Abstract
Klebsiella pneumoniae K1 is a major agent of hepatic abscess with metastatic disease in East Asia, with sporadic reports originating elsewhere. We report a case of abscess complicated by septic endophthalmitis caused by a wzyAKpK1-positive Klebsiella strain in a U.S. resident, raising concern for global emergence.
CASE REPORT
A 58-year-old female resident of Bronx, New York, who was originally from the Dominican Republic presented with a chief complaint of 1 day of decreased vision in her right eye and concomitant symptoms, including weakness, myalgia, low-grade fever, and right upper quadrant pain for 1 week. She had a history of uncomplicated choledochal cyst resection and Roux-en-Y hepaticojejunostomy approximately 5 years prior to presentation but no history of ocular disease or prior intraocular surgery. She reported subsequent travel to the Dominican Republic but denied travel to Asia at any point before or after her surgery. On the initial ophthalmic examination, visual acuity was 20/60 in the affected eye. Slit-lamp examination revealed moderate conjunctival injection in the right eye, along with 4+ cells and hypopyon in the anterior chamber. The fundus view was hazy because of opacity in the anterior segment, but the retina was flat. She was diagnosed with presumed endogenous endophthalmitis. Her vision in the affected eye worsened over the next 24 h. Because of right upper quadrant tenderness on examination, further imaging was performed, revealing a hepatic abscess (7 by 7 by 7 cm) (Fig. 1). The patient reported no history of diabetes, and the serum glucose was normal. She was treated with intravenous levofloxacin, and the abscess was drained percutaneously. Cultures of liver aspirate, blood, and urine grew K. pneumoniae susceptible to expanded- and broad-spectrum cephalosporins, ampicillin-sulbactam, levofloxacin, aminoglycosides, and trimethoprim-sulfamethoxazole. The isolate exhibited a hypermucoviscous phenotype, as exemplified by a positive string test (Fig. 2). On the seventh hospital day, her ophthalmologic exam deteriorated; she was found to have a subretinal abscess in the peripheral temporal retina, and retinal detachment was noted (Fig. 3). A sample of vitreous fluid was obtained, which revealed polymorphonuclear leukocytes on Gram stain but a negative culture, and intravitreal injection of ceftazidime was performed. Over the next several weeks, the vitreous debris cleared, and the retina more clearly assumed the configuration of a bullous rhegmatogenous detachment stemming from a break related to the retinal necrosis at the site of the subretinal abscess. The patient underwent a vitrectomy for retinal detachment 2 months after her initial presentation. The patient's subsequent course was complicated by a relapse of abdominal pain and an increase in size of the liver abscess following a transition to oral therapy. An abdominal CT scan conducted 2 months after the completion of an 8-week antibiotic course demonstrated resolution of her liver abscess.
Fig 1.
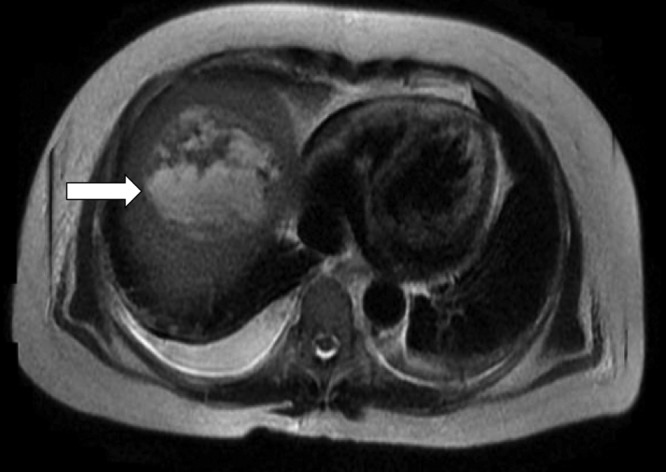
Abdominal magnetic resonance imaging revealed a large hepatic abscess (arrow) with areas of central necrosis.
Fig 2.
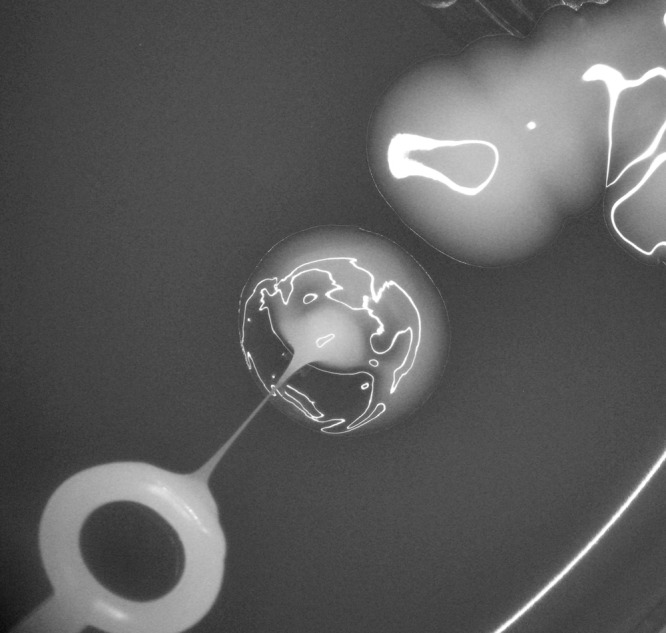
The Klebsiella pneumoniae isolate from this patient exhibited a hypermucoviscous phenotype, as shown by the positive “string test.” For scale, the internal diameter of the pictured inoculation loop is 3.8 mm.
Fig 3.
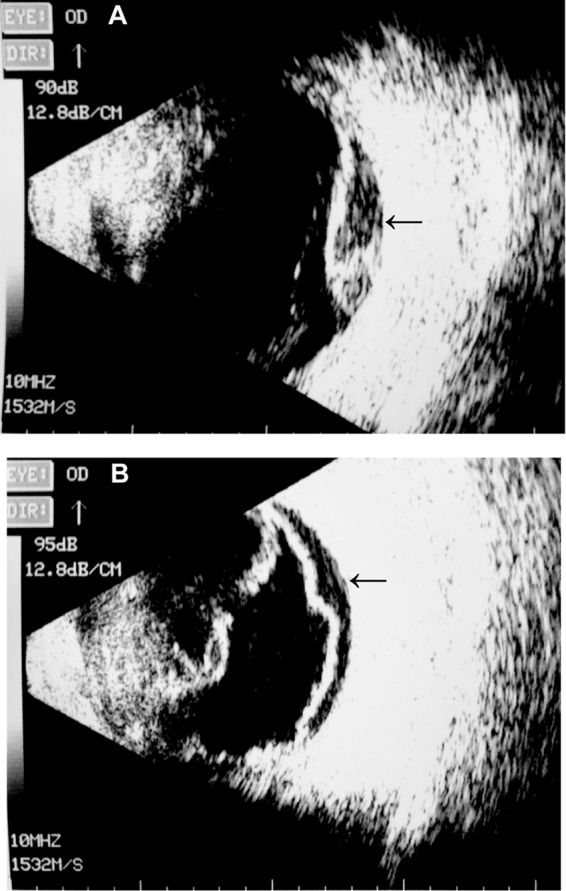
B-scan ultrasonography of the right eye demonstrated subretinal abscess (A) and retinal detachment (B), indicated by arrows.
Because of the similarity of this case to reports from Asia, we conducted PCR to identify the K1-specific wzyA allele (wzyAKpK1) in the K. pneumoniae isolate from this patient. We detected both wzx and wzyAKpK1 alleles using previously published methods and primer sequences (1). Each of these amplicons was purified, and bidirectional Sanger sequencing resulted in >950 bp of sequence for each. For each allele, the generated sequence was 100% identical to the corresponding genes from the published K1 K. pneumoniae NTUH-K2044 strain (GenBank accession no. AP006725.1) (2).
Serotype K1 Klebsiella pneumoniae has emerged as a major cause of pyogenic liver abscess in East Asia, and such infections frequently result in metastatic complications, including endophthalmitis, necrotizing fasciitis, meningitis, and cerebral and pulmonary abscesses (1, 3, 4). The striking emergence of this clinical syndrome has been reviewed in detail recently (5). The implicated K. pneumoniae strains are generally hypermucoviscous, and their virulence is dependent on the wzyAKpK1 (formerly called magA) gene, which is specific to the K1 serotype (6, 7). wzyAKpK1 encodes a polymerase involved in the assembly of the K1 antigen (7, 8). Other important virulence factors in these Klebsiella strains include the rmpA gene, which regulates synthesis of the capsule, and aerobactin, a catecholate siderophore (9–12). Despite numerous reports confirming the association of this distinctive clinical presentation with K. pneumoniae K1 in Asia, descriptions of K1-associated pyogenic abscesses with ophthalmologic complications originating outside this region are less frequent (5, 13).
Laboratory-confirmed invasive K. pneumoniae liver abscess syndrome has been described in North America, primarily in patients of Asian descent (14–18), but only occasional reports have described molecular strain typing to confirm the K1 strain as the causative agent (19). Recently, a Caucasian man from San Diego, CA, was found to have laboratory-confirmed K1-associated recurrent liver abscesses without endophthalmitis (20). There are only sporadic descriptions of ophthalmologic complications of K. pneumoniae hepatic abscess in the United States (21–23), but none of these reported serotyping or molecular confirmation of the K1 strain. The patient presented above represents confirmation of a wzyAKpK1-positive K. pneumoniae K1 strain causing pyogenic liver abscess and endophthalmitis in a U.S. patient. This individual did not report Asian lineage or known close contacts from that region. In prior series, affected patients have generally lacked known immunodeficiency, as was the case with this individual, and have had normal hepatobiliary anatomy. It is unclear what role our patient's prior biliary surgery may have played in the pathogenesis of her disease, though it is conceivable that her altered anatomy may have been a predisposing factor. Gastrointestinal carriage of the K1 strain is likely a risk factor for development of K. pneumoniae liver abscess (24). Diabetes is a frequent underlying illness in reports of K. pneumoniae hepatic abscess (5, 25), but our patient lacked that putative risk factor as well. Clinicians should be aware that the hypervirulent Klebsiella pneumoniae K1 strain is emerging worldwide. Patients presenting with K. pneumoniae pyogenic abscess should be closely evaluated for central nervous system involvement or sight-threatening ophthalmologic complications.
Footnotes
Published ahead of print 9 January 2013
REFERENCES
- 1. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. 2007. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 45:284–293 [DOI] [PubMed] [Google Scholar]
- 2. Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, Fung CP, Chen HJ, Liu YM, Wang JT, Fang CT, Chang SC, Shu HY, Liu TT, Chen YT, Shiau YR, Lauderdale TL, Su IJ, Kirby R, Tsai SF. 2009. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J. Bacteriol. 191:4492–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, Ho M, Siu LK, Liu CY. 2000. A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J. Infect. Dis. 181:2075–2079 [DOI] [PubMed] [Google Scholar]
- 4. Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, Liu CY, Ho M, Siu LK. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50:420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. 2012. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12:881–887 [DOI] [PubMed] [Google Scholar]
- 6. Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, Chen TL, Chang FY, Koh TH. 2007. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J. Clin. Microbiol. 45:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL. 2010. The function of wzy_K1 (magA), the serotype K1 polymerase gene in Klebsiella pneumoniae cps gene cluster. J. Infect. Dis. 201:1268–1269 [DOI] [PubMed] [Google Scholar]
- 8. Yeh KM, Lin JC, Yin FY, Fung CP, Hung HC, Siu LK, Chang FY. 2010. Revisiting the importance of virulence determinant magA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: exact role in serotype K1 capsule formation. J. Infect. Dis. 201:1259–1267 [DOI] [PubMed] [Google Scholar]
- 9. Nassif X, Honore N, Vasselon T, Cole ST, Sansonetti PJ. 1989. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae. Mol. Microbiol. 3:1349–1359 [DOI] [PubMed] [Google Scholar]
- 10. Nassif X, Fournier JM, Arondel J, Sansonetti PJ. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect. Immun. 57:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nassif X, Sansonetti PJ. 1986. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect. Immun. 54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ. 2007. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg. Infect. Dis. 13:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. 2007. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J. Med. Microbiol. 56:593–597 [DOI] [PubMed] [Google Scholar]
- 14. Lederman ER, Crum NF. 2005. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 100:322–331 [DOI] [PubMed] [Google Scholar]
- 15. Pastagia M, Arumugam V. 2008. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med. Infect. Dis. 6:228–233 [DOI] [PubMed] [Google Scholar]
- 16. Nadasy KA, Domiati-Saad R, Tribble MA. 2007. Invasive Klebsiella pneumoniae syndrome in North America. Clin. Infect. Dis. 45:e25–e28 [DOI] [PubMed] [Google Scholar]
- 17. Pomakova DK, Hsiao CB, Beanan JM, Olson R, MacDonald U, Keynan Y, Russo TA. 2012. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur. J. Clin. Microbiol. Infect. Dis. 31:981–989 [DOI] [PubMed] [Google Scholar]
- 18. Rahimian J, Wilson T, Oram V, Holzman RS. 2004. Pyogenic liver abscess: recent trends in etiology and mortality. Clin. Infect. Dis. 39:1654–1659 [DOI] [PubMed] [Google Scholar]
- 19. McCabe R, Lambert L, Frazee B. 2010. Invasive Klebsiella pneumoniae infections, California, USA. Emerg. Infect. Dis. 16:1490–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fierer J, Walls L, Chu P. 2011. Recurring Klebsiella pneumoniae pyogenic liver abscesses in a resident of San Diego, California, due to a K1 strain carrying the virulence plasmid. J. Clin. Microbiol. 49:4371–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saccente M. 1999. Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus. Clin. Infect. Dis. 29:1570–1571 [DOI] [PubMed] [Google Scholar]
- 22. Harris EW, D'Amico DJ, Bhisitkul R, Priebe GP, Petersen R. 2000. Bacterial subretinal abscess: a case report and review of the literature. Am. J. Ophthalmol. 129:778–785 [DOI] [PubMed] [Google Scholar]
- 23. Nye AM, Kirchner JT, Lamendola CE. 1999. Progressive vision loss after pneumonia. Hosp. Pract. (Minneap.) 34:150–152, 157–158 [DOI] [PubMed] [Google Scholar]
- 24. Fung CP, Lin YT, Lin JC, Chen TL, Yeh KM, Chang FY, Chuang HC, Wu HS, Tseng CP, Siu LK. 2012. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg. Infect. Dis. 18:1322–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han SH. 1995. Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West. J. Med. 162:220–224 [PMC free article] [PubMed] [Google Scholar]


