Abstract
Newcastle disease (ND) is a deadly avian disease worldwide. In Africa, ND is enzootic and causes large economic losses, but little is known about the Newcastle disease virus (NDV) strains circulating in African countries. In this study, 27 NDV isolates collected from apparently healthy chickens in live-bird markets of the West African countries Benin and Togo in 2009 were characterized. All isolates had polybasic fusion (F)-protein cleavage sites and were shown to be highly virulent in standard pathogenicity assays. Infection of 2-week-old chickens with two of the isolates resulted in 100% mortality within 4 days. Phylogenetic analysis of the 27 isolates based on a partial F-protein gene sequence identified three clusters: one containing all the isolates from Togo and one from Benin (cluster 2), one containing most isolates from Benin (cluster 3), and an outlier isolate from Benin (cluster 1). All the three clusters are related to genotype VII strains of NDV. In addition, the cluster of viruses from Togo contained a recently identified 6-nucleotide insert between the hemagglutinin-neuraminidase (HN) and large polymerase (L) genes in a complete genome of an NDV isolate from this geographical region. Multiple strains that include this novel element suggest local emergence of a new genome length class. These results reveal genetic diversity within and among local NDV populations in Africa. Sequence analysis showed that the F and HN proteins of six West African isolates share 83.2 to 86.6% and 86.5 to 87.9% identities, respectively, with vaccine strain LaSota, indicative of considerable diversity. A vaccine efficacy study showed that the LaSota vaccine protected birds from morbidity and mortality but did not prevent shedding of West African challenge viruses.
INTRODUCTION
Newcastle disease (ND) is a highly contagious viral disease of birds with worldwide distributions that can cause severe economic losses in the poultry industry (1–4). The causative agent, Newcastle disease virus (NDV), also designated avian paramyxovirus serotype 1 (APMV-1), is a member of the genus Avulavirus in the family Paramyxoviridae (3, 4). NDV is an enveloped virus, and its genome is a nonsegmented, single-stranded, negative-sense RNA that contains six genes encoding the nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion (F) protein, hemagglutinin-neuraminidase protein (HN), large polymerase protein (L), and an additional protein, V, that is expressed by RNA editing of P mRNA (3). The two surface glycoproteins, HN and the F protein, are the viral neutralization antigens and the major protective antigens. HN is responsible for attachment to the host cell, and the F protein mediates fusion of the viral envelope with the cell membrane. The F protein is synthesized as an inactive precursor (F0) that is cleaved by host cell protease into two biologically active F1 and F2 subunits that remain linked by a disulfide bond. Cleavage of the F protein is a prerequisite for virus entry and cell-to-cell fusion (3, 4).
NDV isolates vary widely in their pathogenicity for chickens. NDV strains are classified as highly virulent (velogenic), intermediate (mesogenic), or avirulent (lentogenic) on the basis of their pathogenicity for chickens (1). The sequence of the F-protein cleavage site is a well-characterized, major determinant of NDV pathogenicity in chickens (5, 6). The F protein of mesogenic and velogenic strains of NDV typically contains a polybasic cleavage site [(R/K)RQ(R/K)R↓F, where the arrow indicates the cleavage site] that contains the preferred recognition site for furin [RX(K/R)R↓], which is an intracellular protease present in a wide range of cells and tissues. Consequently, the F protein of these strains can be cleaved in most tissues, conferring the potential for systemic spread. In contrast, avirulent NDV strains typically have basic residues at the −1 and −4 positions in the cleavage site [(G/E)(K/R)Q(G/E)R↓L] and depend on a secreted protease (or, in cell culture, added trypsin or chicken egg allantoic fluid) for cleavage. This typically limits the replication of avirulent strains to the respiratory and enteric tracts, where the secreted protease is found (1, 4).
All NDV strains are classified under a single serotype, but both antigenic and genetic diversities are recognized among NDV strains (7). NDV strains have genome sizes of 15,186 nucleotides (nt), 15,192 nt, or 15,198 nt. NDV strains with a genome size of 15,186 nt were mostly isolated before 1960, while most strains that have been isolated recently have genome sizes of 15,192 or 15,198 nt (8). On the basis of genome length and the sequence of the F-protein gene (F gene), NDV strains have been classified into two major classes. The class I strains have mainly been isolated from wild birds and are generally avirulent, whereas class II strains have been recovered from wild and domestic birds and include virulent and avirulent strains. Class I and II viruses are further divided into 9 and 15 genotypes, respectively (9, 10). The early NDV isolates (class II genotypes I to IV and IX) have a genome size of 15,186 nt, whereas recent NDV isolates (class II genotypes V to VIII and X) have a genome size of 15,192 nt. Class I strains have a genome size of 15,198 nt (8). Epidemiological studies have revealed that genotypes V, VI, and VII of class II NDV strains (i.e., genome size of 15,192 nt) are the most prevalent genotypes currently circulating worldwide. Of these, genotype VII has been associated with many recent outbreaks in Asia, Africa, the Middle East, and South America (7).
In Africa, ND outbreaks are rampant and are the major constraint to poultry farming in villages (11–13). Severe outbreaks of ND have been reported in North African countries, namely, Egypt (14, 15) and Morocco (16); Central African countries, namely, Nigeria, Niger, Sudan, Cameroon, and Burundi (17) and Uganda (13); western African countries, namely, Burkina Faso (17), Mali (18), and Ivory Coast and Mauritania (17); and southern African countries, namely, Mozambique and South Africa (12) and Madagascar (18, 19). Africa is thought to be a reservoir of new virulent strains of NDV (18). Phylogenetically distinguishable unique NDV strains have been reported from eastern and southern Africa, but reports of the molecular epidemiology and genotype distribution of NDV strains from Western and Central Africa are scarce (17, 20). In rural Africa, small backyard farms of indigenous domestic fowl (Gallus gallus domesticus) are the predominant chicken production systems, and the livelihood of most village people depends on these chicken farms (21). Village poultry farmers normally do not vaccinate their birds due to financial constraints, and the birds are sold in live-bird markets when they are approximately 1 year old (22).
The importance and enzootic nature of ND in Africa and the general lack of information available for strains circulating in West African countries prompted efforts to isolate and characterize NDV strains from live-bird markets in the West African countries of Togo and Benin. We previously determined the complete genome sequence of one of the isolates (APMV-1/chicken/Togo/AKO18/2009 [AKO18]) from Togo (Table 1) (23). The present study extends this work by describing the genetic and pathotypic analysis of this virus and 26 other NDV isolates, all 27 of which had been recovered from apparently healthy chickens and ducks from live-bird markets in Benin and Togo in 2009. All of these viruses proved to be velogenic. We have also compared the sequences of these viruses to the sequence of the live vaccine strain LaSota and evaluated the protective efficacy of the LaSota vaccine against these current strains. Our results showed that most of the NDV strains circulating in Benin and Togo form locale-specific phylogenetic clusters, although one of the strains was relatively more divergent. In addition, the strains circulating in Togo have a recently described (23) 6-nt insertion between the HN and L genes, suggesting the emergence of a new length class of the NDV genome. Finally, our results indicate that the commercial vaccine does not provide complete protection against strains circulating in West Africa.
Table 1.
Characteristics of chickens from which samples were taken and isolation history, pathogenicity, F-protein cleavage site sequence, and GenBank accession numbers of 27 West African NDV isolatesa
| Isolate no. | NDV isolateb | Date of isolation (mo/day/yr) | Sex | Country | City | MDT (h) | ICPI | F-protein cleavage site | GenBank accession no.c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | APMV-1/chicken/Benin/432MT/2009! | 02/13/2009 | M | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546255 |
| 2 | APMV-1/chicken/Benin/467MC/2009 | 02/13/2009 | M | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546256 |
| 3 | APMV-1/chicken/Benin/476MT/2009! | 02/13/2009 | M | Benin | Malanville | 56 | ND | GRRQKR↓F | JX546257 |
| 4 | APMV-1/chicken/Benin/480MT/2009!* | 02/13/2009 | F | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546258 |
| 5 | APMV-1/chicken/Benin/415MC/2009* | 02/13/2009 | M | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546259 |
| 6 | APMV-1/chicken/Benin/474MC/2009* | 02/13/2009 | M | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546246# |
| 7 | APMV-1/chicken/Benin/442MT/2009!* | 02/13/2009 | M | Benin | Malanville | 48 | ND | GRRQKR↓F | JX546260 |
| 8 | APMV-1/chicken/Benin/479MT/2009!* | 02/13/2009 | F | Benin | Malanville | 64 | ND | GRRQKR↓F | JX546261 |
| 9 | APMV-1/chicken/Benin/382GT/2009! | 02/01/2009 | M | Benin | Gogounou | 64 | ND | GRRQKR↓F | JX546262 |
| 10 | APMV-1/chicken/Benin/847GC/2009! | 03/13/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546263 |
| 11 | APMV-1/chicken/Benin/380GC/2009* | 02/01/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546264 |
| 12 | APMV-1/chicken/Benin/372GC/2009 | 02/01/2009 | M | Benin | Gogounou | 56 | ND | GRRQKR↓F | JX546265 |
| 13 | APMV-1/chicken/Benin/796GT/2009!* | 03/13/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546266 |
| 14 | APMV-1/chicken/Benin/349GC/2009 | 02/01/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546267 |
| 15 | APMV-1/chicken/Benin/770GT/2009! | 03/13/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546268 |
| 16 | APMV-1/chicken/Benin/379GT/2009! | 02/01/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546269 |
| 17 | APMV-1/chicken/Benin/378GT/2009!* | 02/01/2009 | M | Benin | Gogounou | 56 | ND | GRRQKR↓F | JX546270 |
| 18 | APMV-1/chicken/Benin/846GC/2009* | 03/13/2009 | M | Benin | Gogounou | 48 | ND | GRRQKR↓F | JX546271 |
| 19 | APMV-1/duck/Togo/GBO5/2009! | 03/19/2009 | M | Togo | Gbossime | 48 | ND | GRRQKR↓F | JX546272 |
| 20 | APMV-1/chicken/Togo/AKC12/2009 | 03/17/2009 | F | Togo | Akodessewa | 48 | ND | GRRQKR↓F | JX546273 |
| 21 | APMV-1/duck/Togo/AKC14/2009 | 03/17/2009 | F | Togo | Akodessewa | 48 | ND | GRRQKR↓F | JX546274 |
| 22 | APMV-1/chicken/Togo/AKO11/2009! | 03/17/2009 | F | Togo | Akodessewa | 48 | ND | GRRQKR↓F | JX546275 |
| 23 | APMV-1/chicken/Togo/AKO18/2009! | 03/17/2009 | F | Togo | Akodessewa | 64 | 1.65 | GRRRKR↓F | JX390609§ |
| 24 | APMV-1/chicken/Benin/463MT/2009! | 02/13/2009 | M | Benin | Malanville | 48 | 1.65 | GRRRKR↓F | JX546245# |
| 25 | APMV-1/chicken/Benin/488MT/2009! | 02/13/2009 | F | Benin | Malanville | 56 | 1.62 | GRRQKR↓F | JX546247# |
| 26 | APMV-1/chicken/Benin/376GT/2009! | 02/01/2009 | M | Benin | Gogounou | 64 | 1.62 | GRRQKR↓F | JX546244# |
| 27 | APMV-1/chicken/Benin/373GC/2009* | 02/01/2009 | M | Benin | Gogounou | 56 | 1.51 | GRRQKR↓F | JX546243# |
F, female; M, male; ND, not determined.
NDV was isolated from the oropharyngeal swab samples (!) or from both oropharyngeal and cloacal swab samples from the same bird (*). NDV isolates without any symbolic notation were isolated from cloacal swabs only.
The GenBank accession numbers provided are for the 354-bp partial nucleotide sequence of the corresponding F-protein gene (genome position from nucleotides 4709 to 5062) of West African NDV strains, except for six strains that have the complete nucleotide sequence of the coding region of the F-protein gene (#). Note that the complete genome sequence was previously determined for isolate number 23, strain AKO18(§) (23).
MATERIALS AND METHODS
Cells and viruses.
The chicken embryo fibroblast (DF-1) cell line was grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and maintained in DMEM with 5% FBS. Highly virulent NDV strains GB Texas [APMV-1/chicken/U.S.(TX)/GB/1948] and Fontana [APMV-1/chicken/U.S.(CA)/1083/72] were obtained from the National Veterinary Services Laboratory (Ames, IA). The commercial live LaSota vaccine (Newcastle disease vaccine LaSota strain, live virus; Fort Dodge Animal Health, NY) was purchased commercially and used as provided. The recombinant LaSota virus (LaSota-VF) with virulent F-protein cleavage was described previously (5). This virus was used as a positive control in a plaque reduction assay but was not used to vaccinate birds. Viruses were grown in 9-day-old embryonated specific-pathogen-free (SPF) chicken eggs by injecting each virus into the allantoic cavity. Three days later, the allantoic fluid was harvested and clarified. The presence of the virus was confirmed by hemagglutination (HA) assay (1).
Origin of West African virus strains.
The isolates were collected in Benin and Togo during an avian influenza surveillance program in West Africa in February and March 2009 by the Department of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, TN. The cloacal and oropharyngeal swab specimens were collected from the adult chickens and ducks from live-bird markets in Benin and Togo. The longitude and latitude of each location were noted with a global positioning system. In Togo, the samples were collected from Akodessewa (6°33′94″N and 1°39′64″W) and Gbossime (6°15′N and 1°33′), both from the Lome District. In Benin, the two cities were Gogounou (10°84′31″N and 2°82′83″W) and Malanville (11°86′14″N and 3°38′58″W). Oropharyngeal and cloacal swab samples collected from each bird were placed in transport medium (50% glycerol in physiological saline with penicillin at 2,000 U/ml and amphotericin B [Fungizone] at 2,000 U/ml) and shipped to St. Jude Children's Research Hospital. The samples negative for avian influenza virus and positive for virulent NDV by reverse transcription-PCR (RT-PCR) were sent to the Virginia-Maryland Regional College of Veterinary Medicine, University of Maryland, College Park, MD, for virus isolation and further studies.
Isolation of West African strains.
The virus isolation was performed in a USDA-certified enhanced biosafety level 3 (BSL-3+) laboratory at the Virginia-Maryland Regional College of Veterinary Medicine. A total of 68 swab samples were processed for virus isolation by inoculation into 9-day-old SPF embryonated chicken eggs. The tubes containing oropharyngeal or cloacal swabs were briefly centrifuged, and 100-μl diluted aliquots (1:100) were inoculated into three eggs via the allantoic route. The allantoic fluid from each of the inoculated eggs was harvested 4 days later and assayed for hemagglutinating agent by HA using 1% chicken red blood cells. The HA-negative samples were passaged two times more in eggs and assayed for HA activity to confirm the absence of hemagglutinating agent. Preliminary identification and characterization of these hemagglutinating agents were carried out by hemagglutination inhibition (HI) tests with NDV antisera. All HA-positive allantoic fluid specimens were tested further by RT-PCR using primers specific for NDV (23). All experiments with the West African samples, as well as with the virulent NDV strains GB Texas and Fontana, were performed under BSL-3+ containment. A total of 27 strains were isolated (Table 1). One of these, AKO18 (isolate 23 in Table 1), was previously analyzed by complete genome sequencing (23).
Pathogenicity and tissue tropism of viruses.
The pathogenicity of the viral isolates was determined by two standard pathogenicity index tests described for NDV by the World Organization for Animal Health (OIE) manual (24). These were the mean death time (MDT) in 9-day-old embryonated SPF chicken eggs and the intracerebral pathogenicity index (ICPI) in 1-day-old SPF chicks.
The MDT value was determined following the standard procedure. Briefly, a series of 10-fold (10−6 to 10−9) dilutions of fresh infective allantoic fluid in sterile phosphate-buffered saline (PBS) was made, and 0.1 ml of each dilution was inoculated into the allantoic cavities of five 9-day-old embryonated SPF chicken eggs, which were then incubated at 37°C. Each egg was examined three times daily for 5 days, and the times of the embryo deaths were recorded. The minimum lethal dose was the highest virus dilution that caused all embryos inoculated with that dilution to die. MDT was the mean time (in hours) for the minimum lethal dose to kill all inoculated embryos. The MDT definitions of the NDV pathotypes are as follows: velogenic, less than 60 h; mesogenic, 60 to 90 h; and lentogenic, more than 90 h (1, 24). To determine the ICPI value, 0.05 ml of a 1:10 dilution of fresh infective allantoic fluid (28 HA units) of each virus was inoculated into groups of 10 1-day-old SPF chicks via the intracerebral route. The birds were observed for clinical symptoms and mortality every 8 h for a period of 8 days. At each observation, the birds were scored as follows: 0, healthy; 1, sick; and 2, dead. The ICPI is the mean score per bird per observation over the 8-day period. The most virulent viruses give indices that approach the maximum score of 2.0, whereas lentogenic strains give values close to 0.0. The ICPI values were calculated as described by the OIE protocol (24).
To study tissue tropism and pathogenicity in chickens, 100 μl of fresh infective allantoic fluid of two representative West African strains (AKO18 from Togo and 488MT from Benin) was inoculated into groups of 13 2-week-old SPF chickens by the intraocular and intranasal (oculonasal) routes. The birds were observed for clinical signs and mortality once every 12 h. Each experiment group had mock-inoculated controls that received a similar volume of sterile PBS by the respective routes. Tissue samples from the brain, spleen, nasal turbinate, lung, trachea, kidney, and pancreas were collected from three inoculated chickens per group on day 4 postinfection (p.i.). The oropharyngeal and cloacal swabs were taken on day 4 p.i.
Virus titration.
The virus titers in the tissue samples were determined by limiting dilution assay in DF-1 cells using the Reed and Muench method as described previously (25), and the titers were expressed as the number of 50% tissue culture infectious doses (TCID50s)/g of tissue and the number of TCID50s/ml for swab samples. Briefly, the tissue samples were homogenized in DMEM (1 g/10 ml) and clarified by centrifugation. The supernatant was serially diluted and used to infect DF-1 cells, with duplicate wells used per dilution. Infected wells were identified by HA assay, and the number TCID50s/ml was calculated using the method of Reed and Muench (25).
For challenge NDVs, the 50% chicken lethal dose (CLD50) was determined by infecting three (5-week-old) chickens per group with 100 μl of a 10-fold dilution series of fresh infective allantoic fluid, and the 50% endpoint was determined by the Reed and Muench method (25).
In preparation for the virus neutralization assay, the titers of LaSota-VF and selected West African strains (AKO18, 488MT, 474MC, 463MT, 376GT, and 373GC) were determined by a plaque assay in DF-1 cells using a 0.8% methylcellulose overlay. The infected cells were incubated at 37°C for 3 to 4 days, until the development of plaques was apparent. The cell monolayers were then fixed with methanol and stained with crystal violet for the enumeration of plaques. For virus neutralization assays using LaSota-immunized sera, 100 PFU of virus was mixed with an equal volume of a 4-fold dilution series of serum samples. The serum dilution that inhibited 50% of plaques was considered the neutralization titer.
Immunization and challenge experiments in chickens.
Four groups (n = 13 per group) of 2-week-old SPF chickens were immunized by the oculonasal route with the commercial live LaSota vaccine per the recommendations of the manufacturer (Newcastle disease vaccine strain LaSota, live virus; Fort Dodge Animal Health, NY). In addition, two additional groups (n = 10 birds) were kept as unvaccinated controls. Sera were collected prior to vaccination and at 21 days postvaccination (i.e., immediately before challenge) for evaluation of the serum antibody response. The challenge experiment was carried out in a BSL-3+ containment facility certified by the USDA to work with highly virulent NDV. Each of the LaSota-immunized groups was challenged through the oculonasal route with 100 CLD50s of the highly virulent NDV strains GB Texas, Fontana, AKO18, and 488MT. Two unvaccinated groups were challenged with strains AKO18 and 488MT; GB Texas and Fontana were not evaluated in unvaccinated animals because they have been shown to be uniformly lethal under these conditions. Three chickens from each group were sacrificed on day 4 postchallenge for quantification of challenge virus replication. Tissue samples were collected from the trachea, nasal turbinate, lungs, spleen, cecal tonsils, kidneys, and brain and assayed for challenge virus using the procedure described above. The remaining 10 chickens in each group were observed daily for 14 days for signs of disease and mortality. To monitor shedding of the challenge virus in immunized chickens, oropharyngeal and cloacal swab specimens were collected on days 4 and 7 postchallenge from all chickens. The challenge virus in the swab samples was determined by virus isolation in embryonated egg as described above, and titers in positive samples were determined by limiting dilution assay in DF-1 cells.
RNA isolation, cDNA synthesis, and nucleotide sequencing.
The complete genome sequence of strain AKO18 was described previously (23). For the other 26 isolates, RNA was extracted from infected allantoic fluids using an RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The first-strand cDNA was synthesized by using a SuperScript RT-PCR kit utilizing a random hexamer (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. For genotyping, a 406-bp hypervariable region of the F gene encompassing the cleavage site (positions 4664 to 5070 with reference to the sequence of NDV strain LaSota [GenBank accession number AF077761]) was targeted for RT-PCR. Briefly, the APMV-1 F-gene segment was amplified with primers NDV4664-F (5′-GGCAGGCCTCTTGCAGCTGCAGG-3′) and NDV5070-R (5′-TGCTGCATCTTCCCAACTGCCACTG-3′), and all the PCR products were directly sequenced using these forward and reverse primers.
To determine the presence of the 6-nt insert sequence in the intergenic region between the HN and L genes that was recently identified in strain AKO18 (23), a 615-bp region (from nt positions 7855 to 8469) was amplified with primers NDV7855-F (5′-CGGAATCATACCCTACGAG-3′) and NDV8469-R (5′-TAGGATGGAGTACTCCAATTA-3′). The PCR-amplified product was sequenced by using primers NDV7980-F (5′-CATCAAGGCAGCATACACGACATC-3′) and NDV8642-R (5′-AGCTGTGGTTGAGAGTTTGGTGTA-3′). DNA sequencing was performed in a 3130xl genetic analyzer (Applied Biosystems, Foster City, CA).
To compare the extent of diversity among the West African strains compared to the vaccine strain LaSota, we determined the complete coding sequence of the F and HN genes of six strains. Strain AKO18 had previously been sequenced (23); for the other five strains, we performed RT-PCR to amplify the F and HN genes and sequenced the uncloned PCR products to determine the complete coding sequences of the F and HN genes (23).
Phylogenetic analysis.
Sequence analysis, BLAST searches, and prediction of open reading frames (ORFs) were carried out using the SeqMan and EditSeq programs, and PCR primers were designed using the Primer Select program in DNAStar Lasergene (software suite for sequence analysis, version 8.0.2[13],412). Phylogenetic relationships and nucleotide sequence divergence analyses were established with the MEGA (version 5.1 beta 3) program using the neighbor-joining algorithm with the Kimura 2-parameter model. The statistical significance of the tree topology generated with the neighbor-joining algorithm was evaluated by 1,000 bootstrap resamplings of the data. To determine the phylogenetic relationship among the 27 West African isolates and previously characterized NDVs representing various NDV genotypes, the 354-bp hypervariable region of the F gene (genome position from nt 4709 to 5062) were compared to the corresponding region of representative viruses available in GenBank (accession numbers are mentioned below) for which the genotype was known.
Nucleotide sequence accession numbers.
The partial F-gene sequences of all 27 West African strains used in this study were submitted to GenBank, and their accession numbers are provided in Table 1 (JX546243 to JX546247, JX546255 to JX546275, and JX390609). The complete coding sequences of the F and HN genes of five West African strains were also submitted to GenBank and have the following respective accession numbers: for strain 373GC, JX546243 and JX546249; for strain 376GT, JX546244 and JX546250; for strain 463MT, JX546245 and JX546251; for strain 474MC, JX546246 and JX546252; and for strain 488MT, JX546247 and JX546253. The respective GenBank accession numbers of the F and HN genes of genotype-specific NDV strains are the following: for NDV class I virus, DQ097393 and FJ794269; for class II virus genotype I, AY562991 and AY935499; genotype II, AF0077761 and GU978777; genotype III, FJ436302 and EF201805; genotype IV, FJ986192 and AY741404; genotype V, AY562986 and GQ288386; genotype VI, AY562988 and AY562989; genotype VII, AY562985 and AY865652; genotype VIII, FJ751918 and FJ751919; genotype IX, DQ227252 and AY508514; genotype X, AY372135 and AY372163; and genotype XI, HQ266603 and HQ266605.
RESULTS
Isolation of virus from swab samples from live-bird markets in West Africa.
A total of 68 oropharyngeal and cloacal swab samples positive for NDV were further analyzed. They originated from 34 apparently healthy chickens from live-bird markets in Togo and Benin, West Africa. These specimens were inoculated into 9-day-old embryonated chicken eggs, and allantoic fluid was harvested on day 4 p.i. and tested for hemagglutinating agents by HA assay. The HA titers ranged from 3 to 10 log2 units, and the samples were passaged two times more and stored for further characterization. A total of 37 samples were found to be positive by HA assay and confirmed for NDV by HI assay using NDV-specific serum. In the case of 10 birds, viruses were isolated from both the oropharyngeal and cloacal swab samples, but only one isolate from each bird was chosen for further study (Table 1).
Pathogenicity of NDV strains isolated from live-bird markets.
The pathogenicities of the 27 West African NDV strains were evaluated by a standard pathogenicity assay, namely, the MDT test in 9-day-old embryonated SPF chicken eggs (1, 24) (Table 1). The MDT values of the 27 West African strains ranged from 48 to 64 h, and thus, they are considered velogenic strains (24).
To confirm the pathotype identification, five strains were evaluated by the ICPI test in 1-day-old chickens. We selected five NDV strains, one from Togo and two each from the cities of Malanville and Gogounou in Benin, for ICPI testing. The ICPI values of these five strains ranged from 1.51 to 1.65 (Table 1), consistent with a velogenic pathotype. Taken together, the MDT and ICPI tests showed that all 27 NDV isolates collected from apparently healthy chickens in live-bird markets of Benin and Togo are velogenic.
Determination of F-protein cleavage site amino acid sequence.
As noted, the AKO18 strain was previously analyzed by complete genome sequencing (23). For the remaining 26 strains, a region (from nt 4664 to 5070 in the genome sequence) was amplified by RT-PCR, and the region encompassing the F-protein cleavage site was analyzed by nucleotide sequencing. The deduced amino acid sequences showed that all 27 isolates have a polybasic cleavage site sequence (Table 1). Two versions of a polybasic cleavage site sequence were observed: the majority of isolates (25 out of 27) had the sequence RRQKR↓F, containing four basic residues, and the remaining two strains had the sequence RRRKR↓F, containing five basic residues. Both versions matched the preferred furin cleavage site (R-X-X-R). Thus, the presence of polybasic amino acids and a preferred furin motif at the F-protein cleavage site of all 27 West African isolates is consistent with the velogenic pathotype identified by the MDT and ICPI tests. The two viruses with 5 rather than 4 basic amino acids at the cleavage site had the two highest ICPI values (most pathogenic), although the difference between these and the others was minor (Table 1). With regard to MDT values, 1 of the 2 viruses with 5 basic amino acids had the lowest value among the 27 viruses, while the other had the highest value (Table 1), and thus, there was no clear indication that viruses with the 5-amino-acid cleavage site were phenotypically distinguishable from those with 4 amino acids.
Replication, tissue tropism, and pathogenicity of West African viruses in 2-week-old chickens.
We evaluated the replication and pathogenicity of two of the West African isolates, AKO18 (from Togo; isolate 23 in Table 1) and 488MT (from Benin; isolate 25 in Table 1), in 2-week-old SPF chickens. Chickens in groups of 13 were inoculated with either virus; 3 chickens from each group were euthanized on day 4 p.i. for tissue sample collection and virus titration; the remaining 10 chickens per group were maintained for observation. Interestingly, all of the birds died by day 3 to 4 p.i. without showing any apparent clinical signs. Sudden onset of death without prior indications of illness is the most noteworthy clinical sign of velogenic NDV strains in unvaccinated flocks. Virus titration of the tissue samples from the birds sacrificed on day 4 showed that strain AKO18 replicated substantially more efficiently than strain 488MT in respiratory tissues and the brain and somewhat more efficiently in the pancreas. In contrast, strain 488MT replicated more efficiently in the spleen and cecal tonsil (Fig. 1). The virus titer of strain AKO18 was 8 log10 TCID50s/g in lungs, trachea, and nasal turbinate, whereas strain 463MT had a lung titer of 5.3 ± 1.2 log10 TCID50s/g and trachea and a nasal turbinate titer of 4.6 ± 1.6 log10 TCID50s/g. The brain titers for strains AKO18 and 488MT were 8 log10 TCID50s/g and 2.6 ± 0.3 log10 TCID50s/g, respectively. The pancreas NDV titers were 5.3 ± 0.3 log10 TCID50s/g and 4.3 ± 0.8 log10 TCID50s/g, respectively. In spleen and cecal tonsil, the titers of strain AKO18 (3 log10 TCID50s/g and 5 ± 0.5 log10 TCID50s/g, respectively) were low compared to those of strain 488MT (6.3 ± 1.2 log10 TCID50s/g and 7.3 ± 0.6 log10 TCID50s/g, respectively). The levels of both oral and cloacal shedding for these two viruses were similar, in the range of 4 to 6 log10 TCID50s/ml (Fig. 1). The high level of virus replication in brain, the extensive viral dissemination, and the pattern of tissue tropism indicate that West African NDV strains AKO18 and 463MT are highly virulent.
Fig 1.
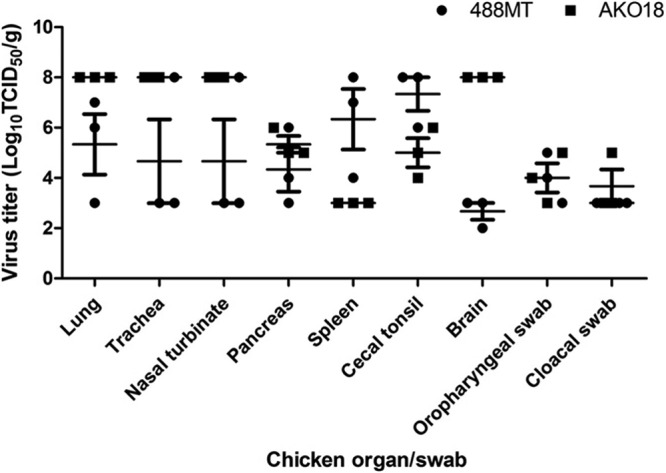
Tissue tropism and titers of West African NDV strains 488MT and AKO18 in different organs of 2-week-old chickens following oculonasal inoculation. Virus titers are expressed in log10 number of TCID50s/g of tissue. The organs were collected at 4 days postinoculation from three chickens infected with each virus and homogenized for virus titration. The virus titers from the swab samples are expressed in log10 number of TCID50s/ml. The mean (short horizontal line) and standard error of the mean (SEM) are provided for virus titers.
Protective efficacy of the commercial vaccine LaSota against West African viruses.
The protective efficacy of the commonly used commercial live vaccine LaSota was evaluated against the same two West African viruses, AKO18 and 488MT, and two known velogenic strains, the neurotropic velogenic strain GB Texas and the viscerotropic velogenic strain Fontana. Four groups of 2-week-old chickens (13 in each group) were vaccinated with the commercial LaSota vaccine following the manufacturer's instructions (Fort Dodge). Two other groups (10 in each group) served as unvaccinated controls. All prevaccination sera were negative for NDV by the HI test, using NDV strain LaSota. HI antibodies to NDV were detected in all immunized birds on day 21 postvaccination. HI antibody titers against the homologous strain LaSota and the two West African challenge viruses were determined (Fig. 2). HI antibody titers against strain GB Texas and strain Fontana were also determined. The HI antibody titer to homologous strain LaSota was 6.4 ± 0.86 log2 units (n = 40). HI titers for different challenge viruses were 6.2 ± 1.03 log2 units for strain AKO18, 5.1 ± 0.92 log2 units for strain 488MT, 6.7 ± 0.83 log2 units for strain GB Texas, and 6.7 ± 1.09 log2 units for strain Fontana.
Fig 2.
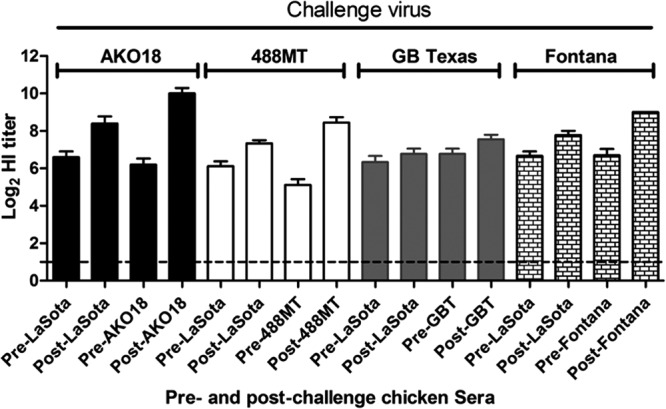
Comparison of HI titers of serum samples in chickens that were immunized with the LaSota vaccine strain and challenged 21 days later with the indicated virulent strains (AKO18, 488MT, GB Texas [GBT], and Fontana). Sera were taken immediately prior to challenge (Pre-) and at 14 days postchallenge (Post-) of survivor birds. The titers in serum samples against the immunizing virus (LaSota) and each respective challenge virus were assayed to compare any increase of serum HI titer. This increase of titer may indicate replication of challenge virus in the vaccinated birds.
The birds were challenged on day 21 postvaccination with 100 CLD50s of challenge virus per chicken: each of the four vaccinated groups was challenged with African strain AKO18 or 488MT or with GB Texas or Fontana, and each of the two unimmunized control groups received West African strain AKO18 or 488MT. Three chickens from each group were sacrificed on day 4 postchallenge; tissue was harvested from the respiratory tract (upper trachea, nasal turbinate, lungs), lymphoid system (spleen), digestive system (cecal tonsil, pancreas), and nervous system (brain) of each chicken; and virus titers were determined by limiting dilution assay (Fig. 3). In the immunized chickens, replication of GB Texas and AKO18 challenge viruses was not detected in any of the above-mentioned organs except the pancreas, whereas one out of three chickens challenged with the 488MT and Fontana viruses had detectable virus in all tissues. It was interesting to note that all of the challenge viruses replicated in the pancreas of immunized chickens to a titer of 4.0 ± 1.7 log10 TCID50s/g. In the nonimmunized animals, the AKO18 and 488MT viruses were detected in all of the sampled tissues: consistent with the previous experiment, the AKO18 virus replicated more efficiently in the respiratory tissue and the brain, whereas the 488MT virus replicated more efficiently in the spleen and cecal tonsil.
Fig 3.
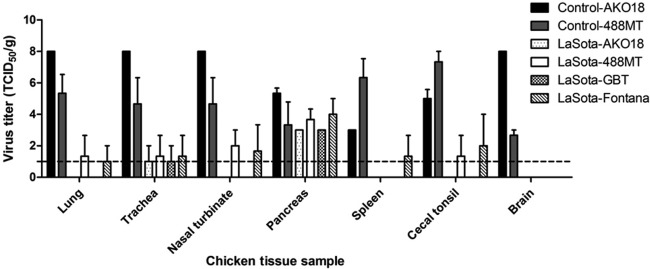
Replication of NDV challenge strains AKO18, 488MT, GB Texas, and Fontana in 2-week-old chickens 21 days following immunization with the NDV LaSota vaccine. Immunized chickens in groups of 3 were infected with challenge virus through the oculonasal route, as described in Materials and Methods. At 4 days p.i., the birds were sacrificed and tissues from the indicated organs were collected and assayed for the presence of virus. Replication of West African NDV strains AKO18 and 488MT in unvaccinated chickens is also provided.
The remaining birds in each group were monitored for disease. All of the immunized birds were fully protected from clinical disease and death with all challenge viruses, whereas all of the unvaccinated control birds succumbed to infection within 3 to 4 days postchallenge (Fig. 4). Swab and tissue samples were collected from the birds of the control groups at the time of death.
Fig 4.
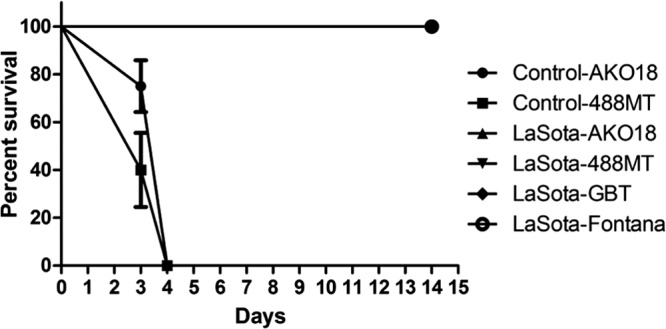
Percent survival curve of NDV LaSota-immunized and control chicken groups challenged with NDV strains AKO18, 488MT, GB Texas, and Fontana. Immunized and control chickens in groups of 10 were challenged with 100 CLD50s of each challenge virus via the oculonasal route and observed for 14 days. All chickens in the control group challenged with West African NDV strains AKO18 and 488MT succumbed to infection by day 4. Note that all of the LaSota-immunized animals survived (the curves are superimposed).
Shedding of challenge viruses was examined from oropharyngeal and cloacal swab specimens taken on day 4 (for all 13 birds per group) and on day 7 (for the remaining 10 birds per group) postchallenge from all of the groups of immunized birds. In the case of the unimmunized control groups, swabs were taken on the day of death, i.e., on either day 3 or day 4. Shedding of challenge virus was determined by virus isolation in embryonated chicken eggs, which yields a positive or a negative result, and also by limiting dilution assay in cell culture, which yields a viral titer (Table 2). In the unimmunized birds, high titers of virus were detected in all of the oropharyngeal and cloacal swab specimens from all of the birds. In the immunized birds, no oral and cloacal shedding was detected for the GB Texas challenge virus, but 3 out of 13 birds were positive for oral shedding of AKO18 virus, whereas no cloacal shedding was detected. Three out of 13 birds were positive for both oral and cloacal shedding of 488MT and Fontana challenge viruses.
Table 2.
Shedding of challenge virus in both vaccinated and unvaccinated chickens on day 4 postchallengea
| Chicken group | No. of chickens challenged with the following virus by the indicated route shedding virus/total no. of chickens (no. of TCID50s/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| AKO18 |
488MT |
GB Texas |
Fontana |
|||||
| Oral | Cloacal | Oral | Cloacal | Oral | Cloacal | Oral | Cloacal | |
| Uninfected control | 10/10 (106) | 10/10 (104) | 10/10 (106) | 10/10 (104) | ND | ND | ND | ND |
| LaSota-immunized group | 3/13 (103) | 0/13 | 3/13 (104) | 3/13 (103) | 0/13 | 0/13 | 3/13 (103) | 3/13 (104) |
Shedding of NDV challenge viruses was determined by inoculation of swab samples in embryonated chicken eggs, and swab samples that were positive by the HA assay were titrated using the endpoint TCID50 assay in DF-1 cells. ND, not determined.
Serum samples were collected for 14 days postchallenge, and titers of antibodies to the immunizing LaSota strain and the respective challenge virus were compared with the prechallenge responses (Fig. 2). An increase in the serum antibody response following challenge was taken as an indication of challenge virus replication. There were substantial increases in HI titer in birds challenged with the two West African viruses AKO18 (3.8-log2-unit increase) and 488MT (3.03-log2-unit increase), whereas the increases for the Fontana (2.3-log2-unit increase) and GB Texas (0.8-log2-unit increase) strains were lower. This was indicative of greater replication of the West African challenge viruses in the LaSota-vaccinated chickens.
Neutralization of West African NDV strains with sera from birds immunized with the LaSota vaccine.
The ability of sera from LaSota-vaccinated chickens to neutralize the West African strains of NDV was assessed by the plaque reduction neutralization test (Fig. 5). Recombinant LaSota virus that had been engineered to contain a polybasic virulent F-protein cleavage site—and thus could form plaques independently of added protease—was used as a homologous virus control in the plaque reduction neutralization test. The West African virus strains used in the preceding challenge experiment (AKO18 and 488MT) were analyzed together with two other strains each from the cities of Malanville (474MC and 463MT) and Gogounou (376GT and 373GC) in Benin. LaSota-immunized chicken sera efficiently neutralized NDV strains AKO18, 474MC, 376GT, and 373GC but less efficiently neutralized strains 488MT and 463MT (Fig. 5). These results suggest that antigenic variation exists among the West African strains and that the LaSota vaccine may not efficiently confer protection against all West African strains.
Fig 5.
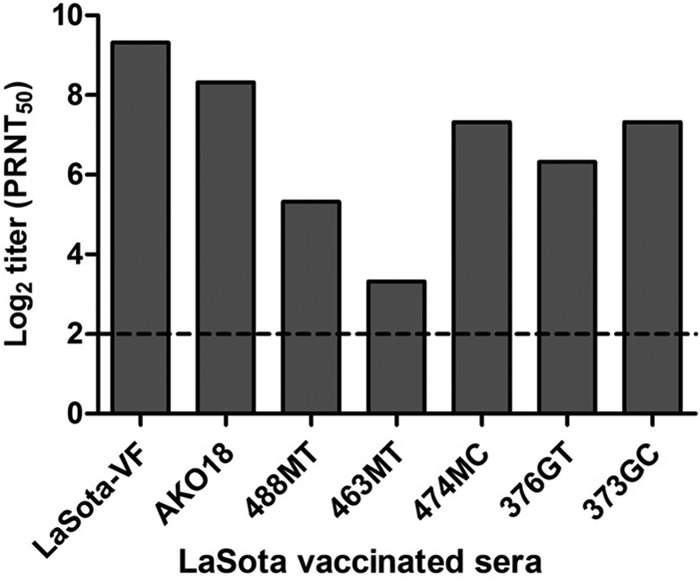
Virus neutralization test for six West African NDV strains using LaSota-immunized chicken sera. A plaque reduction neutralization assay was performed to determine the efficacy of serum from LaSota-vaccinated birds to neutralize in vitro replication of six West African NDV strains. A serially 4-fold-diluted LaSota-immunized chicken serum sample was incubated with 100 PFU of virus for 1 h at 37°C, and plaque assay was performed on a monolayer of chicken embryo fibroblast (DF-1) cells as described in the Materials and Methods section. The serum dilution that inhibited 50% of plaques was considered the neutralization titer (i.e., PRNT50, the 50% plaque reduction neutralization titer).
We compared the extent of diversity among these six West African strains compared to the LaSota vaccine strain. The nucleotide and amino acid sequence identities of these six West African strains with NDV strain LaSota for the F gene ranged from 81.5 to 84.2% and 83.2 to 86.6%, respectively, and for the HN gene ranged from 81.3 to 82.3% and 86.5 to 87.9%, respectively.
Phylogenetic analysis and genotype prediction of West African NDV strains.
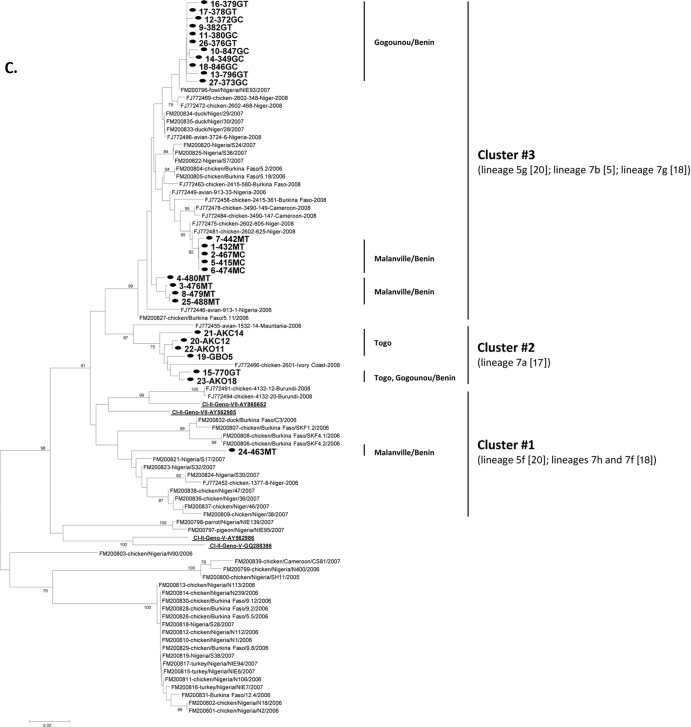
Phylogenetic analysis of the 27 West African strains was carried out by using a 354-nt-long nucleotide sequence of the F-gene hypervariable region (genome nucleotides 4709 to 5062) (7). Representative genotype-specific reference strains were included for constructing a phylogenetic tree (Fig. 6A). The 27 West African strains formed three separate clusters (designated clusters 1 to 3 in Fig. 6A; see Fig. 6B for the strains contained in each cluster) that were interspersed with the representative genotype VII strains. The 5 strains from Togo (cluster 2) were clustered with genotype VII or with lineage 7a NDV strains (17), and 20 strains from Benin (cluster 3) formed a cluster with genotype VII NDV reference strains or with lineage 5f (20) or lineages 7h and 7f (18) but were distantly placed from the Togo strains. One of the West African strains, 463MT (cluster 1), was distantly placed from the other West African strains and was also related to genotype VII or lineages 5g (20), 7b (17), and 7g (18) of previously known NDV strains (Fig. 6C). All the three clusters grouped within genotype VII of class II NDV and showed relatedness to the NDVs previously isolated in Africa.
Fig 6.
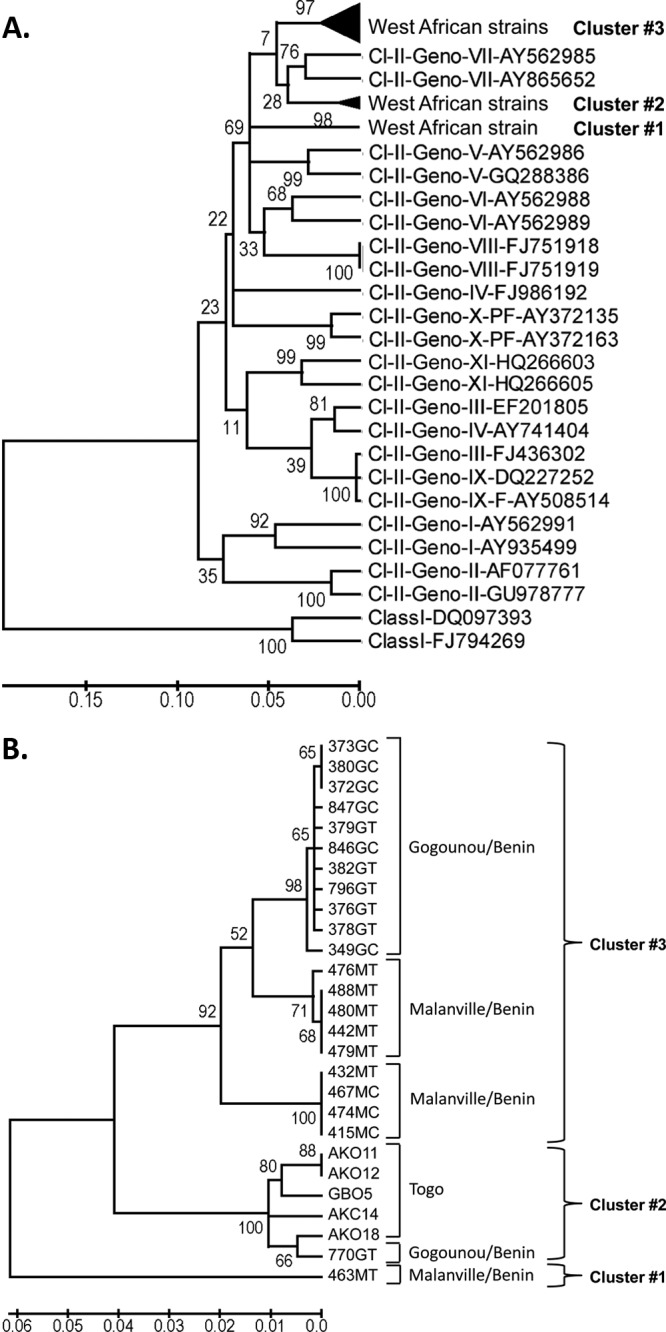
Phylogenetic analysis of West African strains using a partial nucleotide sequence of the fusion-protein gene. (A) Phylogenetic relatedness of the West African strains with representative NDV strains from class I (Cl-I) and class II (Cl-II) genotypes (Geno-) identified by their GenBank accession numbers. The African strains formed three clusters (clusters 1 to 3). (B) Phylogenetic analysis of the 27 West African strains, illustrating the three clusters (clusters 1 to 3). (C) Phylogenetic analysis of the 27 Benin and Togo strains isolated in the present study in relation to previously characterized NDVs from Africa (as described in references 17 and 18). Our 27 viruses are shown in bold font and highlighted with a solid circle. The class II genotype V and VII reference strains shown in panel A are underlined.
To differentiate subclusters or lineages among West African strains, a separate phylogenetic tree was constructed using the 27 strains (Fig. 6B). All isolates from Togo were clustered together (cluster 2), whereas most of the isolates from Benin formed three lineages within cluster 3. Within cluster 3, most of the strains isolated from the city of Gogounou formed a single distinct lineage, and most of the strains from the city of Malanville formed two other lineages. Two Benin strains were outside cluster 3: isolate 770GT from Gogounou clustered together with the Togo isolates, and isolate 463MT from Malanville was relatively distinct, as already noted.
Interestingly, at least 3 distinct viruses were present in the Malanville market in Benin on 13 February 2009: the subcluster of cluster 3 with 415MC, 422MT, 432MT, 467MC, and 474MC; the subcluster of cluster 3 with 476MT, 479MT, 480MT, and 488MT; and 463MT in cluster 1. In Gogounou, while a Togo-like isolate, 770GC (cluster 2), was characterized in mid-March 2009, very similar NDVs seemed to have persisted within a 6-week window: in cluster 3, the partial F-gene sequence of isolate 846GC (specimen collected on 13 March 2009) was identical to that of isolates 376GT, 378GT, 380GC, and 382GT (all collected on 1 February 2009), for example (Table 1).
The genetic variation among West African NDV strains was further studied by comparing the complete coding sequences of the F and HN genes of the six strains noted in the previous section. The nucleotide and amino acid sequence identities for the F gene and the F protein among these six West African strains ranged from 88 to 90% and 87 to 97%, respectively. The nucleotide and amino acid sequence identities for the HN gene and HN ranged from 89 to 99% and 90 to 99%, respectively. Thus, there was greater relatedness among the West African strains than between these strains and the vaccine strain LaSota (with which they had 83 to 86% amino acid sequence identity for the F protein and 86 to 88% amino acid sequence identity for HN).
Determination of a 6-nt insertion between the HN and L genes.
Recently, complete genome sequencing of the West African strain AKO18 identified a novel 6-nt insertion in the intergenic sequence between the HN and L genes (23). This insertion had never been reported for any other NDV strain except strain AKO18. This was of interest because length differences in the genomes of NDV isolates are useful markers for characterizing the natural history of the isolates. Therefore, we analyzed the other 26 West African strains for this 6-nt insertion by sequencing this region (nt 8338 to 8343) of the genome. This 6-nt HN/L insertion was present in all of the strains from Togo but was present in only a single strain from Benin, namely, the 770GT strain from Gogounou, Benin. Interestingly, there were two different versions of this 6-nt insert: four strains (AKO11, AKC12, GBO5 and AKC14) had the insert sequence 8338UUUUUC8343, and two strains (AKO18 and 770GT) had an insertion of 8338UUUUUU8343. Hence, our results identified a novel length class of the NDV genome that contains a 6-nt insertion in the HN and L intergenic region and was present in all of the strains sampled from Togo but was largely absent in the strains from Benin.
DISCUSSION
ND is a major threat to the poultry industry worldwide. The disease particularly affects the livelihood of people across the developing world where poultry and poultry products are the major source of protein and income. In Africa, the disease is enzootic and devastates traditional village poultry farming (26). It is thought that virulent NDV strains are constantly evolving in areas of the world where it is enzootic and that Africa is a major breeding ground for such viruses. NDV is genetically and biologically a diverse virus, with numerous genotypes isolated across the globe. Recent studies suggest that NDV strains from Madagascar in Africa are unique and more divergent from the strains isolated in the other parts of the world (19). In this study, we characterized genetic and biological properties of NDV strains isolated from two countries, Benin and Togo, of West Africa. Oropharyngeal and cloacal swab samples from apparently healthy birds of the live-bird markets were collected as part of an influenza surveillance program. A total of 68 specimens from 34 birds were analyzed and found to be negative for influenza virus. Of these, 37 were positive for NDV by HI and RT-PCR, providing a total of 27 new strains.
Together with our recent sequence analysis of strain AKO18 (23), this is the first study to characterize NDV isolates from Benin and Togo at the molecular level. The genetic analysis of the partial sequences of the fusion-protein gene showed that all 27 West African NDV isolates have a polybasic F-protein cleavage site suggestive of a velogenic pathotype. Twenty-five out of 27 have an F-protein cleavage site of 112RRQKR↓F117 containing 4 basic amino acids, and two strains have 112RRRKR↓F117] with 5 basic amino acids. Virulent cleavage motifs with 5 basic amino acids have been described for NDV isolates from Mali (18), Madagascar (19), and Nigeria (27). As already noted (see Results), there was no clear difference in virulence associated with 5 versus 4 basic residues for these isolates. Our results indicate that virulent strains of NDV are enzootic and highly prevalent in West Africa, notably, in seemingly healthy birds. These results also demonstrate cocirculation of viruses with different F-protein cleavage site sequences at the same time and place in West Africa.
Pathogenicity studies of the 27 West African NDV isolates determined by the MDT assay in embryonated chicken eggs provided values between 48 and 64 h, suggesting that there was some variability in virulence but showing that all of these viruses belong to the highly virulent or velogenic pathotype. This was confirmed for selected strains by the ICPI assay. Two strains, AKO18 and 488MT, isolated from Togo and Benin, respectively, were further characterized by oculonasal inoculation of 2-week-old chickens, mimicking natural infection. Both strains killed chickens in 3 to 4 days, confirming that they are highly virulent. However, the two viruses had differences in tropism: strain AKO18 replicated more efficiently in respiratory tissues and the brain, and strain 488MT replicated more efficiently in visceral organs.
It is surprising that highly virulent NDV strains were isolated from apparently healthy chickens in live-bird markets in a setting where vaccination is not routinely used due to financial constraints. It may be that these chickens were sampled during the incubation period of infection prior to the onset of disease signs, or it may be that these birds had a previous infection with an avirulent NDV strain and were protected. These results may also suggest persistent circulation of virulent NDV strains in local chickens independent of a recognized epizootic. In a recent surveillance study focused on avian influenza, we have shown that 32% of bird serum samples from Togo were positive for NDV antibodies by the HI test (28). The high seroprevalence of NDVs may indeed explain in part the absence of clinical signs, despite their virulent pathotype. However, it is possible that the circulating virus and the local chickens have coevolved to coexist without overt disease. Outbreaks may occur when there is an alteration in their relationship due to a genetic change in the circulating NDV population or to stress of the host or weakened resistance in the host. The contamination of birds in the markets is also a likely possibility. We do not, unfortunately, know much at all about the genetics of local chickens, but we cannot rule out the possibility that they might be less susceptible to disease than SPF chickens.
To test the efficacy of commercial vaccination against the West African NDV strains, 2-week-old chickens were immunized with the commonly used commercial live NDV vaccine LaSota and challenged with strains AKO18 and 488MT as well as with two known velogenic strains of U.S. origin, GB Texas and Fontana. All of the immunized birds were completely protected against clinical disease and mortality by all the four challenge virus strains. However, virus shedding was detected in the AKO18-, 488MT-, and Fontana-challenged group. The postchallenge serum HI titers of AKO18 and 488MT from chickens were higher than those of strains GB Texas and Fontana, indicative of challenge virus replication in the vaccinated birds. The challenge virus shedding and the high postchallenge HI titers suggest that the commonly used vaccine LaSota provided protection against disease but did not prevent infection and virus replication, indicating an antigenic difference between vaccine strain LaSota and West African strains AKO18 and 488MT.
To investigate the genetic relatedness between vaccine strain LaSota and West African strains, the F and HN genes of six West African strains were completely sequenced (these data included those for the recently sequenced strain AKO18). The amino acid sequence identities of the F and HN proteins among these six strains ranged from 87 to 97.1% and 89.8 to 99.5%, respectively, whereas they ranged from 83.2 to 86.6% and 86.5 to 87.6%, respectively, between the West African strains and the vaccine strain LaSota.
It is also noteworthy that three vaccinated birds in the Fontana challenge group had virus shedding. Comparison of the amino acid sequence identities of the F and HN proteins between LaSota and GB Texas were 96.6% and 95.5%, respectively, and thus, the sequences were highly related. In contrast, the amino acid identities between the LaSota and Fontana strains were 90.1% and 88.8% for F and HN, respectively, and thus, the sequences were more divergent. Thus, in the challenge experiment, the three challenge viruses that were relatively more divergent from vaccine strain LaSota, namely, AKO18, 488MT, and Fontana, had breakthrough replication, whereas the challenge virus strain GB Texas that was highly related to LaSota was completely prevented from replication. This supports the idea that the antigenic divergence of NDV strains relative to vaccine strains can reduce vaccine efficacy (2).
Phylogenetic analyses, based on the partial nucleotide sequence of the F gene of the 27 isolates, identified three clusters that were related to the representative strains of genotype VII. All viruses from Togo formed a single cluster. Most of the viruses from Benin formed another cluster containing three subclusters or lineages. However, one Benin strain (770GT) clustered with the Togo strains, and a second Benin strain (463MT) was a more distinct outlier. It may be that these strains and, in particular, the outlier 463MT strain represent genetic divergence between the circulating strains of West Africa within NDV genotype VII. Since the village chickens in Benin and Togo are not regularly vaccinated, it is unlikely that this evolution resulted from vaccine immune pressure. It is also interesting to note that the isolates from Benin formed one genetic cluster and the isolates from Togo formed another genetic cluster, even though they are neighboring countries. These observations suggest that the ecosystem of a location—including the genotype of the virus, the local chicken population, and the local environment—may favor a particular genotype for circulation in that location. Alternatively, evolution may be sufficiently rapid that viruses in different locales quickly become distinct.
The virus diversity in the Malanville, Benin, market in March 2009 may reflect the interesting location of the live-bird market. Malanville is in the north, at the border with Niger, and very close to Nigeria. Commercial exchanges of poultry might explain the cocirculation of different NDV strains in the area. We have also observed similar viruses in a market in Lomé in southern Togo and in the market of Gogounou (northern Benin), suggesting either the cocirculation of similar NDV strains with a slow evolution in West Africa or the importation of new strains due to transport of poultry over hundreds of kilometers or by wild birds. In contrast, we also observed the persistence of very similar viruses in Gogounou (northern Benin) within a 6-week period (Table 1), suggesting the enzootic nature of these viruses in the area.
Our recent complete genome sequence analysis of the West African strain AKO18 revealed an unique 6-nt insertion in the intergenic region between the HN and L genes (23). This prompted us to analyze the HN-L intergenic sequence of the other 26 strains isolated from Togo and Benin. Our results showed that this insertion is present in all of the strains from Togo and in a single strain from Gogounou, Benin (770GT). There was a variation in the 6-nt insert sequence, with strains AKO11, AKC12, GBO5, and AKC14 having 8338UUUUUC8343 and strains AKO18 and 770GT having the insert sequence 8338UUUUUU8343. It is not clear whether this insertion provides any advantage to the virus or whether it is completely incidental. However, the genomes of the NDV strains sequenced to date have few such insertions—namely, a 6-nt insertion in the downstream noncoding region of the N gene and a 12-nt insertion in the P ORF—and these define the different length classes of the NDV genome and are markers for viral evolution. The finding that this insertion was mainly specific to strains from Togo is consistent with the idea that these strains are evolving locally. It is reasonable to suggest that the Togo strains bearing this insert represent the local emergence of a new NDV length class, and the presence of one isolate with this insert in Gogounou, Benin, may be indicative of the beginning of its spread from its locale of origin.
In summary, this study showed that all 27 of the NDV strains that were isolated from apparently healthy chickens in Togo and Benin, West Africa, are highly virulent. Our study also showed that, although the commercial vaccine strain LaSota protected against disease, it did not prevent infection and shedding of the West African strains. Phylogenetic analysis showed that the NDV strains from West Africa form separate clusters of NDV that are related most closely to genotype VII of the known NDV strains. Isolation of NDVs containing a unique 6-nt insert sequence in the HN-L intergenic region and circulating in the Togo region revealed that virulent NDVs with a new genome size of 15,198 nucleotides in class II viruses are unique and might deserve a separate genetic class assignment on the basis of this genetic marker. The occurrence of the more divergent, outlier strain 463MT indicates that genetically diverse viruses circulate in West Africa, in addition to the local lineages. Isolation and characterization of NDV strains from other countries of Africa will help identify the genotypes of indigenous strains and increase our overall understanding of the evolution of virulent NDV strains. These results will also have implications on development of effective NDV vaccines in Africa.
ACKNOWLEDGMENTS
We thank Daniel Rockemann and Bridgett Sharp for their excellent technical assistance and Peggy Barott for proofreading the manuscript.
This work was supported by NIAID contract N01A060009 (85% support) and the NIAID, NIH, Intramural Research Program (15% support).
The views expressed herein do not necessarily reflect the official policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. government.
R. J. Webby, M. F. Ducatez, G. L. Aplogan, and K. A. Awoume were supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, under contract no. HHSN266200700005C and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Published ahead of print 19 December 2012
REFERENCES
- 1. Alexander DJ. 2000. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 19:443–462 [DOI] [PubMed] [Google Scholar]
- 2. Dortmans JC, Peeters BP, Koch G. 2012. Newcastle disease virus outbreaks: vaccine mismatch or inadequate application? Vet. Microbiol. 160:17–22 [DOI] [PubMed] [Google Scholar]
- 3. Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1449–1496 In Knipe DM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 4. Samal SK. 2011. Newcastle disease and related avian paramyxoviruses, p 69–114 In Samal SK. (ed), The biology of paramyxoviruses. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 5. Panda A, Huang Z, Elankumaran S, Rockemann DD, Samal SK. 2004. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 36:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peeters BP, de Leeuw OS, Koch G, Gielkens AL. 1999. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 73:5001–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller PJ, Decanini EL, Afonso CL. 2010. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 10:26–35 [DOI] [PubMed] [Google Scholar]
- 8. Czegledi A, Ujvari D, Somogyi E, Wehmann E, Werner O, Lomniczi B. 2006. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 120:36–48 [DOI] [PubMed] [Google Scholar]
- 9. Diel DG, da Silva LH, Liu H, Wang Z, Miller PJ, Afonso CL. 2012. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 12:1770–1779 [DOI] [PubMed] [Google Scholar]
- 10. Kim LM, King DJ, Guzman H, Tesh RB, Travassos da Rosa AP, Bueno R, Jr, Dennett JA, Afonso CL. 2008. Biological and phylogenetic characterization of pigeon paramyxovirus serotype 1 circulating in wild North American pigeons and doves. J. Clin. Microbiol. 46:3303–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abolnik C, Horner RF, Bisschop SP, Parker ME, Romito M, Viljoen GJ. 2004. A phylogenetic study of South African Newcastle disease virus strains isolated between 1990 and 2002 suggests epidemiological origins in the Far East. Arch. Virol. 149:603–619 [DOI] [PubMed] [Google Scholar]
- 12. Herczeg J, Wehmann E, Bragg RR, Travassos Dias PM, Hadjiev G, Werner O, Lomniczi B. 1999. Two novel genetic groups (VIIb and VIII) responsible for recent Newcastle disease outbreaks in southern Africa, one (VIIb) of which reached southern Europe. Arch. Virol. 144:2087–2099 [DOI] [PubMed] [Google Scholar]
- 13. Otim MO, Christensen H, Jorgensen PH, Handberg KJ, Bisgaard M. 2004. Molecular characterization and phylogenetic study of Newcastle disease virus isolates from recent outbreaks in eastern Uganda. J. Clin. Microbiol. 42:2802–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed MH, Kumar S, Paldurai A, Megahed MM, Ghanem IA, Lebdah MA, Samal SK. 2009. Complete genome sequence of a virulent Newcastle disease virus isolated from an outbreak in chickens in Egypt. Virus Genes 39:234–237 [DOI] [PubMed] [Google Scholar]
- 15. Mohamed MH, Kumar S, Paldurai A, Samal SK. 2011. Sequence analysis of fusion protein gene of Newcastle disease virus isolated from outbreaks in Egypt during 2006. Virol. J. 8:237 doi:10.1186/1743-422X-8-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell JG, Mouahid M. 1987. Vaccination against Moroccan strains of Newcastle disease virus. Trop. Anim. Health Prod. 19:192–196 [DOI] [PubMed] [Google Scholar]
- 17. Cattoli G, Fusaro A, Monne I, Molia S, Le Menach A, Maregeya B, Nchare A, Bangana I, Maina AG, Koffi JN, Thiam H, Bezeid OE, Salviato A, Nisi R, Terregino C, Capua I. 2010. Emergence of a new genetic lineage of Newcastle disease virus in West and Central Africa—implications for diagnosis and control. Vet. Microbiol. 142:168–176 [DOI] [PubMed] [Google Scholar]
- 18. Servan de Almeida R, Maminiaina OF, Gil P, Hammoumi S, Molia S, Chevalier V, Koko M, Andriamanivo HR, Traore A, Samake K, Diarra A, Grillet C, Martinez D, Albina E. 2009. Africa, a reservoir of new virulent strains of Newcastle disease virus? Vaccine 27:3127–3129 [DOI] [PubMed] [Google Scholar]
- 19. Maminiaina OF, Gil P, Briand FX, Albina E, Keita D, Andriamanivo HR, Chevalier V, Lancelot R, Martinez D, Rakotondravao R, Rajaonarison JJ, Koko M, Andriantsimahavandy AA, Jestin V, Servan de Almeida R. 2010. Newcastle disease virus in Madagascar: identification of an original genotype possibly deriving from a died out ancestor of genotype IV. PLoS One 5:e13987 doi:10.1371/journal.pone.0013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snoeck CJ, Ducatez MF, Owoade AA, Faleke OO, Alkali BR, Tahita MC, Tarnagda Z, Ouedraogo JB, Maikano I, Mbah PO, Kremer JR, Muller CP. 2009. Newcastle disease virus in West Africa: new virulent strains identified in non-commercial farms. Arch. Virol. 154:47–54 [DOI] [PubMed] [Google Scholar]
- 21. Ducatez MF, Tarnagda Z, Tahita MC, Sow A, de Landtsheer S, Londt BZ, Brown IH, Osterhaus DM, Fouchier RA, Ouedraogo JB, Muller CP. 2007. Genetic characterization of HPAI (H5N1) viruses from poultry and wild vultures, Burkina Faso. Emerg. Infect. Dis. 13:611–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guèye EF. 1999. Poultry plays an important role in African village life. World Poultry 14:14–17 [Google Scholar]
- 23. Kim S, Nayak S, Paldurai A, Nayak B, Samuel A, Aplogan G, Awoume K, Webby R, Ducatez M, Collins P, Samal SK. 2012. Complete genome sequence of a novel Newcastle disease virus strain isolated from a chicken in West Africa. J. Virol. 86:11394–11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Organization for Animal Health 2009. Newcastle disease. In OIE terrestrial manual 2009. World Organization for Animal Health, Paris, France: http://www.oie.int/Eng./normes/mmanual/2008/pdf/2.03.14_newcastle_dis.pdf [Google Scholar]
- 25. Reed LJ, Muench H. 1938. A simple method of estimation of fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 26. Awan MA, Otte MJ, James AD. 1994. The epidemiology of Newcastle disease in rural poultry: a review. Avian Pathol. 23:405–423 [DOI] [PubMed] [Google Scholar]
- 27. Solomon P, Abolnik C, Joannis TM, Bisschop S. 2012. Virulent Newcastle disease virus in Nigeria: identification of a new clade of sub-lineage 5f from livebird markets. Virus Genes 44:98–103 [DOI] [PubMed] [Google Scholar]
- 28. Couacy-Hymann E, Kouakou VA, Aplogan GL, Awoume F, Kouakou CK, Kakpo L, Sharp BR, McClenaghan L, McKenzie P, Webster RG, Webby RJ, Ducatez MF. 2012. Surveillance for influenza viruses in poultry and swine, West Africa, 2006-2008. Emerg. Infect. Dis. 18:1446–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]