Abstract
A multiplex real-time PCR assay that simultaneously detects the mecA, staphylococcal cassette chromosome (SCCmec)-open reading frame X (orfX) junction, and staphylococcal 16S rRNA genes was developed and evaluated using 444 staphylococcal strains. We demonstrated that this assay resulted in fewer false-positive results than a single-locus real-time PCR assay that amplified the SCCmec-orfX junction. This assay would be useful in a clinical laboratory in a region of high endemicity for methicillin-resistant Staphylococcus aureus (MRSA) infections.
TEXT
The spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospital and community settings poses a threat to public health worldwide. Rapid, accurate detection and appropriate intervention reduce the prevalence of MRSA (1–3). Recently, rapid methods for molecular detection of MRSA have been developed. A single-locus real-time PCR assay that amplifies the staphylococcal cassette chromosome (SCCmec)-open reading frame X (orfX) junction was first proposed by Huletsky et al. (4), and now, there are commercially available assays that identify MRSA based on the detection of the SCCmec-orfX junction (5–7). These assays have an advantage over double-locus assays, based on the simultaneous detection of the mecA gene and a S. aureus-specific gene, for the direct detection of MRSA from screening specimens. Double-locus assays have been associated with false-positive MRSA detections in clinical samples, including nasal swabs that contain both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant coagulase-negative staphylococci (MRCoNS) (8). However, false-positive MRSA results have been also reported in single-locus assays (6, 7, 9–12), for example, due to MSSA isolates containing SCCmec remnants that were misidentified as MRSA (11–16).
South Korea has been a region of MRSA infection endemicity for many years. The rates of methicillin resistance among S. aureus isolates recovered from clinical specimens ranged from 67.8% to 74.1% during the 2000s (17). SCCmec is a mobile element that can be inserted into and excised from the chromosome. It was reported that partial excision of SCCmec from epidemic MRSA strains results in MSSA isolates (13, 15). Thus, in regions of high endemicity, the single-locus assay for direct detection of MRSA may have high false-positive results because of the presence of MRSA-derived MSSA strains that carry remnants of SCCmec elements. To address this, we developed a multiplex real-time PCR assay that simultaneously detects the mecA, SCCmec-orfX junction, and staphylococcal 16S rRNA genes. The assay was based on the hypothesis that a pure MRSA strain has constant mecA, SCCmec-orfX junction, and 16S rRNA copy numbers, represented by the threshold cycle (CT) value, and that the exact relationship between CT values of each target may be established. mecA and SCCmec-orfX were targeted to reduce the false-positive MRSA results caused by the presence of SCCmec remnants among MSSA isolates that do not carry mecA. The staphylococcal 16S rRNA gene was targeted to indicate coexisting staphylococcal strains in clinical samples.
This assay was evaluated using 444 strains, which included both reference strains from various international collections and clinical isolates from laboratory collections. The reference strains were 8 strains of MRSA (CCARM 3792, CCARM 3795, CCARM 3798, CCARM 3803, CCARM 3805, CCARM 3877, CCARM 3897, and CCARM 3911), 4 strains of MSSA (KCTC 1621, KCTC 1916, KCTC 1928, and ATCC 29213), and 11 strains of methicillin-susceptible coagulase-negative staphylococci (MSCoNS) (Staphylococcus epidermidis, KCCM 35494; Staphylococcus simulans, KCCM 41686; Staphylococcus capitis, KCCM 41466; Staphylococcus warneri, KCTC 3340; Staphylococcus haemolyticus, KCTC 3341; Staphylococcus xylosus, KCTC 3342; Staphylococcus intermedius, KCTC 3344; Staphylococcus saprophyticus, KCTC 3345; Staphylococcus cohnii, KCTC 3574; Staphylococcus caprae, KCTC 3583; and Staphylococcus auricularis, KCTC 3584). Twenty-nine MSSA isolates carrying SCCmec remnants, which had been confirmed by SCCmec typing (18, 19), were tested as control strains. The clinical isolates consisted of 209 MRSA, 109 MSSA, and 74 MRCoNS strains and were recovered mostly from wound, sputum, blood, and urine samples. Identification and susceptibility testing of these staphylococcal isolates were performed using the MicroScan WalkAway 96 (Siemens Healthcare Diagnostics Inc., West Sacramento, CA) and the Vitek 2 (bioMérieux Inc., Durham, NC) automated identification and susceptibility testing systems.
The reference strains and the clinical isolates were grown on blood agar plates (Asan Pharmaceutical, Seoul, South Korea) at 37°C for 24 h. Two or three bacterial colonies of the reference strains and isolates were harvested with a 1-μl loop and suspended in 0.5 ml of distilled water. The suspension was heated in a boiling water bath for 10 min and centrifuged at 13,000 × g for 5 min. The supernatant was used for the real-time PCR.
Base sequences of the SCCmec-orfX junction, mecA, and staphylococcal 16S rRNA genes were obtained from NCBI GenBank and aligned with Sequencher 5.0 software (Gene Codes Co., Ann Arbor, MI). Based on sequence alignment, we identified regions of interest and designed primers and probes manually or with the Primer 3 program (http://frodo.wi.mit.edu/primer3/). The real-time PCR primers and probes designed and used in this study are shown in Table 1.
Table 1.
The real-time PCR primers and probes for the detection of MRSA
| Oligonucleotide | Sequence (5′→3′)a | Concn (μM) | Target(s)b |
|---|---|---|---|
| FSCC_A | GCGGAGGCTAACTATGTCAA | 0.5 | I, II, IVa, IVb, IVc, IVg, VI, VIII |
| FSCC_B | ATATGTAATTCCTCCACATCTCATT | 0.5 | III, V, VII |
| FSCC_C | GGCTGAAGTAACCGCATCA | 0.5 | IVe |
| FSCC_D | TTCATAATATGTGCTACGCAATC | 0.5 | X |
| FSCC_E | CGGCAATTCTCATAAACCTC | 0.5 | IX, XI |
| BorfX | GCAAAATGACATTCCCACA | 0.5 | orfX |
| PorfX | HEX-TCAATTAACACAACCCGCATCAT-BHQ1 | 0.2 | orfX |
| FmecA | GAATGCAGAAAGACCAAAGC | 0.5 | mecA |
| BmecA | TTCTTTGGAACGATGCCTAT | 0.5 | mecA |
| PmecA | FAM-TTGGCCAATACAGGAACAGCA-BHQ1 | 0.2 | mecA |
| F16SrRNA | CTTACCAAATCTTGACATCCTTT | 0.5 | 16S rRNA |
| B16SrRNA | CTCGTTGCGGGACTTAAC | 0.5 | 16S rRNA |
| P16SrRNA | Cy5.5-CGTCAGCTCGTGTCGTGAGAT-BHQ2 | 0.2 | 16S rRNA |
HEX, hexachloro-6-carboxyfluorescein; FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1; BHQ2, black hole quencher 2.
The Roman numerals indicate the SCCmec types amplified by the primer.
The real-time PCRs were conducted with a Rotor-Gene Q real-time PCR instrument (Qiagen Inc., Germantown, MD). The PCR mixture contained 0.5 μl of primer-probe mix, 5 μl of 2× Rotor-Gene Multiplex PCR master mix (Qiagen Inc., Germantown, MD), and 1.0 μl of template DNA in a total volume of 10 μl. The PCR parameters were 95°C for 5 min followed by 40 cycles of 95°C for 15 s and 60°C for 15 s, and green, yellow, and crimson fluorescence were measured. After completion of PCR, CT values of the mecA, SCCmec-orfX, and 16S rRNA genes were recorded from the Rotor-Gene Q software. Statistical tests, including determinations of the r correlation coefficient and descriptive statistics, were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). P values below the 5% level were considered statistically significant.
The analytical sensitivity of the real-time PCR was determined by 10-fold serial dilutions of a subculture of MRSA strain CCARM 3792. The strain was grown overnight on a blood agar plate, suspended in saline to a density equivalent to a 0.5 McFarland turbidity number, and serially diluted 10-fold from 102 to 107. DNA was extracted from 200 μl of the bacterial dilutions using the QIAcube with a QIAamp DNA minikit (Qiagen Inc., Germantown, MD) and eluted in 50 μl. In parallel, 100 μl of the dilutions was plated on blood agar and incubated at 37°C for 24 h. Thereafter, CFU were counted.
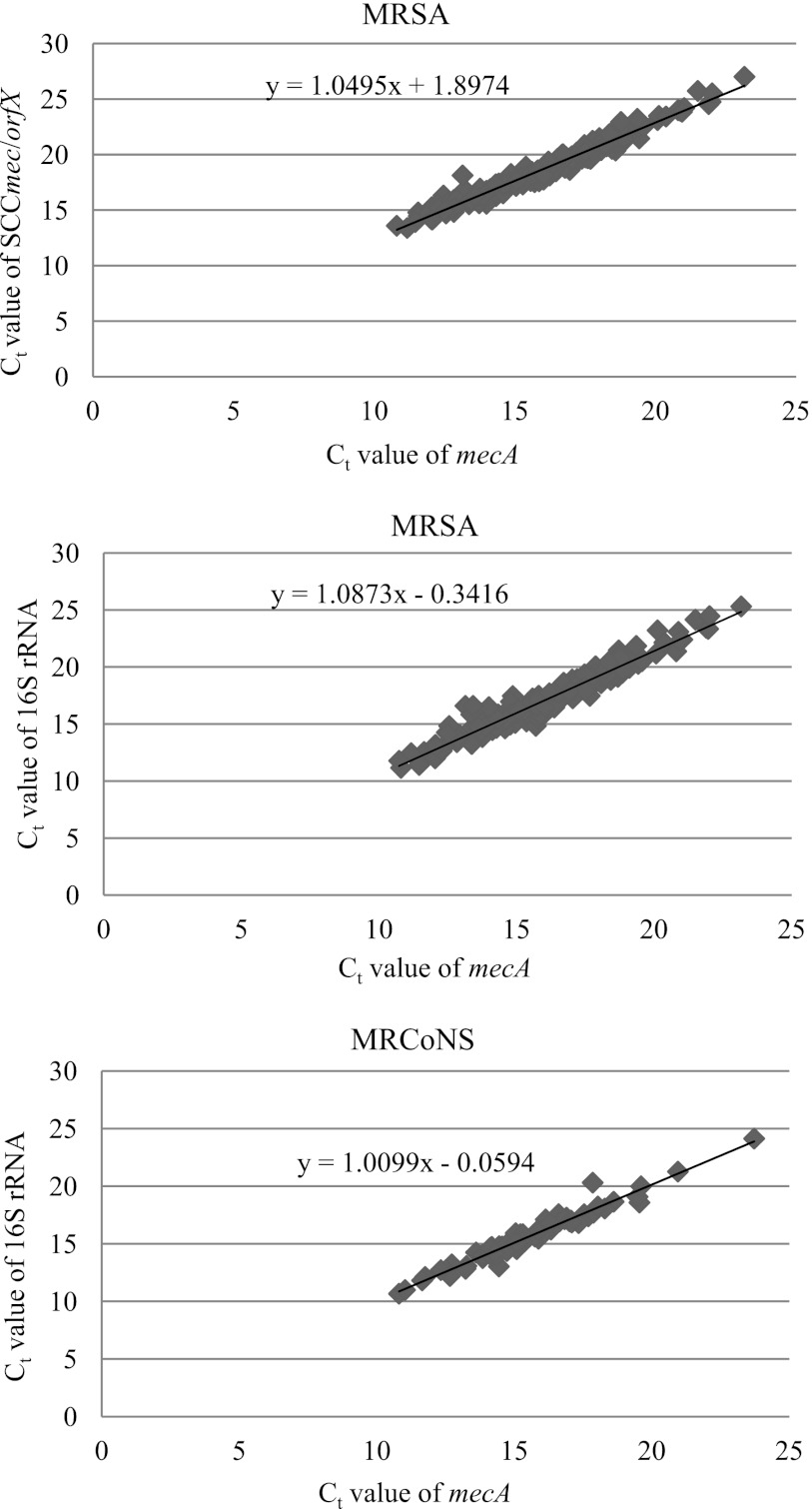
The real-time PCR assay was initially evaluated with 8 MRSA, 4 MSSA, and 11 MSCoNS reference strains and 29 MSSA control strains carrying SCCmec remnants. Only the expected PCR products were amplified from each reference strain. However, SCCmec-orfX was not detected in 6 of 29 control strains. The results of the evaluation of 392 clinical isolates were as follows. Three targets were simultaneously detected in all 209 (100%) MRSA isolates and in 4 (5.4%) MRCoNS isolates. Of the 109 MSSA isolates, the mecA and 16S rRNA genes were detected at the same time in 2 (1.8%) isolates, and both the SCCmec-orfX and 16S rRNA genes were detected in 11 (10.1%) isolates. The CT values of mecA were compared to the CT values of the SCCmec-orfX and 16S rRNA genes. The correlation coefficient determined between mecA CT values and SCCmec-orfX CT values was high for MRSA isolates (r = 0.959; P < 0.0001), and correlation coefficients determined between mecA CT values and 16S rRNA CT values were high for MRSA isolates (r = 0.970; P < 0.0001) and MRCoNS isolates (r = 0.963; P < 0.0001). The results are shown in Fig. 1 and 2 and Table 2. Thus, the CT differences between the SCCmec-orfX and mecA genes (CTSCC) and between the 16S rRNA and mecA genes (CT16S) were used to assess the presence of MRSA. A CTSCC ≥ 4.7 (mean + 4 standard deviations [SD]) indicated that MRSA and staphylococci other than MRSA were present simultaneously, whereas a CT16S ≤ −1.72 (mean − 4 SD) indicated that MRSA and staphylococci lacking the mecA gene coexisted.
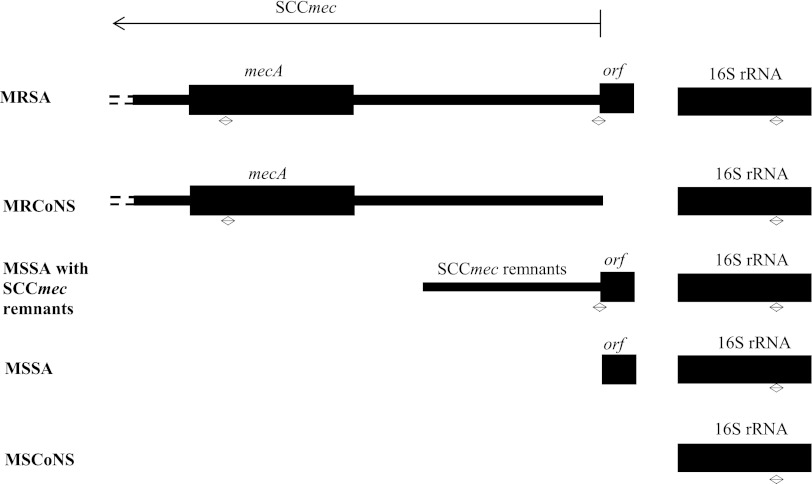
Fig 1.
Schematic diagram showing the relevant genetic elements detected by the multiplex real-time PCR assay in MRSA, MRCoNS, MSSA with SCCmec remnants, MSSA, and MSCoNS. The two-way arrows indicate the amplified regions.
Fig 2.
Correlation between CT values of mecA, SCCmec-orfX, and 16S rRNA genes in 209 MRSA and 74 MRCoNS isolates.
Table 2.
MRSA detection results for clinical isolates by multiplex real-time PCRa
| Species | No. of isolates |
mecA |
SCCmec-orfX |
16S rRNA |
CT difference between SCCmec-orfX and mecA (CTSCC) | CT difference between 16S rRNA and mecA (CT16S) | |||
|---|---|---|---|---|---|---|---|---|---|
| No. of isolates showing positive result | Threshold cycle (CT) value | No. of isolates showing positive result | Threshold cycle (CT) value | No. of isolates showing positive result | Threshold cycle (CT) value | ||||
| MRSA | 209 | 209 | 16.71 ± 2.44 | 209 | 19.02 ± 2.59 | 209 | 17.35 ± 2.73 | 2.70 ± 0.50 (1.58 ∼ 4.99) | 1.08 ± 0.70 (−0.86 ∼ 3.45) |
| MSSA | 109 | 2 | 35.40 ± 1.36 | 11 | 29.32 ± 2.45 | 109 | 17.95 ± 2.87 | −18.58 ± 1.28 (−19.48 ∼ −17.67) | |
| MRCoNS | 74 | 74 | 15.48 ± 2.36 | 4 | 34.98 ± 2.35 | 74 | 15.61 ± 2.41 | 17.78 ± 1.84 (15.37 ∼ 19.69) | 0.10 ± 0.51 (−1.41 ∼ 2.46) |
The mecA, SCCmec-orfX, and 16S rRNA threshold cycle values represent the means ± standard deviations. The CTSCC and CT16S threshold cycle values represent ranges of means ± standard deviations.
The detection limit of the assay was determined using genomic DNA purified from a 1:104 dilution of a stock solution of MRSA strain CCARM 3792 and was found to be 20 CFU per PCR.
Real-time PCR assays, including the BD GeneOhm MRSA assay and Cepheid's Xpert MRSA assay that target the orfX of S. aureus and the right-extremity junction of SCCmec, are currently used for infection control (20–23). Previous studies reported false-negative and -positive results in these single-locus assays ranging from 0.0% to 7.3% and from 0.0% to 5.4%, respectively (4, 6, 24–26). False-negative and -positive results can affect the whole infection control program, bringing about the spread of MRSA in the hospital and unnecessary isolation and decolonization procedures. If a MRSA with an unknown SCCmec type is present in the samples, there could be false-negative results. Currently, 11 different types of SCCmec have been recognized in S. aureus (http://www.sccmec.org/); we designed 5 forward primers, 1 reverse primer, and 1 probe to detect all known types of SCCmec. SCCmec is a 21- to 67-kb genetic fragment that integrates into the chromosome of MRSA at the integration site sequence for SCC (ISS), which is located at the 3′ end of orfX, and carries the central determinant for broad-spectrum β-lactam resistance encoded by the mecA gene (27–29). It is unstable and able to be excised. Excision of the SCCmec can be complete or partial, with some elements left behind at the ISS. Since MRSA strains are resistant to multiple drugs, the excision of SCCmec from such isolates results in MSSA isolates retaining resistance to antibiotics other than ß-lactams (12–16, 30). In the study on determining the proportion and diversity of multidrug-resistant MSSA (MR-MSSA) strains derived from MRSA strains, Donnio et al. investigated 247 MR-MSSA isolates from 60 French hospitals using the IDI-MRSA real-time PCR assay, the forerunner of the BD GeneOhm MRSA assay, and found that 68% of isolates were positive (15). According to Shore's study, 7 MR-MSSA isolates harboring SCCmec remnants identified by SCCmec typing PCR were tested with the BD GeneOhm MRSA and Xpert MRSA assays, and 3 isolates yielded positive results in both assays (14). In the present study, 29 MSSA isolates tested as control strains were MR-MSSA and carried SCCmec remnants that had been confirmed by the multiplex PCR-based SCCmec typing. In 23 of 29 MR-MSSA control strains, the SCCmec-orfX junction was detected. Six control strains with negative results might contain SCCmec remnants that lacked the target-specific region of 5 forward primers. The possibility of the presence of SCCmec remnants in MR-MSSA should always be considered when a real-time PCR assay targeting the SCCmec-orfX junction is used for the rapid detection of MRSA from clinical specimens, since this might give a high number of false-positive results.
To our knowledge, the incidence of false positives has not been reported in South Korea for single-locus real-time PCR assays. We expected the false-positive results to be higher than in countries of low MRSA infection endemicity; as predicted, they were as high as 10.1% in the clinical isolates of staphylococci that had been consecutively collected in our laboratory over 3 months. If such a high rate of false-positive results occurs, the diagnostic value of single-locus real-time PCR assays seems unsatisfactory for a laboratory in a region of high MRSA infection endemicity. Therefore, we considered simultaneous amplification of the mecA gene and SCCmec-orfX junction to rule out MSSA isolates that carry SCCmec remnants and lack the mecA gene. However, this could also lead to a false-positive result when MRCoNS and MSSA carrying SCCmec remnants coexist in the clinical samples. Thus, the staphylococcal 16S rRNA gene was added to the targets to lessen false-positive results. In the case of a pure MRSA strain, relative quantifications of the three target genes would be constant, whereas in cases of mixed populations of MRCoNS and MSSA carrying SCCmec remnants, they would be mostly variable.
Three primer-probe pairs targeting the 16S rRNA gene were designed. Of those, the pair having a CT value very close to the CT of mecA was chosen. In most of the MRSA isolates, the CT of SCCmec was the largest, followed by those of the 16S rRNA and mecA genes. The mecA gene was chosen as a reference gene for relative quantifications. Consequently, the CT differences between the SCCmec-orfX and mecA genes (CTscc) and between the 16S rRNA and mecA genes (CT16S) were constant. We tested whether mixed populations of MRCoNS and MSSA carrying SCCmec remnants can be distinguished by relative quantifications. Mixed cocktails of staphylococcal genomic DNA samples were made to amplify the three targets, including mixtures of genomic DNA from MRSA and staphylococci other than MRSA and mixtures of MRCoNS and MSSA carrying SCCmec remnants. Then, the simulated samples were analyzed. The results of analysis for mixtures of genomic DNA from MRCoNS and MSSA carrying SCCmec remnants are shown in Table 3. The data showed that MRCoNS and MSSA carrying SCCmec remnants were simultaneously present only with CTSCC ≥ 4.7 and CT16S ≤ −1.72, and mixed populations of MRSA and MRCoNS could not be differentiated from those of MRCoNS and MSSA carrying SCCmec remnants with CTSCC ≥ 4.7 and CT16S ≥ −1.71. The data from other mixed DNA samples were in accord with proposed CT calculations; a CTSCC ≥ 4.7 indicated that MRSA and staphylococci other than MRSA were present simultaneously, whereas a CT16S ≤ −1.72 indicated that MRSA and staphylococci lacking the mecA gene coexisted.
Table 3.
Results of the multiplex real-time PCR assay for mixtures of genomic DNA from MRCoNS and MSSA carrying SCCmec remnants
| DNA samplea | Proportion |
CT difference between SCCmec-orfX and mecA (CTSCC)b | CT difference between 16S rRNA and mecA (CT16S)b | |
|---|---|---|---|---|
| MRCoNS (%) | MSSA (%) | |||
| A | 95 | 5 | 15.99 ± 1.48 (13.03 ∼ 16.94) | 0.87 ± 0.50 (0.20 ∼ 1.34) |
| B | 90 | 10 | 14.60 ± 1.52 (11.75 ∼ 16.15) | 0.74 ± 0.17 (0.51 ∼ 0.92) |
| C | 80 | 20 | 13.15 ± 1.74 (10.33 ∼ 15.34) | 0.90 ± 0.44 (0.36 ∼ 1.38) |
| D | 70 | 30 | 12.32 ± 1.96 (9.36 ∼ 15.00) | 0.59 ± 0.23 (0.19 ∼ 0.85) |
| E | 60 | 40 | 11.54 ± 1.79 (8.79 ∼ 14.29) | 0.45 ± 0.51 (−0.13 ∼ 1.10) |
| F | 50 | 50 | 12.25 ± 4.42 (8.34 ∼ 20.75) | 0.46 ± 0.62 (−0.65 ∼ 1.01) |
| G | 40 | 60 | 10.41 ± 2.41 (7.38 ∼ 14.59) | 0.27 ± 0.22 (−0.01 ∼ 0.57) |
| H | 30 | 70 | 9.25 ± 2.23 (6.46 ∼ 12.43) | −0.19 ± 0.40 (−0.72 ∼ 0.22) |
| I | 20 | 80 | 7.81 ± 2.00 (4.94 ∼ 9.71) | −0.56 ± 0.91 (−1.85 ∼ 0.56) |
| J | 10 | 90 | 6.91 ± 1.64 (4.85 ∼ 8.59) | −1.55 ± 1.00 (−2.81 ∼ −0.47) |
| K | 5 | 95 | 5.66 ± 1.34 (3.84 ∼ 7.01) | −2.06 ± 0.69 (−2.99 ∼ −0.95) |
Six samples each of 11 types of DNA were used.
The threshold cycle values represent ranges of means ± standard deviations.
In this study, unexpected amplimers were obtained in 4 MRCoNS isolates and 2 MSSA isolates. A total of 4 MRCoNS isolates yielded simultaneous amplification of the three targets, and the CTSCC values were 19.69, 17.50, 18.55, and 15.37; CT16S values were 0.61, −0.97, −0.31, and −0.09, respectively. In 2 MSSA isolates, the mecA was amplified and the CT16S values were −17.67 and −19.48, respectively. In order to know whether or not these 6 isolates were unusual genotypic strains, stored isolates were regrown on blood agar plates for 24 h. Template DNA was extracted from a single colony. Only expected products were amplified from the reprepared samples; both the mecA and 16S rRNA genes were detected in 4 MRCoNS isolates and the 16S rRNA gene in 2 MSSA isolates. The results showed that the unexpected amplimers were due to the mixed staphylococci. Consequently, 4 MRCoNS isolates showing amplification of all of the three targets would be mixtures of MRSA and MRCoNS or of MRCoNS and MSSA carrying SCCmec remnants. In South Korea, it is assumed that MSSA strains carrying SCCmec remnants comprise approximate 3% of the S. aureus isolates recovered from clinical specimens because about 30% of S. aureus isolates are MSSA and 10% of MSSA carry SCCmec remnants. Becker's study reported that nasal cocolonization by MSSA and MRCoNS was observed in 3.4% of patients (8). Furthermore, it was known from analyzing simulated samples that MRCoNS must comprise more than 90% of a mixed population to have a CTSCC value over 15. Accordingly, it is reasonable to assume that there is very little chance of amplifying all three of the targets from mixed populations of MRCoNS and MSSA carrying SCCmec remnants. In the case of 2 MSSA isolates showing amplification of the mecA, it would seem that they contained mixed populations of MRCoNS and MSSA.
In summary, as a preliminary study for the introduction of a direct MRSA molecular detection system to our laboratory in a region with high MRSA infection endemicity, a multiplex real-time PCR assay that simultaneously detects the mecA, SCCmec-orfX junction, and staphylococcal 16S rRNA genes was developed and evaluated using 444 staphylococcal strains. The key issue was whether this assay can reduce false positives caused by MSSA carrying SCCmec remnants. The evaluation data showed that this assay resulted in fewer false-positive results than a single-locus real-time PCR assay that amplified the SCCmec-orfX junction. This assay would be useful in a clinical laboratory in a region with high MRSA infection endemicity, although further evaluation with clinical specimens is necessary before it can be applied in the laboratory.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Bootsma MC, Diekmann O, Bonten MJ. 2006. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc. Natl. Acad. Sci. U. S. A. 103:5620–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harbarth S, Masuet-Aumatell C, Schrenzel J, Francois P, Akakpo C, Renzi G, Pugin J, Ricou B, Pittet D. 2006. Evaluation of rapid screening and pre-emptive contact isolation for detecting and controlling methicillin-resistant Staphylococcus aureus in critical care: an interventional cohort study. Crit. Care 10:R25 doi:10.1186/cc3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chowers MY, Paitan Y, Gottesman BS, Gerber B, Ben-Nissan Y, Shitrit P. 2009. Hospital-wide methicillin-resistant Staphylococcus aureus control program: a 5-year follow-up. Infect. Control Hosp. Epidemiol. 30:778–781 [DOI] [PubMed] [Google Scholar]
- 4. Huletsky A, Giroux R, Rossbach V, Gagnon M, Vaillancourt M, Bernier M, Gagnon F, Truchon K, Bastien M, Picard FJ, van Belkum A, Ouellette M, Roy PH, Bergeron MG. 2004. New real-time PCR assay for rapid detection of methicillin-resistant Staphylococcus aureus directly from specimens containing a mixture of staphylococci. J. Clin. Microbiol. 42:1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson LR, Liesenfeld O, Woods CW, Allen SD, Pombo D, Patel PA, Mehta MS, Nicholson B, Fuller D, Onderdonk A. 2010. Multicenter evaluation of the LightCycler methicillin-resistant Staphylococcus aureus (MRSA) advanced test as a rapid method for detection of MRSA in nasal surveillance swabs. J. Clin. Microbiol. 48:1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossney AS, Herra CM, Brennan GI, Morgan PM, O'Connell B. 2008. Evaluation of the Xpert methicillin-resistant Staphylococcus aureus (MRSA) assay using the GeneXpert real-time PCR platform for rapid detection of MRSA from screening specimens. J. Clin. Microbiol. 46:3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossney AS, Herra CM, Fitzgibbon MM, Morgan PM, Lawrence MJ, O'Connell B. 2007. Evaluation of the IDI-MRSA assay on the SmartCycler real-time PCR platform for rapid detection of MRSA from screening specimens. Eur. J. Clin. Microbiol. Infect. Dis. 26:459–466 [DOI] [PubMed] [Google Scholar]
- 8. Becker K, Pagnier I, Schuhen B, Wenzelburger F, Friedrich AW, Kipp F, Peters G, von Eiff C. 2006. Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods? J. Clin. Microbiol. 44:229–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurent C, Bogaerts P, Schoevaerdts D, Denis O, Deplano A, Swine C, Struelens MJ, Glupczynski Y. 2010. Evaluation of the Xpert MRSA assay for rapid detection of methicillin-resistant Staphylococcus aureus from nares swabs of geriatric hospitalized patients and failure to detect a specific SCCmec type IV variant. Eur. J. Clin. Microbiol. Infect. Dis. 29:995–1002 [DOI] [PubMed] [Google Scholar]
- 10. Oberdorfer K, Pohl S, Frey M, Heeg K, Wendt C. 2006. Evaluation of a single-locus real-time polymerase chain reaction as a screening test for specific detection of methicillin-resistant Staphylococcus aureus in ICU patients. Eur. J. Clin. Microbiol. Infect. Dis. 25:657–663 [DOI] [PubMed] [Google Scholar]
- 11. Stamper PD, Louie L, Wong H, Simor AE, Farley JE, Carroll KC. 2011. Genotypic and phenotypic characterization of methicillin-susceptible Staphylococcus aureus isolates misidentified as methicillin-resistant Staphylococcus aureus by the BD GeneOhm MRSA assay. J. Clin. Microbiol. 49:1240–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong H, Louie L, Lo RY, Simor AE. 2010. Characterization of Staphylococcus aureus isolates with a partial or complete absence of staphylococcal cassette chromosome elements. J. Clin. Microbiol. 48:3525–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnio PY, Oliveira DC, Faria NA, Wilhelm N, Le Coustumier A, de Lencastre H. 2005. Partial excision of the chromosomal cassette containing the methicillin resistance determinant results in methicillin-susceptible Staphylococcus aureus. J. Clin. Microbiol. 43:4191–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shore AC, Rossney AS, O'Connell B, Herra CM, Sullivan DJ, Humphreys H, Coleman DC. 2008. Detection of staphylococcal cassette chromosome mec-associated DNA segments in multiresistant methicillin-susceptible Staphylococcus aureus (MSSA) and identification of Staphylococcus epidermidis ccrAB4 in both methicillin-resistant S. aureus and MSSA. Antimicrob. Agents Chemother. 52:4407–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donnio PY, Fevrier F, Bifani P, Dehem M, Kervegant C, Wilhelm N, Gautier-Lerestif AL, Lafforgue N, Cormier M, Le Coustumier A. 2007. Molecular and epidemiological evidence for spread of multiresistant methicillin-susceptible Staphylococcus aureus strains in hospitals. Antimicrob. Agents Chemother. 51:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindqvist M, Isaksson B, Grub C, Jonassen TO, Hallgren A. 2012. Detection and characterisation of SCCmec remnants in multiresistant methicillin-susceptible Staphylococcus aureus causing a clonal outbreak in a Swedish county. Eur. J. Clin. Microbiol. Infect. Dis. 31:141–147 [DOI] [PubMed] [Google Scholar]
- 17. Korea Centers for Disease Control and Prevention 2010. 2010 laboratory surveillance for vancomycin resistant staphylococcus aureus. VRSA Newsl. 10:3 [Google Scholar]
- 18. Chen L, Mediavilla JR, Oliveira DC, Willey BM, de Lencastre H, Kreiswirth BN. 2009. Multiplex real-time PCR for rapid Staphylococcal cassette chromosome mec typing. J. Clin. Microbiol. 47:3692–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milheiriço C, Oliveira DC, de Lencastre H. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3374–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho TH, Huang YC, Lin TY. 2011. Evaluation of the BD GeneOhm StaphSR assay for detection of Staphylococcus aureus in patients in intensive care units. J. Microbiol. Immunol. Infect. 44:310–315 [DOI] [PubMed] [Google Scholar]
- 21. Keshtgar MR, Khalili A, Coen PG, Carder C, Macrae B, Jeanes A, Folan P, Baker D, Wren M, Wilson AP. 2008. Impact of rapid molecular screening for meticillin-resistant Staphylococcus aureus in surgical wards. Br. J. Surg. 95:381–386 [DOI] [PubMed] [Google Scholar]
- 22. Kluytmans J. 2007. Control of meticillin-resistant Staphylococcus aureus (MRSA) and the value of rapid tests. J. Hosp. Infect. 65(Suppl 2):100–104 [DOI] [PubMed] [Google Scholar]
- 23. Schulz M, Nonnenmacher C, Mutters R. 2009. Cost-effectiveness of rapid MRSA screening in surgical patients. Eur. J. Clin. Microbiol. Infect. Dis. 28:1291–1296 [DOI] [PubMed] [Google Scholar]
- 24. Kolman S, Arielly H, Paitan Y. 2010. Evaluation of single and double-locus real-time PCR assays for methicillin-resistant Staphylococcus aureus (MRSA) surveillance. BMC Res. Notes 3:110 doi:10.1186/1756-0500-3-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ornskov D, Kolmos B, Bendix Horn P, Nederby Nielsen J, Brandslund I, Schouenborg P. 2008. Screening for methicillin-resistant Staphylococcus aureus in clinical swabs using a high-throughput real-time PCR-based method. Clin. Microbiol. Infect. 14:22–28 [DOI] [PubMed] [Google Scholar]
- 26. Söderquist B, Neander M, Dienus O, Zimmermann J, Berglund C, Matussek A, Molling P. 2012. Real-time multiplex PCR for direct detection of methicillin-resistant Staphylococcus aureus (MRSA) in clinical samples enriched by broth culture. APMIS 120:427–432 [DOI] [PubMed] [Google Scholar]
- 27. Hiramatsu K, Cui L, Kuroda M, Ito T. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9:486–493 [DOI] [PubMed] [Google Scholar]
- 28. Hiramatsu K, Katayama Y, Yuzawa H, Ito T. 2002. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 292:67–74 [DOI] [PubMed] [Google Scholar]
- 29. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donnio PY, Louvet L, Preney L, Nicolas D, Avril JL, Desbordes L. 2002. Nine-year surveillance of methicillin-resistant Staphylococcus aureus in a hospital suggests instability of mecA DNA region in an epidemic strain. J. Clin. Microbiol. 40:1048–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]