Abstract
Although antiretroviral treatment availability has improved, the virological monitoring of patients remains largely uneven across regions. In addition, viral quantification tests are suffering from human immunodeficiency virus type 1 (HIV-1) genetic diversity, fueled by the emergence of new recombinants and of lentiviruses from nonhuman primates. We developed a real-time reverse transcription-PCR (RT-PCR) assay that is relatively inexpensive and able to detect and quantify all circulating forms of HIV-1 and its simian immunodeficiency virus (SIV) precursors, SIVcpz and SIVgor. Primers and a probe were designed to detect all variants of the HIV-1/SIVcpz/SIVgor lineage. HIV-1 M plasma (n = 190; 1.68 to 7.78 log10 copies/ml) representing eight subtypes, nine circulating recombinant forms, and 21 unique recombinant forms were tested. The mean PCR efficiency was 99%, with low coefficients of intra- and interassay variation (<5%) and a limit of quantification of <2.50 log10 copies/ml, with a 200-μl plasma volume. On the studied range, the specificity and the analytical sensitivity were 100 and 97.4%, respectively. The viral loads were highly correlated (r = 0.95, P < 0.0001) with the reference method (generic HIV assay; Biocentric) and had no systematic difference, irrespective of genotype. Furthermore, 22 HIV-1 O plasmas were screened and were better quantified compared to the gold-standard RealTime HIV-1 assay (Abbott), including four samples that were only quantified by our assay. Finally, we could quantify SIVcpzPtt and SIVcpzPts from chimpanzee plasma (n = 5) and amplify SIVcpz and SIVgor from feces. Thus, the newly developed real-time RT-PCR assay detects and quantifies strains from the HIV-1/SIVcpz/SIVgor lineage, including a wide diversity of group M strains and HIV-1 O. It can therefore be useful in geographical areas of high HIV diversity and at risk for the emergence of new HIV variants.
INTRODUCTION
In 2009, about 5.2 million people in low- and middle-income countries were receiving antiretroviral therapy (ART) (UNAIDS, 2010). Programs to scale-up ART in resource-limited countries have increased the number of people receiving treatment. Nevertheless, viral load (VL) monitoring for patients on ART or for early viral detection in exposed children is only rarely available in resource-limited settings. Scale-up of laboratory monitoring such as VL measurement in low-income countries is a priority, and it has been defined as a recommendation by UNAIDS in 2010 to improve the efficiency and quality of human immunodeficiency virus (HIV) antiretroviral treatment and care.
Today, different viral load assays are available and use different techniques of molecular biology such as real-time reverse transcription-PCR (RT-PCR), nucleic acid sequence based amplification, or branched-chain DNA signal amplification (bDNA) (reviewed in reference 1). If HIV quantification assays are rarely present or only in reference laboratories of resource-limited countries, it is mainly due to their high cost which can reach 50 to 100 dollars per sample (i.e., higher than the cost of ART) and their need for specific instruments and trained staff (2). Two cheaper alternatives to classical molecular methods, heat-dissociated HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA) (3, 4) and the Cavidi ExaVir reverse transcriptase activity assay (5, 6), have been proposed but have demonstrated inconclusive results for the first and need further improvement and evaluation for the second (7–9). Dipstick and chip technologies are still in development (2, 10, 11). Therefore, recent “in-house” or generic real-time RT-PCR assays have shown diverse advantages and are relatively inexpensive (the real-time technology is widely used in HIV and other viral infection detections) (12–14).
The high genetic diversity of HIV-1 is a major challenge for the development of efficient and sensitive quantification assays (1, 2, 14, 15). All commercial quantitative tests were primarily designed on subtype B viruses and, even if they are continuously optimized to detect a broad range of variants, they still do not quantify effectively all circulating strains (1, 16–18). This drawback is particularly an issue in sub-Saharan African areas, where non-B strains predominate, and where many and highly diverse HIV-1 variants cocirculate. Due to globalization, this heterogeneity can also be found in different geographic regions in which the common viral load assays may not be able to detect these “unusual” strains (19–21). Furthermore, highly divergent viruses, such as HIV-1 groups O, N, and P, which also circulate and have a clinical course similar to that of HIV-1 group M, need appropriate monitoring tools that are not often available (22, 23). Thus, HIV diversity and molecular epidemiology still impacts on the management and monitoring of HIV-infected patients. Some recent generic or “in-house” tests have designed their assay to target a wide variety of HIV-1 strains and could validate their technique on multiple samples from different geographic areas (reviewed in reference 24). However, the main limit of the majority of these tests is that their designed primers and probe have numerous mismatches with HIV-1 groups O, N, and P (alignment with HIV-1 sequences from the HIV.lanl database [data not shown]), implying the possible nondetection or underquantification of these circulating strains (25). On the other hand, a real-time PCR assay developed by Gueudin and coworkers specifically quantifies HIV-1 group O but does not detect HIV-1 group M strains (22, 26).
To date, no viral load assay has been designed to possibly detect new zoonotic simian immunodeficiency virus (SIV)/HIV viruses emerging from SIVs naturally infecting chimpanzees and gorillas, such as HIV-1 group P identified in 2009 from SIVgor (27). Furthermore, recent studies have shown that SIVcpz, ancestors of HIV-1, can be pathogenic for their natural host (28, 29), and we showed previously how difficult it can be to follow such viral infection in plasma and fecal samples. To monitor SIV infection in naturally infected apes, a new tool should be available to detect SIV RNA in fecal samples and to quantify SIV viral load in plasma over the course of infection.
Here, our goal was to design a new quantitative RT-PCR (RT-qPCR) assay, relatively inexpensive and at least equal to generic or “in-house” tests regarding technical characteristics and performance, but with the capacity to virtually detect and quantify all HIV-1 circulating strains. Furthermore, there is still a risk for SIV emergence from infected apes to humans. It will then be important to be able to detect with this same test any hypothetic new emerging SIVcpz or SIVgor viruses in the human population. Finally, a detection and quantification test would be useful for monitoring SIV infection in great apes from both fecal and plasma samples in order to understand better the course of SIVcpz and SIVgor infections in their natural hosts, the reservoir of the ancestors of HIV-1. Thus, our aim was to design a single quantitative viral load assay satisfying these different goals.
MATERIALS AND METHODS
HIV-negative plasma samples.
For negative control and specificity determinations, 72 HIV-negative plasma samples from patients attending a hospital in Yaoundé, Cameroon, for an HIV test were available. The negativity of these samples was determined by diverse HIV serological tests (ICE HIV-1.0.2 [Murex Biotech, Ltd., Dartford, United Kingdom]; Wellcozyme HIV Recombinant [Murex]; and Determine HIV-1/2 [Abbott Laboratories, Tokyo, Japan]). These samples were processed similarly as the HIV-positive samples.
HIV-1 group M plasma samples.
A total of 190 HIV-1 group M RNA extracts from plasma samples were available for viral quantification (Table 1). All of them were previously analytically detected with the Generic HIV-1 Viral Load Biocentric kit (Biocentric) for clinical studies and conserved at −80°C. Their VLs ranged between 1.68 and 7.78 log10 copies/ml, with 12 of the 190 plasma samples being analytically detected (PCR amplification) but under the threshold of quantification determined by Biocentric (Biocentric quantification threshold, 2.5 log10 copies/ml) (30). The remaining 178 plasma samples were clinically positives as quantified by the Biocentric technique (>2.5 log10 copies/ml).
Table 1.
Selected HIV-1 group M plasma samples tested with the new RT-qPCR assay
| Subtype/CRF | No. of samples |
|||||
|---|---|---|---|---|---|---|
| Burundi | Cameroon | DRCa | Togo | France | Total | |
| A | 2 | 6 | 8 | |||
| B | 14 | 14 | ||||
| C | 16 | 1 | 3 | 20 | ||
| D′ | 3 | 3 | ||||
| F2 | 3 | 3 | ||||
| G | 2 | 3 | 5 | |||
| H | 3 | 3 | ||||
| J | 3 | 3 | ||||
| U | 1 | 1 | ||||
| CRF01 | 2 | 1 | 3 | |||
| CRF02 | 34 | 3 | 25 | 62 | ||
| CRF06 | 2 | 3 | 5 | |||
| CRF11 | 3 | 1 | 4 | |||
| CRF13 | 1 | 1 | ||||
| CRF14 | 1 | 1 | ||||
| CRF18 | 1 | 1 | ||||
| CRF22 | 8 | 8 | ||||
| CRF37 | 1 | 1 | ||||
| URF | 3 | 12 | 6 | 21 | ||
| Totalb | 18 | 65 | 39 | 54 | 14 | 190 |
DRC, Democratic Republic of the Congo.
The total includes the 23/190 samples with unknown genotypes.
For 167/190 HIV-1 group M-positive plasma samples that had a VL superior to 3 log10 copies/ml, a region of ∼1,865 bp in pol (protease and reverse transcriptase regions) was amplified and sequenced as previously described, primarily to determine the drug resistance profile and genotypes for previous studies (31–33; M. Peeters, unpublished data). The different subtypes and circulating recombination forms (CRFs) are shown in Table 1. Our evaluation included plasma samples from four different countries of Africa with different HIV-1 subtype/CRF distributions: 18 from Burundi and 54 from Togo with a relatively low genotypic heterogeneity (mainly subtype C and CRF02, respectively) and 65 from Cameroon and 39 from the Democratic Republic of the Congo (DRC) with highly diverse subtypes and CRFs. We also included 14 plasma samples with HIV-1 subtype B strains from the hospital of Montpellier, France (34). The panel covered the heterogeneity of subtypes and CRFs of HIV-1 group M circulating strains: all subtypes, with the exception of subtype K, were represented, major CRFs were also present, and 21 unique recombinant forms (URFs) were included (19). This panel also comprised 23 samples from which genotyping was not possible or not performed due to their low viral loads (<3 log10 copies/ml), but they were kept in the study since we wanted to test our assay on samples with very low viral loads too.
HIV-1 group O samples.
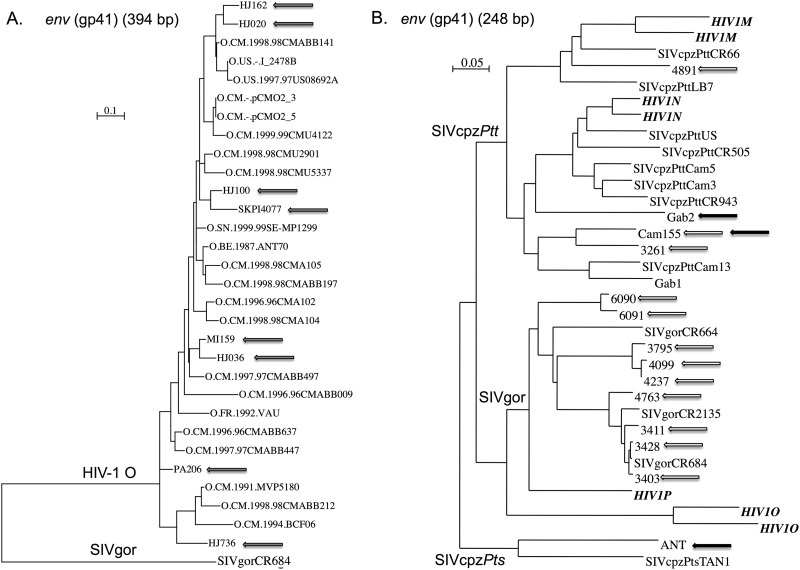
We tested 31 HIV-1 group O samples with our new RT-qPCR assay. All samples were identified as HIV-1 O by a previously reported in-house ELISA using V3-loop peptides from HIV-1 groups M, N, and O (35). Twenty-two samples were plasma samples from Cameroon conserved at −80°C and previously quantified by the Abbott RealTime test (Abbott Molecular, Inc., Des Plaines, IL) in Yaoundé, Cameroon. For the nine remaining samples, only DNA extracts, with HIV-1 group O sequence confirmation, conserved at −20°C, were available for detection, but they allowed us to further test our assay on various HIV-1 group O strains. For HIV-1 group O-positive samples, a small region in env (gp41) of ∼450 bp and/or a region in pol (reverse transcriptase) of ∼1,800 bp were amplified and sequenced when enough material was available (14/31 samples). The sequences were aligned with CLUSTAL W (36), and the phylogenies were performed using PhyML (37) with the GTR model with four gamma distributed rate categories. The group O strains sequenced and tested here covered the HIV-1 group O genetic diversity (Fig. 1A).
Fig 1.
(A and B) Phylogenetic relationships between HIV-1 group O strains (A) and SIVcpz/SIVgor viruses (B), tested by our RT-qPCR assay with reference strains. (A) Phylogenetic tree derived from an env region (gp41; 394 bp). Gray arrows highlight strains from our panel. Some strains were not sequenced because of material limitations and others were available only in pol region. (B) The tree is derived from a small env region (gp41; 248 bp), constructed by BioNJ (66). White arrows highlight strains from our fecal sample panel (except from CR4112 amplified in the pol region only, and CR6278, -6466, -6495, -6534, and -6682 amplified in a small 195-bp region of gp41), and black arrows highlight strains from plasma samples. All of the sequences were retrieved from the HIV database (http://www.hiv.lanl.gov/).
SIVcpz plasma samples from chimpanzees.
We tested plasma samples from three previously described SIVcpz-infected chimpanzees and one uninfected chimpanzee as a negative control. Two SIVcpz+ chimpanzees (Gab2 and Ch-Go) are from Pan troglodytes subsp. troglodytes and were infected with SIVcpzPttGab2 (38, 39) and SIVcpzPttCam155 (28), respectively. These strains cluster in the SIVcpzPtt/HIV-1 M/HIV-1N lineage close to other SIVcpzPtt-infecting chimpanzees from Cameroon and Gabon (black arrows in Fig. 1B). Ch-No, the third SIVcpz-positive chimpanzee, is from Pan troglodytes subsp. schweinfurthii and was infected with SIVcpzPts-ant that clusters in the monophyletic lineage of SIVcpzPts strains, of the SIVcpzPtt/SIVgor/HIV-1 lineage (40, 41) (see the black arrow in Fig. 1B). Sequential plasma samples were available for Ch-Go (two time points 7 years apart) and for Ch-No (four time points between October 1989 and January 1991).
HIV/SIV RNA extraction from plasma samples.
HIV and SIV RNA were extracted from 200 μl of plasma, conserved at −80°C, using a QIAamp viral RNA minikit (Qiagen, Courtaboeuf, France), and eluted with 60 μl of elution buffer. Standards and the reproductive control, provided by generic HIV-1 viral load Biocentric kit (Biocentric, Bandol, France), were inactivated culture supernatants of HIV-1 subtype B and extracted according to the same protocol.
Fecal samples from wild-living chimpanzees and gorillas infected with SIV.
We tested 78 fecal samples, conserved in RNAlater, from chimpanzees (n = 24) and gorillas (n = 54) from Cameroon previously described to have HIV-1 cross-reactive antibodies (42, 43). In these previous studies, we were able to amplify and sequence fragments in the pol and/or gp41 viral regions from five chimpanzee samples (5/24) and from 15 gorilla samples (15/54), after 2 to 10 independent RNA extractions and subsequent RT-PCR attempts. These strains represented the genetic diversity of SIVcpzPtt and SIVgor viruses (white arrows in Fig. 1B). Here, we extracted total RNA from 1.5 ml of each ape's fecal sample, using a NucliSens magnetic extraction kit (bioMérieux, Craponne, France) as previously described (43), to obtain a final RNA extract volume of 50 μl.
Development of a real-time RT-qPCR assay for detection and quantification of viral strains from the HIV-1/SIVcpz/SIVgor lineage.
We designed the primers and probe using an alignment of sequences from various HIV-1 strains from all four groups (M, N, O, and P), SIVcpzPtt and SIVcpzPts, and SIVgor viruses. The long terminal repeat (LTR) region was first explored according to previous studies, which choose this region because of its low variability across HIV-1 strains (26, 44–46). The alignment we made with more divergent viruses (SIVcpz and SIVgor) and with all sequences available from divergent HIV-1 groups (N, O, and P), including some not available at the time of the previously reported assays in the LTR region, highlighted the numerous mismatches of previously described primers and probes. Thus, we designed new ones that were set to amplify HIV-1 groups M, N, O, and P, as well as SIVcpzPtt, SIVcpzPts, and SIVgor viruses. The reverse primer (HXB2 positions 622 to 642, 5′-AAAATCTCTAGCAGTGGCGCC-3′) was similar to a previously described primer (30), and the forward primer (HXB2 positions 523 to 539, 5′-SSCTCAATAAAGCTTGCC-3′) had a position and length similar to that previously described (46), but we changed two nucleotides at the 5′ end to match all of the divergent strains. These primers, matching all aligned sequences with 100% homology, amplified a small fragment of 120 bp. The new probe (HXB2 positions 588 to 603, 5′-CTAGAGATCCCTCAGA-3′) was designed in a position similar to that previously reported (45) but was 10 bp shorter and had different characteristics; it was a reverse internal TaqMan probe carrying a 5′ FAM reporter and a 3′ minor groove binding/nonfluorescent quencher (Applied Biosystems, Foster City, CA). It should be noted that one mismatched nucleotide residue at the 3′ end was observed for three SIVcpzPts strains (SIVcpzPtsTAN1, -2, and -3).
We performed all runs in a 20-μl reaction volume containing 10 μl of RNA extract, the primers and the probe at 500 nM, 1× of TaqMan Fast Virus 1-Step master mix (Applied Biosystems), and RNase-free water to the final volume. The thermal cycling conditions were as follows: RT at 50°C for 5 min, RT inactivation and initial denaturation at 95°C for 20 s, and amplification with 50 cycles of 95°C for 3 s and 58°C for 30 s (total duration, ∼70 min). Cycling and data acquisition were carried out using the 7500 Real-Time PCR system (Applied Biosystems). We used five standards from the OptiQuant HIV-1 RNA quantification panel (2.78, 3.78, 4.78, 5.78, and 6.78 log10 copies/ml) and the OptiQuant HIV-1 RNA low-positive control (3.78 log10 copies/ml) (Biocentric). We assessed the maximum lower-limit at which a sample can be correctly quantified by diluting the 3.78-log10 copies/ml standard to two lower concentrations (2.50 and 1.78 log10 copies/ml) and tested them in eight replicates.
RT-qPCR reference techniques.
The generic HIV-1 viral load Biocentric assay was used as a reference for group M detection and quantification according to the manufacturer's instructions. This Biocentric assay was previously validated compared to the Versant bDNA HIV RNA kit (v3.0; Siemens Healthcare Diagnostics, Inc., Deerfield, IL) and the Amplicor HIV-1 Monitor standard RT-PCR assay (v1.5; Roche Molecular Systems, Pleasanton, CA) (30, 46) and can detect a wide diversity of HIV-1 group M subtypes and CRFs. Using 200 μl of plasma, the threshold of the Biocentric assay was set at 2.50 log10 copies/ml. The total duration of the amplification was ca. 120 to 140 min. Cycling and data acquisition were carried out using an ABI Prism 7000 sequence detection system or the 7500 Real-Time PCR system (Applied Biosystems). The generic Biocentric technique was performed in the IRD laboratory in Montpellier, France. The Abbott m2000rt RealTime HIV-1 assay (Abbott Molecular) was used as a reference for group O detection and quantification, since it was previously validated for HIV-1 group O sample quantification (18, 23). This technique was performed in the IMPM/IRD laboratory in Yaoundé, Cameroon, according to the manufacturer's instructions.
Statistical analyses.
The STATA software package version 10.1 (Stata Corp., College Station, TX) was used for all statistical analyses described. The standards and the low-positive control were tested in 10 independent runs to determine the reproducibility, the linearity, and the between-run variability of our RT-qPCR technique. We assessed within-run variability by testing six different samples (five standards and the low-reproductive control) in eight replicates in the same run. The specificity of our assay was calculated as the number of negative samples out of the total number of tested samples from uninfected individuals. The analytical sensitivity for HIV-1 M RNA was calculated as the number of samples detected with our technique divided by the number of samples detected with the Biocentric generic assay, including samples under the quantification threshold. Correlation between results from the generic Biocentric test and our new RT-qPCR assay were measured by a Pearson correlation coefficient and by a Spearman rank correlation coefficient for results from each country. We generated a Bland-Altman difference plot for bias and agreement measurements, including the limits of agreement (47).
RESULTS
Reproducibility and variations between and within runs.
The interassay reproducibility of the standard curve with our new RT-qPCR method was assessed on 10 independent assays. In all cases, there was a strong linear correlation between the cycle threshold values found in each experiment and the viral load (log10 copies/ml) with a median correlation coefficient of 1.00 (range, 0.99 to 1.00). The mean slope of the standard curve was −3.33 (range, −3.44 to −3.15), corresponding to a mean amplification efficiency of 99.2%. The standard with the lowest concentration (2.78 log10 copies/ml) was always detected and amplified. The diluted sample at 2.50 log10 copies/ml was always detected and quantified with a low coefficient of variation (inferior to 15%), whereas the diluted sample at 1.78 log10 copies/ml was detected in six of eight replicates. Thus, our RT-qPCR assay has a quantification threshold inferior to 2.50 log10 copies/ml using an input volume of 200 μl of plasma (limit included in the 1.78- to 2.50-log10 copies/ml interval). The low-positive control at 3.78 log10 copies/ml was added to each test and was used to further assess the reproducibility and determine the between-run variation. The mean value of this positive control was 3.83 log10 copies/ml (standard deviation [SD] of ±0.19) with a coefficient of variation of 4.8%. These data are highly similar to what has been determined for the generic HIV viral load Biocentric assay, confirming a good inter-run reproducibility.
To assess the within-run variation, the standards (n = 5) and the low-positive control were each replicated eight times in the same experiment. The low-positive control and the standards were always detected and correctly quantified with a mean coefficient of variation of 4.0% ± 0.2%.
The analyses of HIV-1 group M and HIV negative samples show that the assay has a good sensitivity and specificity.
For the analytical evaluation, a total of 190 HIV-1 group M-positive plasma samples were detected by generic HIV viral load Biocentric assay and tested with the new RT-qPCR test (VL range, 1.68 to 7.78 log10 copies/ml). Of these, 185 plasma samples were effectively detected with our technique (VL range, 2.14 to 8.07 log10 copies/ml). The analytical sensitivity of our assay could be estimated at 97.4% (95% confidence interval [CI] = 94.0 to 99.1%). Five samples, with a Biocentric viral load between 2.18 and 3.04 log10 copies/ml, were not detected with our assay, including three under the Biocentric quantification threshold (2.5 log10 copies/ml). Two samples had a viral load superior to the quantification threshold: one from Cameroon [VL(Biocentric), 3.04 log10 copies/ml] from which no genotype could be obtained despite two amplification attempts in the conserved pol region, and one from the DRC (VL, 2.94 log10 copies/ml) with no genotyping available. The specificity of the test was assessed with 72 HIV negative samples from Cameroon. All samples yielded negative results with our test. Thus, the specificity of the assay was 100% (95% CI = 95.9 to 100%).
Good correlation between the new test and the reference Biocentric assay for the quantification of HIV-1 group M, irrespective of the genotype.
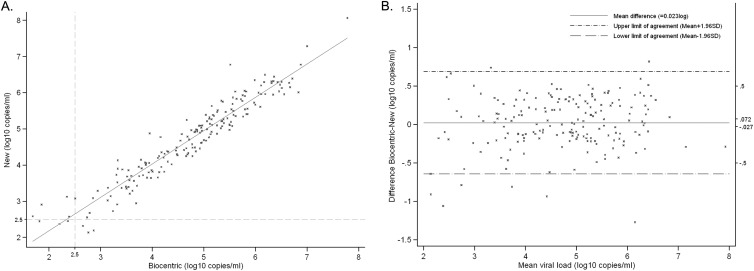
Biocentric HIV viral load results and new RT-qPCR assay viral load measures were both available for 185 HIV-1 group M plasma samples. As shown in Fig. 2A, an excellent correlation was found between the results of both assays (Pearson correlation coefficient r = 0.95; P < 0.0001). Considering the quantification threshold of 2.5 log10 copies/ml, the Biocentric assay and our RT-qPCR test quantified 178 and 179 samples above this limit, respectively. Three samples were under the threshold with both techniques, while four and three samples were under the threshold with Biocentric and our assay, respectively (Fig. 2A). We determined the agreement between the two assays by the Bland-Altman difference plot (Fig. 2B) (47). The mean viral load difference between the two tests was of 0.02 log10 copies/ml and was not significantly different from 0 (P = 0.37). Importantly, the difference between both assays did not increase at low or high viral loads (r = 0.09; P = 0.24). The SD was of 0.34 log10 copies/ml, and the 95% CI ranged from −0.03 to 0.07, included in the ±0.50-log10 copies/ml limits (48). In total, 95.1% of the 185 quantified samples were inside the limits of agreement (mean ± 1.96). Four of the nine samples outside of the limits of agreement (Table 2) had a viral load inferior to the quantification threshold of 2.5 log10 copies/ml with the Biocentric technique, whereas only one was detected under this threshold in our assay (Table 2). Two samples, subtype H and CRF02, had higher viral loads with the Biocentric technique (Table 2). However, seven samples—one subtype B, one CRF22, one CRF37, two CRF02, and two with unknown genotype—had higher viral loads with our new RT-qPCR method, with a mean difference of −0.92 log10 copies/ml. Overall, our test was validated for the quantification of HIV-1 group M RNA since it demonstrated no significant difference with the reference assay.
Fig 2.
HIV-1 group M RNA viral load quantified by the generic HIV-1 viral load Biocentric kit and our new RT-qPCR assay. (A) Samples (n = 185) detected with both techniques were plotted on this linearity plot. The solid line represents the fitted regression. Pearson correlation r, 0.95 (P < 0.0001). The 2.50-log10 copies/ml limit of detection is represented by gray dashed lines. (B) The 185 samples detected with both techniques were plotted on this Bland-Altman difference plot. The vertical axis indicates the difference between the Biocentric and the new RT-qPCR assay viral load, and the horizontal axis shows the mean viral load between the two techniques. The mean bias on the difference (solid line, ∂ = 0.023 log10 copies/ml) and limits of agreement (dashed lines) are shown on the graphic. On the right vertical axis are represented two main limits: the ±0.5 log10 copies/ml limit, and the 95% CI interval (−0.027 to 0.072 log10 copies/ml).
Table 2.
HIV-1 group M samples out of the limits of agreement between our new RT-qPCR method and the generic Biocentric assaya
| Sample | Country | Genotype | VL (log10 copies/ml) |
∂ | |
|---|---|---|---|---|---|
| VL(Biocentric) | VL(new RT-qPCR) | ||||
| Group 1 | |||||
| 3355 | Cameroon | CRF02 | 5.51 | 6.78 | –1.27 |
| 3080 | Cameroon | 1.85* | 2.91 | –1.06 | |
| 625 | DRCb | CRF02 | 3.95 | 4.88 | –0.93 |
| 5004 | Togo | 1.68* | 2.59 | –0.91 | |
| 596 | DRC | CRF37 | 3.23 | 4.13 | –0.81 |
| 3023 | Cameroon | CRF22 | 2.34* | 3.13 | –0.79 |
| 24 | France | B | 1.81* | 2.45* | –0.64 |
| Group 2 | |||||
| 543 | DRC | H | 3.69 | 2.95 | 0.74 |
| 5053 | Togo | CRF02 | 6.83 | 6.01 | 0.82 |
Seven samples, out of the limits of agreement (mean ± 1.96 [standard deviation]), had a higher viral load (VL) with our quantification assay compared to the generic Biocentric test (group 1) and two had a lower viral load (group 2). For each sample, the country of origin and the genotype are given with the viral loads obtained from both techniques and the difference between them: ∂ = VL(Biocentric) − VL(new RT-qPCR). *, Viral load inferior to the 2.5-log10 copies/ml threshold.
DRC, Democratic Republic of the Congo.
Our panel of HIV-1 group M samples was very diverse and covered the genetic diversity of HIV-1 group M subtypes and CRFs. Each subtype or CRF was represented by 1 to 62 samples. Samples for the study came from four different countries of Africa with very distinct HIV-1 circulating strains (i.e., our panel from Burundi had samples mostly from subtype C, whereas our panel from the DRC harbored a high genetic diversity with 11 different subtypes or CRFs, 12 URFs, and 2 from an unknown genotype [Table 1] and from one hospital in France) (Table 1). To determine whether the molecular epidemiological situations, i.e., different HIV-1 subtypes circulating at different frequencies, would impact negatively on our quantitative assay, we assessed the correlation between Biocentric and our technique for each studied country. We found that the Spearman correlation coefficient between the two techniques was superior to 0.95 (P < 0.0001), irrespective of the country. Our test thus showed very good capacity to quantify the viral load of HIV-1 group M plasma samples, irrespective of the molecular epidemiological situation and the HIV-1 group M genotype.
Detection and quantification of HIV-1 group O strains.
We tested 31 HIV-1 group O samples for viral detection with our new RT-qPCR assay, 22 were plasma samples previously tested with the Abbott RealTime kit, and 9 were DNA extracts with HIV-1 group O sequence confirmation (Table 3). With our method, we detected all 10 HIV-1 group O plasma samples that were previously detected with the Abbott kit, showing that our assay can readily detect HIV-1 group O viruses. We then compared viral loads of each plasma sample assessed with our technique and with the Abbott RealTime assay. We found that our new RT-qPCR assay correlated well with the Abbott test for 7 of 10 HIV-1 group O samples, with a mean viral load difference of −0,05 log10 copies/ml for these seven samples [range of ∂ VL(Abbott RealTime) − VL(new RT-qPCR), −0.35 to 0.37 log10 copies/ml]. We quantified three samples with a significantly higher viral load than for the Abbott technique (∂ = −0.72, −1.19, and −1.69 log10 copies/ml). Importantly, we could detect four additional HIV-1 group O strains that were not detected by the reference method. The viral loads of these four samples ranged from 2.18 to 3.64 log10/ml (Table 3). Thus, our technique allowed us to detect and quantify more HIV-1 group O viral RNA than the reference method (higher analytical sensitivity, 64% versus 45%), and some samples detected by both methods had higher viral loads with the new method.
Table 3.
HIV-1 group O detection and quantification by RT-qPCRa
| Sample | VL (log10 copies/ml) |
∂ | |
|---|---|---|---|
| VL(Abbott RealTime) | VL(new RT-qPCR) | ||
| Plasma samples for quantification | |||
| YD1396 | 2.28 | 2.43 | –0.15 |
| C1/378/LIMA | 2.47 | 2.11 | 0.37 |
| YD1431 | 2.53 | 4.22 | –1.69 |
| CM2080 | 3.04 | 3.38 | –0.35 |
| 03/096/A66 | 3.12 | 3.84 | –0.72 |
| YD656 | 3.14 | 4.32 | –1.19 |
| CI973 | 3.18 | 3.21 | –0.03 |
| CM1070 | 3.29 | 3.51 | –0.22 |
| C1/251/NKPI | 3.65 | 3.70 | –0.06 |
| 2778/07 | 3.68 | 3.61 | 0.07 |
| MR140 | – | 3.64 | New +/Abbott – |
| HJ2464 | – | 3.16 | New +/Abbott – |
| HJ2653 | – | 2.18 | New +/Abbott – |
| HJ2656 | – | 3.25 | New +/Abbott – |
| YD593 | – | – | |
| YD594 | – | – | |
| YD603 | – | – | |
| 1689/09 | – | – | |
| 2634/08 | – | – | |
| CI706 | – | – | |
| up0041 | – | – | |
| HJ2722 | – | – | |
| DNA samples for detection | |||
| HJ020 | 3.64 | ||
| HJ036 | 4.46 | ||
| HJ100 | 4.00 | ||
| HJ162 | 3.92 | ||
| HJ736 | – | ||
| MI159 | 4.72 | ||
| PA206 | 3.60 | ||
| SKPI4077 | 3.32 | ||
| SKPI1015 | 5.22 | ||
The table is divided into two main parts: (i) plasma samples, to test HIV-1 group O detection and quantification, and (ii) DNA samples, to test for HIV-1 group O detection. Sample identifications are indicated in column 1. For each sample, the viral loads (VL) in log10 copies/ml obtained from both techniques are given if it could be detected (the minus signs in the VL columns reflect nondetection of samples), and the difference between them was calculated: ∂ = VL(Abbott RealTime) − VL(new RT-qPCR), given as the log10 copies/ml. “New +/Abbott −” indicates that only the new assay could detect and quantify the corresponding strains. Values indicated in boldface represent significantly different VLs or different results between our assay and the reference assay.
HIV-1 group O viruses harbor a high genetic diversity, but no subtypes have been determined, such as group M classification. Here, as shown by our phylogenetic tree in the env region (Fig. 1A) and also by analyses in pol region (data not shown), the strains from our panel covered the HIV-1 group O diversity. These data suggest that our technique provides a better quantification of HIV-1 group O viruses than the gold standard commercial test, irrespective of the genetic diversity.
SIVcpzPtt and SIVcpzPts RNA detection and quantification.
The SIVcpz strains from the three infected chimpanzees were readily detected with our assay, while the two plasma samples from Ch-Ni, the SIV-negative chimpanzee, were negative (Table 4), showing that our RT-qPCR assay was able to specifically detect both SIVcpzPts and SIVcpzPtt strains. Plasma samples from Cam155 were all detected and quantified and had viral loads of ∼5 log10 copies/ml (Table 4). Previously, the quantification of the plasmatic SIVcpzPttCam155 RNA concentration was also carried out with the bDNA assay in 2004 (Versant HIV-1 RNA 3.0) and the Abbott RealTime test in 2011 (28). In 2004, the VL(Versant) was 5.09 log10 copies/ml, which was not significantly different from the viral load found with the new RT-qPCR assay. However, a viral load of 3.76 log10 copies/ml was found with the Abbott test in 2011, which was significantly lower than with our technique (∂ = −1.02 log10 copies/ml). We could detect and quantify SIVcpzPttGab2 from a plasma sample of chimpanzee Gab2 drawn in April 1988. We performed two independent RNA extractions and quantifications and found similar viral loads ∼3 log10 copies/ml (Table 4). The strain infecting Ch-No is from the SIVcpzPts lineage (Fig. 1B), a clade more divergent from HIV-1 than SIVcpzPtt. However, our test was still able to detect and quantify this divergent variant (Table 4). For Ch-No, we had four sequential blood samples taken between September 1989 and January 1991: the first two at the end of 1989 had an undetectable viral load, and the last two in April 1990 and January 1991 had detectable viral loads of 2.68 and 3.98 log10 copies/ml, respectively. Previously, Kestens et al. observed a fluctuating pattern with the measurement of virus titers in plasma varying from undetected to 1,000 tissue culture infectious doses/ml (49). From the end of 1997 to 2001 (i.e., dates after our panel), Ondoa et al. quantified viral RNA from Ch-No plasma samples with a specific in-house assay, and the values varied from 3.93 to 5.80 log10 copies/ml (50, 51), in the range of our viral loads. Therefore, despite the high genetic distances between SIVcpz strains, our assay was able to detect and quantify the SIVcpzPtt and SIVcpzPts RNA from chimpanzee plasma samples.
Table 4.
SIVcpzPtt and SIVcpzPts detection and quantification by RT-qPCR in plasma samplesa
| Chimpanzee subspecies | SIVcpz | Plasma sample | RNA extraction date (day.mo.yr) | Serology | Detection | Quantification (log10 copies/ml)c |
|---|---|---|---|---|---|---|
| Pan troglodytes subsp. troglodytes | ||||||
| Ch-Go | SIVcpzPtt-Cam155 | CAM155-01.05.04 | 26.02.09 | + | + | 5.12 |
| CAM155-31.03.11 | 01.04.11b | + | + | 4.64* | ||
| + | 4.92* | |||||
| Gab2 | SIVcpzPtt-Gab2 | GAB2-01.04.88-1 | 12.04.11 | + | + | 3.26 |
| GAB2-01.04.88-2 | 12.04.11 | + | + | 3.29 | ||
| Pan troglodytes subsp. schweinfurthii | ||||||
| Ch-No | SIVcpzPts-ANT | Ch-No-29.09.89 | 12.04.11 | + | - | |
| Ch-No27.10.89 | 12.04.11 | + | - | |||
| Ch-No-14.04.90 | 12.04.11 | + | + | 2.68 | ||
| Ch-No-08.01.91 | 12.04.11 | + | + | 3.98† | ||
| Ch-Ni | Negative | Ch-Ni-07.11.89 | 12.04.11 | – | – | |
| Ch-Ni-07.11.89 | 12.04.11 | – | – |
Two to four plasma samples per individual from sequential blood tests from SIVcpz-infected chimpanzees (Ch-Go, Gab2, and Ch-No), as well as an SIV-negative chimpanzee (negative control, Ch-Ni), were tested for the presence of SIV antibodies (serology) and the detection and quantification of viral RNA by the new RT-qPCR assay.
RNA extraction was performed in Cameroon, and quantification was performed in Montpellier, France.
*, Duplicates; †, viral titer in the plasma, 100 tissue culture infected doses/ml (49).
SIVcpz and SIVgor detection from fecal samples.
Here, we tested the detection of SIVcpzPtt and SIVgor viral RNA from fecal samples (i) to determine whether our real-time RT-PCR assay was able to detect both types of viruses, direct ancestors of all HIV-1 groups, and (ii) to test whether this assay was sensitive enough for viral detection in fecal samples.
We showed previously that our test was able to detect SIVcpzPtt virus in plasma samples. Here, we tested 24 fecal samples from nine different Pan troglodytes subsp. troglodytes chimpanzees previously shown, by serology, to be infected with SIVcpzPtt (28, 42). Since these chimpanzees were from four different locations in south Cameroon and because of the phylogeographic clustering of SIVcpzPtt, the viruses tested were expected to have high genetic distances between them (52), which could be confirmed for four of them (28, 42) (Fig. 1B). Here, from a unique RNA extract, we could amplify SIVcpzPtt from only three fecal samples (corresponding to two individuals) with the conventional RT-PCR, while we could detect SIVcpzPtt from eight fecal samples (corresponding to four individuals) with the real-time RT-PCR assay (Table 5). We confirmed by sequence analyses that the amplified LTR fragments corresponded to SIVcpzPtt (data not shown). It is thus possible to detect SIVcpzPtt in fecal samples using this real-time RT-PCR system. Although, the sensitivity of detection on this small number of samples could not be determined and further studies are needed, the sensitivity of the new assay seemed better than with conventional RT-PCR.
Table 5.
SIVcpzPtt and SIVgor detection from fecal samples with our real-time RT-PCR assaya
| Fecal sample | Individual | Extraction dateb | RT-PCR amplification after multiple attempts on various RNA extracts | RT-PCR amplification on the given RNA extract | Real-time RT-PCR detection on the given RNA extract | “VL” if detected |
|---|---|---|---|---|---|---|
| Chimpanzees | ||||||
| Cam155-1 | Ch-Go | 20110311 | – | – | – | |
| Cam155-4 | Ch-Go | 20110311 | – | – | – | |
| Cam155-2 | Ch-Go | 20110311 | Pos | Pos | Pos | 1.9 |
| Cam155-3 | Ch-Go | 20110311 | Pos | Pos | Pos | 2.56 |
| CR4891 | BYc-ID1 | 20100402 | Pos | – | Pos | 1.63 |
| CR3261 | DJc-ID1 | 20090401 | Pos | – | – | |
| CR6369 | DJc-ID3 | – | – | – | ||
| CR5137 | MBc-ID4 | 20100526 | – | – | – | |
| CR5138 | MBc-ID4 | 20100526 | – | – | – | |
| CR6232 | MBc-ID8 | – | – | – | ||
| CR6233 | MBc-ID8 | Pos | Pos | – | ||
| CR6234 | MBc-ID8 | – | – | – | ||
| CR6235 | MBc-ID8 | – | – | – | ||
| CR6236 | MBc-ID8 | – | – | – | ||
| CR6386 | MBc-ID8 | – | – | – | ||
| CR6387 | MBc-ID8 | – | – | Pos | 2,75* | |
| CR6388 | MBc-ID8 | – | – | Pos | 2.71 | |
| CR6254 | MBc-ID9 | – | – | – | ||
| CR6405 | MBc-ID11 | – | – | Pos | 2.45* | |
| CR6406 | MBc-ID11 | – | – | – | ||
| CR6407 | MBc-ID11 | – | – | Pos | 2.34 | |
| CR6413 | MBc-ID11 | – | – | – | ||
| CR6414 | MBc-ID11 | – | – | Pos | 2.46 | |
| CR6411 | MBc-ID10 | – | – | – | ||
| Total | 5 | 3 | 8 | |||
| Gorillas | ||||||
| CR6684 | CPg-ID? | – | – | – | ||
| CR3428 | CPg-ID01 | 20091021 | Pos | – | – | |
| CR3428 | CPg-ID01 | 20090619 | Pos | – | Pos | 1.66 |
| CR6101 | CPg-ID02 | 20110124 | – | – | – | |
| CR6435 | CPg-ID04 | – | – | – | ||
| CR6437 | CPg-ID04 | – | – | – | ||
| CR6438 | CPg-ID04 | – | – | – | ||
| CR6451 | CPg-ID04 | – | – | – | ||
| CR6473 | CPg-ID04 | – | – | – | ||
| CR6477 | CPg-ID04 | – | – | – | ||
| CR6481 | CPg-ID04 | – | – | Pos | 2.55 | |
| CR6485 | CPg-ID04 | – | – | – | ||
| CR6486 | CPg-ID04 | – | – | – | ||
| CR6495 | CPg-ID04 | Pos | – | – | ||
| CR6640 | CPg-ID04 | – | – | – | ||
| CR6641 | CPg-ID04 | – | – | Pos | 2.57 | |
| CR6682 | CPg-ID04 | Pos | – | – | ||
| CR5752 | CPg-ID05 | 20100505 | – | – | – | |
| CR5803 | CPg-ID05 | 20101019 | – | – | – | |
| CR5804 | CPg-ID05 | 20100818 | – | – | – | |
| CR5849 | CPg-ID05 | 20100817 | – | – | – | |
| CR6442 | CPg-ID05 | – | – | Pos | 1.56 | |
| CR6453 | CPg-ID05 | – | – | – | ||
| CR6465 | CPg-ID05 | – | – | Pos | 2.17 | |
| CR6466 | CPg-ID05 | Pos | – | – | ||
| CR6476 | CPg-ID05 | – | – | – | ||
| CR6478 | CPg-ID05 | – | – | Pos | 2.21 | |
| CR6488 | CPg-ID05 | – | – | – | ||
| CR6489 | CPg-ID05 | – | – | – | ||
| CR6685 | CPg-ID05 | – | – | – | ||
| CR2744 | CPg-ID11 | 20081211 | – | – | – | |
| CR2749 | CPg-ID11 | 20081211 | – | – | – | |
| CR3018 | CPg-ID13 | 20080630 | – | – | – | |
| CR3403 | CPg-ID30 | 20090304 | Pos | – | Pos | 1.65 |
| CR3411 | CPg-ID31 | 20090421 | Pos | – | – | |
| CR6631 | CPg-ID37 | – | – | – | ||
| CR6635 | CPg-ID37 | – | – | – | ||
| CR4763 | CPg-ID38 | 20100402 | Pos | Pos | – | |
| CR5832 | CPg-ID60 | 20100817 | – | – | – | |
| CR5816 | CPg-ID65 | 20101019 | – | – | – | |
| CR6484 | CPg-ID65 | – | – | – | ||
| CR6534 | CPg-ID66 | Pos | – | – | ||
| CR6555 | CPg-ID66 | – | – | – | ||
| CR5810 | CPg-ID67 | 20101019 | – | – | – | |
| CR6688 | CPg-ID72 | – | – | – | ||
| CR6090 | CPg-mixed | 20110124 | Pos | Pos | Pos | 1.66 |
| CR6091 | CPg-mixed | 20110124 | Pos | Pos | – | |
| CR3795 | DJg-ID1 | 20090304 | Pos | – | – | |
| CR4099b | DJg-ID2 | 20091021 | Pos | – | Pos | 1.64 |
| CR5265 | DJg-ID3 | 20100402 | – | – | Pos | 1.66 |
| CR4112 | DJg-ID4 | 20090505 | Pos | – | – | |
| CR6259 | DJg-IDx | – | – | Pos | 2.84 | |
| CR6278 | DJg-IDx | Pos | Pos | Pos | 2.76 | |
| CR6279 | DJg-IDx | – | – | Pos | 2.59 | |
| Total | 15 | 4 | 13 |
Fecal samples from chimpanzees and gorillas from Cameroon, positive in the HIV-1/-2 Innolia serological assay, were subjected to our new real-time RT-PCR test. For each sample tested, the sample number is given with the identification of the corresponding individual (e.g., CPg-ID1, abbreviation of the collection site followed by a letter for the species (g, gorilla; c, chimpanzee). Some samples were previously confirmed as SIV positive by conventional RT-PCR after multiple extraction and amplification attempts (the result of 2 to 10 amplification attempts from 1 to 10 different RNA extracts). For each sample and for a given RNA extract, we performed a conventional RT-PCR in gp41 region and a real-time RT-PCR with our new protocol. For information, values obtained with the real-time RT-PCR ranged between 1.63 and 2.84 log10 copies/ml. Pos, positive; −, negative.
Extraction date numerals indicate the year (YYYY), month (MM), and day (DD) as a single number: “YYYYMMDD.”
In addition, we tested 54 fecal samples from 22 Gorilla gorilla subsp. gorilla individuals from Cameroon previously shown, by serology, to be infected with SIVgor. In previous studies (28, 42), we could amplify and sequence SIVgor small fragments with a conventional RT-PCR in only 15 samples after multiple extractions and amplification attempts (Fig. 1B). Here, on a unique RNA extract, we could amplify SIVgor viruses from only four fecal samples (corresponding to three infected gorillas) with the conventional RT-PCR, whereas we could detect SIVgor viruses in 13 fecal samples (corresponding to eight individuals) with the real-time RT-PCR assay (Table 5). We confirmed by LTR sequencing analyses that the amplified fragments were corresponding to SIVgor. Our test is thus able to detect SIVgor viruses in fecal samples. After one attempt, the new real-time assay was able to detect SIVgor strains on 24% of the samples, compared to only 7% with the conventional method.
What is striking is that SIVcpz and SIVgor from various fecal samples from the same infected individual were not systematically detected. Thus, the main limit for SIV detection and amplification in fecal samples from great apes is not the genetic diversity of the viruses but the extremely low viral load in these samples, at the limit of detection of any PCR assay, which is certainly due to the sample degradation and/or the low viral shedding in feces.
DISCUSSION
We were able to develop a new real-time RT-PCR assay with the capacity to detect and quantify a wide range of HIV-1 variants and their progenitors SIVcpz and SIVgor, infecting chimpanzees and gorillas, respectively. The cost per reaction was comparable to the costs of other generic or “in-house” assays (lower than $15 for reagents and consumables) and, most importantly, was substantially less than the cost of commercial tests at around $50 to $100 per reaction (2).
Our assay had a high PCR efficiency with low variations between and within runs. The quantification threshold was inferior to 2.50 log10 copies/ml (range, 1.68 to 2.50) with an input volume of 200 μl, which is comparable with commercial and “in-house” assays with the same input volume (18, 24). As shown previously, a higher plasma input volume could lower the quantification threshold our assay (18, 24), but the test was validated here on the same RNA extraction method as for the Biocentric assay to ensure reproducibility and eliminate bias related to different extraction procedures. The 100% specificity of our test shows the unlikeliness of false-positive results that have adverse consequences for a patient on ART with a normally undetectable viral load. Steegen et al. reported, for example, a false-positive result out of 20 HIV-negative samples tested with the generic Biocentric assay (specificity, 95.0%; 95% CI, 73.1 to 99.7%) (53). The analytical sensitivity of our real-time assay, 97.4%, was calculated on 190 HIV-1 group M-positive samples previously tested with the generic Biocentric kit with a VL range of 1.68 to 7.78 log10 copies/ml. Only two samples with a viral load superior to the Biocentric quantification threshold were not detected with our technique, possibly because of their low viral load close to the quantification limit. However, 15 other samples with a low to very low viral load (<3 log10 copies/ml) were effectively detected by our test. Alternative explanations for false-negative results would be mismatches with the primers or the probe or a PCR inhibition. An interesting perspective would be to add an internal control to this “in-house” assay to identify false-negative results due to PCR inhibition; Drosten et al. showed that PCR inhibition could concern 3.7% of reactions (45). The impact of these two nondetections comparing to the low Biocentric VL results would not have major clinical consequences in resource-limited settings since, with both results, treated patients would not have switched their ART regimens. Actually, the threshold to determine treatment failure in resource-limited countries as recommended by the World Health Organization in 2010 is 3.7 log10 copies/ml (54).
We showed that the generic Biocentric assay and our new RT-qPCR test were highly correlated with no significant difference between their mean viral load, albeit the wide viral load range tested (1.68 to 7.78 log10 copies/ml), and 95.1% of quantified samples were within the limits of agreement between the two methods (47). Two samples had higher viral load with Biocentric, whereas seven samples from various subtypes were significantly better quantified with our method, including three that had viral loads under the Biocentric threshold. Our panel included HIV-1 group M samples from five different countries, with very diverse HIV-1 subtype/CRF distribution (19), including 39 samples from the DRC and 65 from Cameroon, two countries with an extensive genetic diversity (20, 55, 56). For each country, we found an excellent correlation between both VL methods' results, showing that HIV-1 group M diversity did not impact negatively on our viral quantification. This aspect is of major importance, and VL assays should always be validated and further evaluated in different countries with different molecular epidemiological features, as has been done for previous “in-house” assays developed for resource-limited settings (30, 45). The later criticisms on commercial viral load assays and their lack of validation on “unusual” strains for developed countries (1, 16, 17, 46, 57, 58) have induced some changes in the development of industrial assays (2); i.e., the Abbott real-time assay or the last Roche Cobas TaqMan kit were recently validated and evaluated on HIV-1 group M diversity and a few HIV-1 group O strains (18, 23, 59–62).
Unlike previously described generic or “in-house” tests, the designed probe did not bear numerous mismatches with described HIV-1 group O sequences, and our RT-qPCR assay was able to detect and quantify HIV-1 group O viruses from plasma samples. Importantly, of 22 group O samples, we were able to detect and quantify four samples that were not detected by the Abbott RealTime assay, and we measured higher viral loads in three samples (∂, −1.69 to −0.72), showing that our method may be more sensitive than this commercial assay. In the present study, we were able to test a high number of HIV-1 group O strains (22 plasma samples and nine DNA extracts representing 31 different HIV-1 O strains). In most studies, including the ones performed for commercial assays, only 2 to 11 HIV-1 group O samples were tested, which is low to assess the quality of the quantification (18, 23, 61, 62). Plantier and coworkers were the only team able to test a large number of group O samples (77 different strains) to validate their specific HIV-1 group O “in-house” real-time assay (22, 26, 63). Furthermore, our group O panel covered HIV-1 group O genetic diversity, reflecting that the high genetic diversity of this group did not have an impact on our detection. Since other highly divergent strains (HIV-1 groups N and P) have been found in only few cases in humans, we could not test this new assay on all HIV-1 groups, but we are fairly confident that our test has this capacity since we could detect genetically distant SIVcpz and SIVgor strains.
Because of the ongoing risk of cross-species transmissions of SIVs from apes to humans (64) and the necessity to follow SIVcpz and SIVgor infection in their natural hosts to better understand the pathogenicity of these HIV-1 progenitors (28, 29), our goal was to develop an assay that can detect and quantify all viruses from the HIV-1/SIVcpz/SIVgor clade. We showed here that our test is able to detect and quantify SIVcpzPtt and SIVcpzPts viruses, which are highly divergent strains, in plasma samples from western and eastern central African chimpanzees, respectively. We found that plasmatic SIVcpz viral loads in naturally infected chimpanzees are in the range of HIV-1 viral loads in humans. The test could also detect SIVgor viruses, precursors of HIV-1 group P and probable ancestors of HIV-1 group O (27, 42, 65). The detection of SIVcpz and SIVgor RNA in fecal samples by this method was possible and more efficient than with conventional specific RT-PCR, showing this assay can be a good complement to confirm viral presence in seropositive fecal samples. However, it seems that it cannot be used for SIV screening in fecal samples to replace SIVcpz/SIVgor serological detection because of the numerous false-negative results. By this method, we confirmed that SIVcpz and SIVgor viral loads are very low in fecal samples. In general, the viral loads retrieved from infected chimpanzee plasma samples were higher than the ones obtained from fecal samples. Interestingly, SIVcpzPtt viral loads from both plasma and fecal samples could be tested for Ch-Go, and we found a >100-fold difference between both compartments (Tables 4 and 5, first rows). However, the sample availability was a limit here to analyze in details this pattern on a panel of associated plasma and fecal samples. Amplification of divergent variants such as SIVcpz and SIVgor was not possible with the Biocentric technique due to numerous mismatches with the probe and the primers, and we found that SIVcpz quantification was suboptimal with Abbott RealTime assay. Therefore, this real-time RT-PCR test is a new opportunity to detect possible new emerging SIVs from apes to humans.
In conclusion, we developed a relatively low-cost real-time RT-PCR assay able to detect and quantify all viral strains from the HIV-1/SIVcpz/SIVgor clade, meaning that HIV-1 diversity is covered and that HIV-1 precursors can also be monitored. This new test is thus a major step in the field of viral load quantification since it could monitor any HIV-1 strains currently circulating in humans but could also detect new emergences of SIVcpz/SIVgor in humans. After this validation, an evaluation of this RT-qPCR assay on reference panels and a larger panel of samples from a broad range of variants is needed. Also, a parallel study in a resource-limited country would confirm its use in such settings.
ACKNOWLEDGMENTS
We thank Fatima Mouacha for technical support.
The funding of this study was supported in part by grants from the National Institutes of Health (R01 AI50529), the Agence Nationale de Recherches sur le SIDA, France (ANRS 12182 and ANRS 12255), and the Institut de Recherche pour le Développement. Lucie Etienne was supported by a Ph.D. grant from Sidaction and Fonds de Dotation Pierre Bergé.
Footnotes
Published ahead of print 19 December 2012
REFERENCES
- 1. Fiscus SA, Cheng B, Crowe SM, Demeter L, Jennings C, Miller V, Respess R, Stevens W. 2006. HIV-1 viral load assays for resource-limited settings. PLoS Med. 3:e417 doi:10.1371/journal.pmed.0030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang S, Xu F, Demirci U. 2010. Advances in developing HIV-1 viral load assays for resource-limited settings. Biotechnol. Adv. 28:770–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonard D, Rouet F, Toni TA, Minga A, Huet C, Ekouevi DK, Dabis F, Salamon R, Rouzioux C. 2003. Field evaluation of an improved assay using a heat-dissociated p24 antigen for adults mainly infected with HIV-1 CRF02_AG strains in Cote d'Ivoire, West Africa. J. Acquir. Immune. Defic. Syndr. 34:267–273 [DOI] [PubMed] [Google Scholar]
- 4. Ribas SG, Ondoa P, Schupbach J, van der Groen G, Fransen K. 2003. Performance of a quantitative human immunodeficiency virus type 1 p24 antigen assay on various HIV-1 subtypes for the follow-up of human immunodeficiency type 1 seropositive individuals. J. Virol. Methods 113:29–34 [DOI] [PubMed] [Google Scholar]
- 5. Braun J, Plantier JC, Hellot MF, Tuaillon E, Gueudin M, Damond F, Malmsten A, Corrigan GE, Simon F. 2003. A new quantitative HIV load assay based on plasma virion reverse transcriptase activity for the different types, groups and subtypes. AIDS 17:331–336 [DOI] [PubMed] [Google Scholar]
- 6. Malmsten A, Shao XW, Aperia K, Corrigan GE, Sandstrom E, Kallander CF, Leitner T, Gronowitz JS. 2003. HIV-1 viral load determination based on reverse transcriptase activity recovered from human plasma. J. Med. Virol. 71:347–359 [DOI] [PubMed] [Google Scholar]
- 7. Labbett W, Garcia-Diaz A, Fox Z, Clewley GS, Fernandez T, Johnson M, Geretti AM. 2009. Comparative evaluation of the ExaVir Load version 3 reverse transcriptase assay for measurement of human immunodeficiency virus type 1 plasma load. J. Clin. Microbiol. 47:3266–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart P, Cachafeiro A, Napravnik S, Eron JJ, Frank I, van der Horst C, Bosch RJ, Bettendorf D, Bohlin P, Fiscus SA. 2010. Performance characteristics of the Cavidi ExaVir viral load assay and the ultra-sensitive P24 assay relative to the Roche Monitor HIV-1 RNA assay. J. Clin. Virol. 49:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ondoa P, Vereecken C, Asahchop EL, Litzroth A, Diallo A, Fransen K, Dieye T, Ryder R, Mboup S, Kestens L. 2009. Proof of principle: an HIV p24 microsphere immunoassay with potential application to HIV clinical diagnosis. Cytometry B Clin. Cytometry 76:231–236 [DOI] [PubMed] [Google Scholar]
- 10. Panhotra BR, Hassan ZU, Joshi CS, Bahrani A. 2005. Visual detection of multiple viral amplicons by dipstick assay: its application in screening of blood donors a welcome tool for the limited resource settings. J. Clin. Microbiol. 43:6218–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu H, Yaglidere O, Su TW, Tseng D, Ozcan A. 2011. Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab Chip 11:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li G, Li W, Guo F, Xu S, Zhao N, Chen S, Liu L. 2010. A novel real-time PCR assay for determination of viral loads in person infected with hepatitis B virus. J. Virol. Methods 165:9–14 [DOI] [PubMed] [Google Scholar]
- 13. Li P, Ruel T, Fujimoto K, Hatano H, Yukl S, Eller LA, Liegler T, Kamya M, Gassasira A, Dorsey G, Rosenthal PJ, Havlir DV, Wong JK. 2010. Novel application of locked nucleic acid chemistry for a TaqMan assay for measuring diverse human immunodeficiency virus type 1 subtypes. J. Virol. Methods 170:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rouet F, Rouzioux C. 2007. HIV-1 viral load testing cost in developing countries: what's new? Expert Rev. Mol. Diagn. 7:703–707 [DOI] [PubMed] [Google Scholar]
- 15. Preiser W, Drexler JF, Drosten C. 2006. HIV-1 viral load assays for resource-limited settings: clades matter. PLoS Med. 3:e538; author reply e550. doi:10.1371/journal.pmed.0030538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peeters M, Aghokeng AF, Delaporte E. 2010. Genetic diversity among human immunodeficiency virus-1 non-B subtypes in viral load and drug resistance assays. Clin. Microbiol. Infect. 16:1525–1531 [DOI] [PubMed] [Google Scholar]
- 17. Swanson P, de Mendoza C, Joshi Y, Golden A, Hodinka RL, Soriano V, Devare SG, Hackett J., Jr 2005. Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol. 43:3860–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang N, Huang S, Salituro J, Mak WB, Cloherty G, Johanson J, Li YH, Schneider G, Robinson J, Hackett J, Jr, Swanson P, Abravaya K. 2007. A RealTime HIV-1 viral load assay for automated quantitation of HIV-1 RNA in genetically diverse group M subtypes A-H, group O and group N samples. J. Virol. Methods 146:236–245 [DOI] [PubMed] [Google Scholar]
- 19. Hemelaar J, Gouws E, Ghys PD, Osmanov S. 2011. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS 25:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor BS, Hammer SM. 2008. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 359:1965–1966 [DOI] [PubMed] [Google Scholar]
- 21. Treadwell TL, Fleisher J. 2003. Underestimation of HIV-1 plasma viral burden in patients who acquire infection abroad: the experience in a community hospital clinic. Arch. Intern. Med. 163:1613–1614 [DOI] [PubMed] [Google Scholar]
- 22. Gueudin M, Leoz M, Lemee V, De Oliveira F, Vessiere A, Kfutwah A, Plantier JC. 2012. A new real-time quantitative PCR for diagnosis and monitoring of HIV-1 group O infection. J. Clin. Microbiol. 50:831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swanson P, Huang S, Abravaya K, de Mendoza C, Soriano V, Devare SG, Hackett J., Jr 2007. Evaluation of performance across the dynamic range of the Abbott RealTime HIV-1 assay as compared to VERSANT HIV-1 RNA 3.0 and AMPLICOR HIV-1 MONITOR v1.5 using serial dilutions of 39 group M and O viruses. J. Virol. Methods 141:49–57 [DOI] [PubMed] [Google Scholar]
- 24. Rouet F, Menan H, Viljoen J, Ngo-Giang-Huong N, Mandaliya K, Valea D, Lien TX, Danaviah S, Rousset D, Ganon A, Nerrienet E. 2008. In-house HIV-1 RNA real-time RT-PCR assays: principle, available tests, and usefulness in developing countries. Expert Rev. Mol. Diagn. 8:635–650 [DOI] [PubMed] [Google Scholar]
- 25. Pyne MT, Brown KL, Hillyard DR. 2010. Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance. J. Clin. Microbiol. 48:2852–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gueudin M, Plantier JC, Damond F, Roques P, Mauclere P, Simon F. 2003. Plasma viral RNA assay in HIV-1 group O infection by real-time PCR. J. Virol. Methods 113:43–49 [DOI] [PubMed] [Google Scholar]
- 27. Plantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemee V, Damond F, Robertson DL, Simon F. 2009. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15:871–872 [DOI] [PubMed] [Google Scholar]
- 28. Etienne L, Nerrienet E, LeBreton M, Bibila GT, Foupouapouognigni Y, Rousset D, Nana A, Djoko CF, Tamoufe U, Aghokeng AF, Mpoudi-Ngole E, Delaporte E, Peeters M, Wolfe ND, Ayouba A. 2011. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodytes chimpanzee with AIDS-related symptoms. Retrovirology 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, Danel C, Anglaret X, Leroy V, Msellati P, Dabis F, Rouzioux C. 2005. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J. Clin. Microbiol. 43:2709–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vergne L, Snoeck J, Aghokeng A, Maes B, Valea D, Delaporte E, Vandamme AM, Peeters M, Van Laethem K. 2006. Genotypic drug resistance interpretation algorithms display high levels of discordance when applied to non-B strains from HIV-1 naive and treated patients. FEMS Immunol. Med. Microbiol. 46:53–62 [DOI] [PubMed] [Google Scholar]
- 32. Dagnra AY, Vidal N, Mensah A, Patassi A, Aho K, Salou M, Monleau M, Prince-David M, Singo A, Pitche P, Delaporte E, Peeters M. 2011. High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lome, Togo. J. Int. AIDS Soc. 14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muwonga J, Edidi S, Butel C, Vidal N, Monleau M, Okenge A, Mandjo JL, Mukumbi H, Muyembe JJ, Mbayo F, Nzongola DK, Delaporte E, Boillot F, Peeters M. 2011. Resistance to antiretroviral drugs in treated and drug-naive patients in the Democratic Republic of Congo. J. Acquir. Immune Defic. Syndr. 57(Suppl 1):S27–S33 [DOI] [PubMed] [Google Scholar]
- 34. Monleau M, Butel C, Delaporte E, Boillot F, Peeters M. 2010. Effect of storage conditions of dried plasma and blood spots on HIV-1 RNA quantification and PCR amplification for drug resistance genotyping. J. Antimicrob. Chemother. 65:1562–1566 [DOI] [PubMed] [Google Scholar]
- 35. Vergne L, Bourgeois A, Mpoudi-Ngole E, Mougnutou R, Mbuagbaw J, Liegeois F, Laurent C, Butel C, Zekeng L, Delaporte E, Peeters M. 2003. Biological and genetic characteristics of HIV infections in Cameroon reveals dual group M and O infections and a correlation between SI-inducing phenotype of the predominant CRF02_AG variant and disease stage. Virology 310:254–266 [DOI] [PubMed] [Google Scholar]
- 36. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321 [DOI] [PubMed] [Google Scholar]
- 38. Bibollet-Ruche F, Bailes E, Gao F, Pourrut X, Barlow KL, Clewley JP, Mwenda JM, Langat DK, Chege GK, McClure HM, Mpoudi-Ngole E, Delaporte E, Peeters M, Shaw GM, Sharp PM, Hahn BH. 2004. New simian immunodeficiency virus infecting De Brazza's monkeys (Cercopithecus neglectus): evidence for a cercopithecus monkey virus clade. J. Virol. 78:7748–7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peeters M, Honore C, Huet T, Bedjabaga L, Ossari S, Bussi P, Cooper RW, Delaporte E. 1989. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS 3:625–630 [DOI] [PubMed] [Google Scholar]
- 40. Peeters M, Fransen K, Delaporte E, Van den Haesevelde M, Gershy-Damet GM, Kestens L, van der Groen G, Piot P. 1992. Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild-captured chimpanzee. AIDS 6:447–451 [DOI] [PubMed] [Google Scholar]
- 41. Vanden Haesevelde MM, Peeters M, Jannes G, Janssens W, van der Groen G, Sharp PM, Saman E. 1996. Sequence analysis of a highly divergent HIV-1-related lentivirus isolated from a wild captured chimpanzee. Virology 221:346–350 [DOI] [PubMed] [Google Scholar]
- 42. Neel C, Etienne L, Li Y, Takehisa J, Rudicell RS, Bass IN, Moudindo J, Mebenga A, Esteban A, Van Heuverswyn F, Liegeois F, Kranzusch PJ, Walsh PD, Sanz CM, Morgan DB, Ndjango JB, Plantier JC, Locatelli S, Gonder MK, Leendertz FH, Boesch C, Todd A, Delaporte E, Mpoudi-Ngole E, Hahn BH, Peeters M. 2010. Molecular epidemiology of simian immunodeficiency virus infection in wild-living gorillas. J. Virol. 84:1464–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Etienne L, Locatelli S, Ayouba A, Esteban A, Butel C, Liegeois F, Aghokeng A, Delaporte E, Mpoudi Ngole E, Peeters M. 2012. Noninvasive follow-up of simian immunodeficiency virus infection in wild-living nonhabituated Western lowland gorillas in Cameroon. J. Virol. 86:9760–9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Baar MP, van Dooren MW, de Rooij E, Bakker M, van Gemen B, Goudsmit J, de Ronde A. 2001. Single rapid real-time monitored isothermal RNA amplification assay for quantification of human immunodeficiency virus type 1 isolates from groups M, N, and O. J. Clin. Microbiol. 39:1378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drosten C, Panning M, Drexler JF, Hansel F, Pedroso C, Yeats J, de Souza Luna LK, Samuel M, Liedigk B, Lippert U, Sturmer M, Doerr HW, Brites C, Preiser W. 2006. Ultrasensitive monitoring of HIV-1 viral load by a low-cost real-time reverse transcription-PCR assay with internal control for the 5′ long terminal repeat domain. Clin. Chem. 52:1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rouet F, Chaix ML, Nerrienet E, Ngo-Giang-Huong N, Plantier JC, Burgard M, Peeters M, Damond F, Ekouevi DK, Msellati P, Ferradini L, Rukobo S, Marechal V, Schvachsa N, Wakrim L, Rafalimanana C, Rakotoambinina B, Viard JP, Seigneurin JM, Rouzioux C. 2007. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA Quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J. Acquir. Immune Defic. Syndr. 45:380–388 [DOI] [PubMed] [Google Scholar]
- 47. Bland JM, Altman DG. 1999. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 8:135–160 [DOI] [PubMed] [Google Scholar]
- 48. Saag MS, Holodniy M, Kuritzkes DR, O'Brien WA, Coombs R, Poscher ME, Jacobsen DM, Shaw GM, Richman DD, Volberding PA. 1996. HIV viral load markers in clinical practice. Nat. Med. 2:625–629 [DOI] [PubMed] [Google Scholar]
- 49. Kestens L, Vingerhoets J, Peeters M, Vanham G, Vereecken C, Penne G, Niphuis H, van Eerd P, van der Groen G, Gigase P, et al. 1995. Phenotypic and functional parameters of cellular immunity in a chimpanzee with a naturally acquired simian immunodeficiency virus infection. J. Infect. Dis. 172:957–963 [DOI] [PubMed] [Google Scholar]
- 50. Ondoa P, Kestens L, Davis D, Vereecken C, Willems B, Fransen K, Vingerhoets J, Zissis G, ten Haaft P, Heeney J, van der Groen G. 2001. Longitudinal comparison of virus load parameters and CD8 T-cell suppressive capacity in two SIVcpz-infected chimpanzees. J. Med. Primatol. 30:243–253 [DOI] [PubMed] [Google Scholar]
- 51. Ondoa P, Davis D, Willems B, Heyndrickx L, Kestens L, van der Berg I, Coppens S, Janssens W, Heeney J, van der Groen G. 2001. Genetic variability of the V1 and V2 env domains of SIVcpz-ant and neutralization pattern of plasma viruses in a chimpanzee infected naturally. J. Med. Virol. 65:765–776 [DOI] [PubMed] [Google Scholar]
- 52. Van Heuverswyn F, Li Y, Bailes E, Neel C, Lafay B, Keele BF, Shaw KS, Takehisa J, Kraus MH, Loul S, Butel C, Liegeois F, Yangda B, Sharp PM, Mpoudi-Ngole E, Delaporte E, Hahn BH, Peeters M. 2007. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology 368:155–171 [DOI] [PubMed] [Google Scholar]
- 53. Steegen K, Luchters S, De Cabooter N, Reynaerts J, Mandaliya K, Plum J, Jaoko W, Verhofstede C, Temmerman M. 2007. Evaluation of two commercially available alternatives for HIV-1 viral load testing in resource-limited settings. J. Virol. Methods 146:178–187 [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization 2010. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2010 revision. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 55. Vidal N, Peeters M, Mulanga-Kabeya C, Nzilambi N, Robertson D, Ilunga W, Sema H, Tshimanga K, Bongo B, Delaporte E. 2000. Unprecedented degree of human immunodeficiency virus type 1 (HIV-1) group M genetic diversity in the Democratic Republic of Congo suggests that the HIV-1 pandemic originated in Central Africa. J. Virol. 74:10498–10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, Kabongo JM, Kalengayi RM, Van Marck E, Gilbert MT, Wolinsky SM. 2008. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature 455:661–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drexler JF, de Souza Luna LK, Pedroso C, Pedral-Sampaio DB, Queiroz AT, Brites C, Netto EM, Drosten C. 2007. Rates of and reasons for failure of commercial human immunodeficiency virus type 1 viral load assays in Brazil. J. Clin. Microbiol. 45:2061–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Holguin A, Lopez M, Molinero M, Soriano V. 2008. Performance of three commercial viral load assays, Versant human immunodeficiency virus type 1 (HIV-1) RNA bDNA v3.0, Cobas AmpliPrep/Cobas TaqMan HIV-1, and NucliSens HIV-1 EasyQ v1.2, testing HIV-1 non-B subtypes and recombinant variants. J. Clin. Microbiol. 46:2918–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Bel A, Marissens D, Debaisieux L, Liesnard C, Van den Wijngaert S, Lauwers S, Pierard D. 2010. Correction of underquantification of human immunodeficiency virus type 1 load with the second version of the Roche Cobas AmpliPrep/Cobas TaqMan assay. J. Clin. Microbiol. 48:1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sire JM, Vray M, Merzouk M, Plantier JC, Pavie J, Maylin S, Timsit J, Lascoux-Combe C, Molina JM, Simon F, Delaugerre C. 2011. Comparative RNA quantification of HIV-1 group M and non-M with the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0 and Abbott Real-Time HIV-1 PCR assays. J. Acquir. Immune Defic. Syndr. 56:239–243 [DOI] [PubMed] [Google Scholar]
- 61. Sizmann D, Glaubitz J, Simon CO, Goedel S, Buergisser P, Drogan D, Hesse M, Kroh M, Simmler P, Dewald M, Gilsdorf M, Fuerst M, Ineichen R, Kirn A, Pasche P, Wang Z, Weisshaar S, Young K, Haberhausen G, Babiel R. 2010. Improved HIV-1 RNA quantitation by COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 using a novel dual-target approach. J. Clin. Virol. 49:41–46 [DOI] [PubMed] [Google Scholar]
- 62. Swanson P, Holzmayer V, Huang S, Hay P, Adebiyi A, Rice P, Abravaya K, Thamm S, Devare SG, Hackett J., Jr 2006. Performance of the automated Abbott RealTime HIV-1 assay on a genetically diverse panel of specimens from London: comparison to VERSANT HIV-1 RNA 3.0, AMPLICOR HIV-1 MONITOR v1.5, and LCx HIV RNA Quantitative assays. J. Virol. Methods 137:184–192 [DOI] [PubMed] [Google Scholar]
- 63. Plantier JC, Gueudin M, Damond F, Braun J, Mauclere P, Simon F. 2003. Plasma RNA quantification and HIV-1 divergent strains. J. Acquir. Immune Defic. Syndr. 33:1–7 [DOI] [PubMed] [Google Scholar]
- 64. Locatelli S, Peeters M. 2012. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS 26:659–673 [DOI] [PubMed] [Google Scholar]
- 65. Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, Ngolle EM, Sharp PM, Shaw GM, Delaporte E, Hahn BH, Peeters M. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. [DOI] [PubMed] [Google Scholar]
- 66. Gascuel O. 1997. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol. Biol. Evol. 14:685–695 [DOI] [PubMed] [Google Scholar]