall effectiveness (32). In the meantime, although capsular switching and emergence of vaccine escape strains are unlikely to have any major impact on the efficacy of pneumococcal conjugate vaccination (33), continued epidemiological and molecular surveillance across all ages is essential in order to monitor the long-term impact of pneumococcal conjugate vaccines.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded through a competitive grant awarded by the Meningitis Research Foundation, a nonprofit charitable organization that funds research to prevent meningitis and septicemia and improve survival rates and outcomes. The Department of Health also funded part of this study.
The funding bodies had no role in the study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
We thank the general practitioners, hospital doctors, and laboratory staff in England and Wales who provided information on their patients, Rashmi Malkani at Health Protection Services for following up all of the cases, Anna Sharma for assistance with collection of clinical data, Androulla Efstratiou, Siobhan Martin, and Carmen Sheppard of the Respiratory and Systemic Infection Laboratory for serotyping and laboratory support, and Malcolm Guiver from the Health Protection Agency, Manchester (United Kingdom), for laboratory support. We also thank Derrick Crook and Dona Foster of the John Radcliffe Hospital Oxford for reporting IPD cases in Southern England serotyped by his laboratory.
S.N.L. and M.P.E.S. have performed contract research for vaccine manufacturers on behalf of St. George's University of London and the Health Protection Agency, respectively, but have received no personal remuneration. None of the other authors has a conflict of interest.
Footnotes
Published ahead of print 26 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01917-12.
REFERENCES
- 1. Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A. 2011. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 364:2016–2025 [DOI] [PubMed] [Google Scholar]
- 2. Jit M. 2010. The risk of sequelae due to pneumococcal meningitis in high-income countries: a systematic review and meta-analysis. J. Infect. 61:114–124 [DOI] [PubMed] [Google Scholar]
- 3. Johnson AP, Waight P, Andrews N, Pebody R, George RC, Miller E. 2007. Morbidity and mortality of pneumococcal meningitis and serotypes of causative strains prior to introduction of the 7-valent conjugant pneumococcal vaccine in England. J. Infect. 55:394–399 [DOI] [PubMed] [Google Scholar]
- 4. Department of Health 2006. Important changes to the childhood immunisation programme. PL CMO 2006/1. Department of Health, United Kingdom: http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Professionalletters/Chiefmedicalofficerletters/DH_4137171 [Google Scholar]
- 5. Andrews N, Waight PA, Borrow R, Ladhani S, George RC, Slack MP, Miller E. 2011. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS One 6:e28435 doi:10.1371/journal.pone.0028435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. 2011. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect. Dis. 11:760–768 [DOI] [PubMed] [Google Scholar]
- 7. Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, Farley MM, Jorgensen JH, Lexau CA, Petit S, Reingold A, Schaffner W, Thomas A, Whitney CG, Harrison LH. 2009. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988–1995 [DOI] [PubMed] [Google Scholar]
- 10. Reinert R, Jacobs MR, Kaplan SL. 2010. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine 28:4249–4259 [DOI] [PubMed] [Google Scholar]
- 11. Coffey TJ, Enright MC, Daniels M, Morona JK, Morona R, Hryniewicz W, Paton JC, Spratt BG. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73–83 [DOI] [PubMed] [Google Scholar]
- 12. Sheppard CL, Harrison TG, Smith MD, George RC. 2011. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J. Med. Microbiol. 60:49–55 [DOI] [PubMed] [Google Scholar]
- 13. Pichon B, Bennett HV, Efstratiou A, Slack MP, George RC. 2009. Genetic characteristics of pneumococcal disease in elderly patients before introducing the pneumococcal conjugate vaccine. Epidemiol. Infect. 137:1049–1056 [DOI] [PubMed] [Google Scholar]
- 14. Platt S, Pichon B, George R, Green J. 2006. A bioinformatics pipeline for high-throughput microbial multilocus sequence typing (MLST) analyses. Clin. Microbiol. Infect. 12:1144–1146 [DOI] [PubMed] [Google Scholar]
- 15. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dagan R, Melamed R, Muallem M, Piglansky L, Yagupsky P. 1996. Nasopharyngeal colonization in southern Israel with antibiotic-resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J. Infect. Dis. 174:1352–1355 [DOI] [PubMed] [Google Scholar]
- 17. Brueggemann AB, Pai R, Crook DW, Beall B. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 3:e168 doi:10.1371/journal.ppat.0030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beall BW, Gertz RE, Hulkower RL, Whitney CG, Moore MR, Brueggemann AB. 2011. Shifting genetic structure of invasive serotype 19A pneumococci in the United States. J. Infect. Dis. 203:1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore MR, Gertz RE, Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, Hadler JL, Bennett NM, Thomas AR, McGee L, Pilishvili T, Brueggemann AB, Whitney CG, Jorgensen JH, Beall B. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016–1027 [DOI] [PubMed] [Google Scholar]
- 20. Ansaldi F, Canepa P, de Florentiis D, Bandettini R, Durando P, Icardi G. 2011. Increasing incidence of Streptococcus pneumoniae serotype 19A and emergence of two vaccine escape recombinant ST695 strains in Liguria, Italy, 7 years after implementation of the 7-valent conjugated vaccine. Clin. Vaccine Immunol. 18:343–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. 2011. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 29:9127–9131 [DOI] [PubMed] [Google Scholar]
- 22. Ruckinger S, von Kries R, Siedler A, van der Linden M. 2009. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr. Infect. Dis. J. 28:118–122 [DOI] [PubMed] [Google Scholar]
- 23. Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, George R, Miller E. 2011. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 8:e1001017 doi:10.1371/journal.pmed.1001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hussain M, Melegaro A, Pebody RG, George R, Edmunds WJ, Talukdar R, Martin SA, Efstratiou A, Miller E. 2005. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol. Infect. 133:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews N, Kaye P, Slack M, George R, Miller E. 2012. Effectiveness of the 13 valent pneumococcal conjugate vaccine against IPD in England and Wales, poster 148. Eighth Int. Symp. Pneumococci Pneumococcal Dis., Iguacu Falls, Brazil, 11 to 15 March 2012 http://www2.kenes.com/ISPPD/Scientific/Documents/FinalAbstractbook.pdf [Google Scholar]
- 26. Andrews NJ, Waight PA, George RC, Slack MP, Miller E. 2012. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 30:6802–6808 [DOI] [PubMed] [Google Scholar]
- 27. Vakevainen M, Eklund C, Eskola J, Kayhty H. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789–793 [DOI] [PubMed] [Google Scholar]
- 28. Yu X, Gray B, Chang S, Ward JI, Edwards KM, Nahm MH. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 180:1569–1576 [DOI] [PubMed] [Google Scholar]
- 29. Thomas JC, Figueira M, Fennie KP, Laufer AS, Kong Y, Pichichero ME, Pelton SI, Pettigrew MM. 2011. Streptococcus pneumoniae clonal complex 199: genetic diversity and tissue-specific virulence. PLoS One 6:e18649 doi:10.1371/journal.pone.0018649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Hoek AJ, Andrews N, Waight PA, George R, Miller E. 2012. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One 7:e39150 doi:10.1371/journal.pone.0039150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salisbury D. 2010. Introduction of Prevenar 13 into the childhood immunisation programme. Gateway reference 13581. Department of Health, United Kingdom: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_112192.pdf [Google Scholar]
- 32. Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, Gavon Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331:430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Temime L, Boelle PY, Opatowski L, Guillemot D. 2008. Impact of capsular switch on invasive pneumococcal disease incidence in a vaccinated population. PLoS One 3:e3244 doi:10.1371/journal.pone.0003244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.