Abstract
We report an autochthonous hepatitis E virus (HEV)-hepatitis B virus co-primary infection in a 41-year-old man having sex with men and infected with human immunodeficiency virus (HIV). This case prompts testing for HEV in HIV-infected patients with acute hepatitis even if primary infection with another hepatitis virus is diagnosed.
CASE REPORT
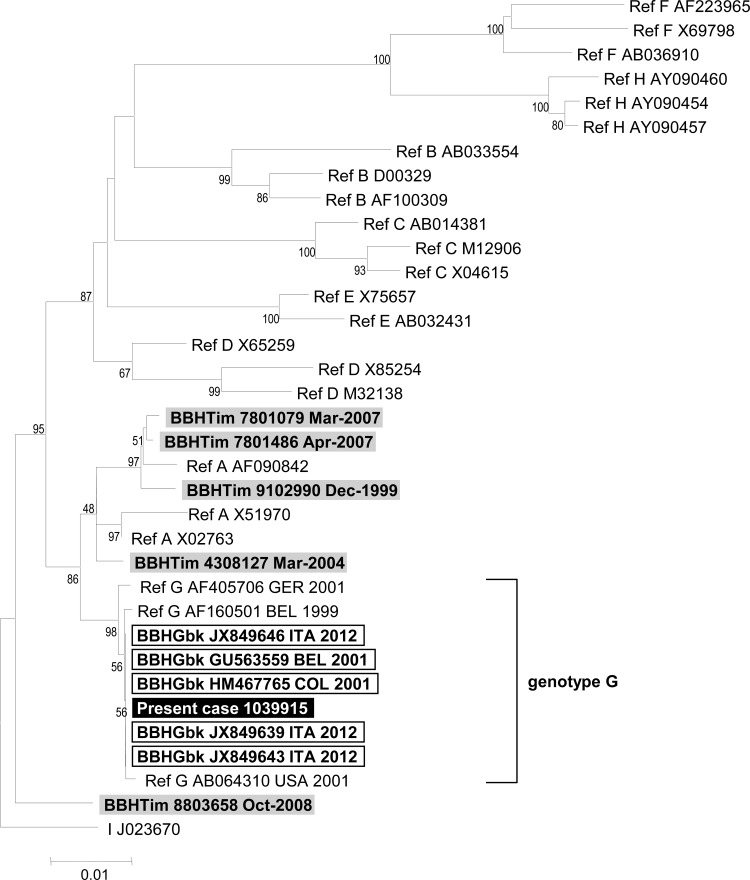
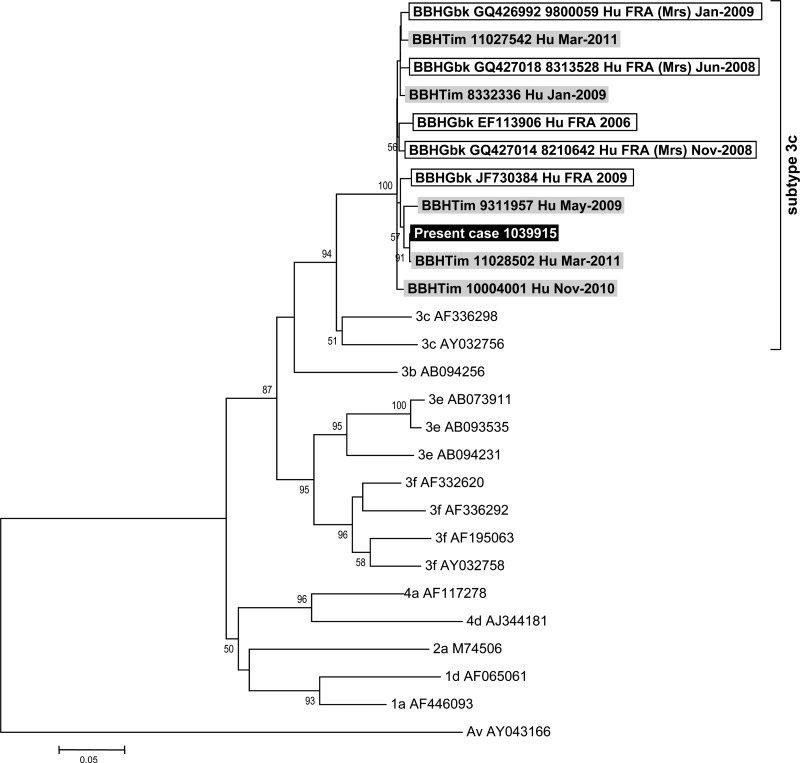
A 41-year-old man having sex with men (MSM) diagnosed in 1997 with human immunodeficiency virus type 1 (HIV-1) infection (1) presented asthenia, abdominal pain, and dark urine specimens in September 2010. His CD4+ T-lymphocyte count (CD4 count) was 222/mm3, and his plasma HIV-1 RNA level was <40 copies/ml (RealTime HIV; Abbott, Wiesbaden, Germany) under raltegravir, darunavir, and ritonavir. In June 2009, he was seronegative for hepatitis C virus (HCV) and hepatitis B virus (HBV) (Architect; Abbott), while primary hepatitis A virus (HAV) infection was diagnosed by detecting anti-HAV IgM (Architect; Abbott) and HAV RNA, as described previously (2). At admission, the alanine aminotransferase level was 2,620 IU/ml, bilirubinemia was 72 μmol/liter, and the prothrombin index was 100% (Table 1). His antiretroviral drugs had not been modified since June 2009. Infections with HCV (HCV serology, Architect [Abbott]; HCV RNA, RealTime HCV [Abbott]), Delta agent (DiaSorin, Saluggia, Italy), Epstein-Barr virus (DiaSorin Liaison), cytomegalovirus (Vidas; bioMérieux, Meylan, France), and herpes simplex virus (Siemens, Marburg, Germany) were ruled out by serology and PCR testing. Acute hepatitis B was diagnosed by detection in serum of HBV DNA (>9 log10 IU/ml) (RealTime HBV; Abbott), hepatitis B surface antigen (HBsAg; Abbott), HBe antigen (HBeAg; Abbott), and anti-HB core (anti-HBc) IgM (titer of >200 IU/ml) (Vidas; bioMérieux) (Table 1). Retrospective HBsAg and anti-HBc testing showed negativity in December 2009 and positivity in June 2010; HBV DNA testing was negative in July 2009. The HBV genotype, determined as described previously (3), was G (Fig. 1). Concurrently with HBV diagnosis, hepatitis E virus (HEV) testing on the serum sample collected in September 2010 showed positivity for anti-HEV IgM (Adaltis, Casalecchio di Reno, Italy), and for HEV RNA using in-house assays as described previously (4). Retrospective testing showed absence of HEV RNA and anti-HEV IgM in June 2010. HEV RNA sequencing (4) identified genotype 3c (Fig. 2).
Table 1.
Longitudinal follow-up of biological and virological parameters
| Parametera | Result for date (mo/day/yr) shownb |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12/18/09 | 6/25/10 | 9/2/10 | 9/7/10 | 9/10/10 | 9/20/10 | 9/27/10 | 10/1/10 | 10/4/10 | 10/19/10 | 1/14/11 | 5/19/11 | 11/18/11 | |
| ALT (IU/liter) | 49 | 82 | 623 | 2,223 | 2,620 | 1,129 | 1,001 | 842 | 675 | 161 | 34 | 19 | 13 |
| AST (IU/liter) | 37 | 47 | 315 | 1,259 | 1,489 | 428 | 413 | 293 | 272 | 59 | 30 | 22 | 18 |
| GGT (IU/liter) | 26 | 30 | 259 | 441 | 381 | 278 | 227 | 192 | 182 | 98 | 15 | 19 | 19 |
| Bilirubinemia (μmol/liter) | 16 | 11 | 22 | 43 | 72 | 44 | 8 | 19 | 17 | 13 | 13 | 8.7 | 7 |
| PI (%) | 100 | 100 | 100 | 100 | 100 | 94 | 100 | 93 | 90 | 86 | 100 | 100 | 100 |
| Platelet count (109/liter) | 230 | 217 | 212 | 210 | 161 | 256 | 240 | 233 | 248 | 278 | 216 | 213 | 256 |
| CD4 count/mm3 | 141 | 149 | 175 | 188 | 222 | 226 | NP | NP | NP | 183 | 210 | NP | 284 |
| HEV RNA | Neg. | Neg. | Pos. | NP | Pos. | Pos. | NP | NP | Neg. | Neg. | Neg. | Neg. | Neg. |
| Anti-HEV IgM (SCOR) | NA | NA | 15.1 | NP | 14.3 | NP | NP | NP | NP | >10 | 1.0 | Neg. | |
| Anti-HEV IgG (SCOR) | NA | NA | 0.7 | NP | 10.7 | NP | NP | NP | NP | 9.3 | 6.8 | Pos. | |
| HBV DNA (log10 IU/ml) | NAc | NA | >9.0 | NP | NP | 6.15 | NP | NP | 4.15 | 3.13 | 2.15 | 1.92 | Neg. |
| HBsAg (pg/ml) | Neg. | >250d | >250 | NP | >250 | NP | NP | NP | NP | >250 | 32 | 25 | 0 |
| HBeAg (SCOR) | NP | 1,900 | 1,825 | NP | 1,293 | NP | NP | NP | NP | 82.9 | 5.5 | 5.6 | NP |
| Total anti-HBc Ab (SCOR) | Neg. | 3.8 | 9.4 | NP | 9.5 | NP | NP | NP | NP | 10.6 | 9.8 | 9.6 | 10.5 |
| Anti-HBc IgM (IU/ml) | >200 | ||||||||||||
| Anti-HBs Ab (IU/liter) | <10 | <10 | <10 | NP | <10 | NP | NP | NP | NP | <10 | <10 | <10 | 37.9 |
| Anti-HBe | NP | 0 | Neg. | NP | Neg. | NP | NP | NP | NP | Neg. | Neg. | Neg | NP |
| HIV RNA (log10 copies/ml) | Neg. | <40 | Neg. | NP | <40 | <40 | NP | NP | NP | NP | NP | NP | Neg. |
| Anti-HCV Ab | Neg. | Neg. | Neg. | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP |
ALT, alanine aminotransferase level; AST, aspartate aminotransferase level; CD4 count, CD4+ T-lymphocyte count; GGT, gammaglutamyltransferase; PI, prothrombin index; HCV, hepatitis C virus; HEV, hepatitis E virus; SCOR, signal/cutoff ratio; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HbeAg, hepatitis B e antigen; HBc, hepatitis B core; Ab, antibody; HIV, human immunodeficiency virus.
The antiviral therapy from 18 December 2009 through 10 September 2010 included raltegravir (RGV), darunavir (DRV), and ritonavir (RTV). The antiviral therapy from 20 September 2010 through 19 October 2010 included RGV, DRV, RTV, tenofovir-emtricitabine (TDF/FTC), and ribavirin (RBV). The antiviral therapy from 14 January 2011 through 18 November 2011 included RGV, DRV, RTV, and TDF/FTC. Neg., negative; Pos., positive; NP, not performed; NA, not available.
Negative on 24 July 2009.
Performed retrospectively.
Fig 1.
Phylogenetic tree based on a 943-nucleotide (nt) fragment of the hepatitis B virus genome encoding the reverse transcriptase/hepatitis B surface antigen (nt 131 to 1073 in reference to GenBank sequence accession no. AF405706). HBV DNA from the present case is indicated by a boldface white font on a black background. Other sequence names in boldface indicate sequences with the highest BLAST scores (http://blast.ncbi.nlm.nih.gov/Blast.cgi) when searching in the NCBI GenBank nucleotide sequence database (BBHGbk; black frame) or our laboratory nucleotide sequence database (BBHTim; gray background); reference sequences with known genotypes and subtypes are indicated (3). Nucleotide sequence alignments were performed by using ClustalX version 2.0 (www.clustal.org/download/current). The tree was constructed by using MEGA5 (www.megasoftware.net) and the neighbor-joining method. Branches were obtained from 1,000 resamplings of the data; those with bootstrap values of >50% are labeled on the tree. The avian HEV sequence AY043166 was used as an out-group. The scale bar indicates the number of nucleotide substitutions per site. HEV sequences are labeled with GenBank accession number or laboratory number (for BBHTim and the present case), host, country where isolated, and collection or submission date. Av, avian; Hu, human; BBH, best BLAST hit; FRA, France; Gbk, GenBank; Mrs, Marseille; Tim, Timone laboratory.
Fig 2.
Phylogenetic tree based on partial (281-nucleotide [nt]) sequences of open reading frame 2 of the hepatitis E virus (HEV) genome (nt 6034 to 6314 in reference to GenBank sequence accession no. AB291961). HEV RNA from the present case is indicated by a boldface white font on a black background. Other sequence names in boldface indicate sequences with the highest BLAST scores (http://blast.ncbi.nlm.nih.gov/Blast.cgi) when searching in the NCBI GenBank nucleotide sequence database (BBHGbk; black frame) or our laboratory nucleotide sequence database (BBHTim; gray background); reference sequences with known genotypes and subtypes are indicated (4). Nucleotide sequence alignments were performed by using ClustalX version 2.0 (www.clustal.org/download/current). The tree was constructed by using MEGA5 (www.megasoftware.net) and the neighbor-joining method. Branches were obtained from 1,000 resamplings of the data; those with bootstrap values of >50% are labeled on the tree. The avian HEV sequence AY043166 was used as an out-group. The scale bar indicates the number of nucleotide substitutions per site. HEV sequences are labeled with GenBank accession number or laboratory number (for BBHTim and the present case), host, country where isolated, and collection or submission date. Av, avian; BEL, Belgium; Hu, human; BBH, best BLAST hit; COL, Colombia; FRA, France; Gbk, GenBank; GER, Germany; ITA, Italy; Mrs, Marseille; Tim, Timone laboratory; USA, United States of America.
The patient did not travel abroad in 2010 but reported multiple male sexual partners and frequent consumption of uncooked pig liver sausage (PLS) in the 9-week period before hepatitis onset. Tenofovir-emtricitabine and ribavirin (12 mg/kg of body weight/day) were introduced to help control HBV and HEV, respectively. HBV viremia and HBsAg titers progressively became undetectable (<10 IU/ml and <0.05 pg/ml, respectively) 14 months post-HBV diagnosis (Table 1). Anti-HBs antibodies became detectable at this time point. HEV RNA became undetectable within 2 weeks post-ribavirin introduction. Liver biochemical markers normalized within 4 months after HBV-HEV diagnosis.
We report here, to our knowledge, the first observation of HBV/HEV co-primary infection in an HIV-infected patient. Otherwise, co-primary infections with other hepatitis viruses sharing the same transmission routes have been described. One case of HAV-HEV dual infection was recently reported in association with aseptic meningitis in India, but diagnosis relied only on positive IgM to both viruses (5). In contrast, co-primary infections with HBV and HCV or delta agent were more frequently described (6–8). Besides, concurrent acute hepatitis E and HBV reactivation has been described in an HIV-infected person (9).
Compared to the general population, HIV-infected persons are at higher risk of viral hepatitis, including with HBV, HCV, or delta agent, due to common risk factors for these infections, including intravenous drug use or sexual intercourse (8, 10–13). Regarding HEV, it is an emerging cause of autochthonous hepatitis in Europe (14) and a new causative agent of acute and chronic hepatitis in HIV-infected persons (15–19). Nonetheless, HEV seroprevalence has not been found to date to differ statistically significantly between HIV-seropositive and -seronegative patients and in HIV-infected patients according to CD4 count, gender, or HIV transmission mode (18–21). HEV genotype 3c identified here is among the most frequently described in autochthonous cases in Europe, including France (4, 22, 23). Best matches in the NCBI nucleotide sequence database were HEV sequences obtained in France from humans in whom HIV status was not documented, while best matches in our laboratory sequence database were HEV sequences from HIV-negative persons (Fig. 2). Consumption of uncooked PLS reported by the case has been documented as an HEV source in southern France, and eating raw or undercooked pork has been identified as a risk factor predictive of anti-HEV seropositivity in HIV-infected persons (4, 19, 24, 25). This habit may explain the greater incidence and prevalence of HEV infections reported in southern France than northern France, including in HIV-infected persons (26, 27). HEV transmission through sexual intercourse between MSM had been suspected in the 1990s but was not confirmed thereafter (19, 21, 28). Nonetheless, HEV transmission may potentially occur through sexual intercourse between MSM, as demonstrated for HAV or HCV, particularly in HIV-seropositive persons (29–31), and HEV infection has been described previously in several MSM and a bisexual man infected with HIV (9, 17, 21, 32, 33). Here, the patient reported sexual intercourse with several men and concurrently acquired HBV as well as HAV and HIV earlier in his life. Of note, he became infected with HBV genotype G, which is rare in France and worldwide but has been described to be more frequent in HIV-infected patients (34–36) and particularly among MSM (37–39). Also, we searched for top hits in the NCBI sequence database with the HAV sequence recovered from a patient's serum collected 1 year before HEV-HBV primary infection, and we found three HAV sequences with 100% nucleotide identity that were obtained from serum samples of MSM in Barcelona, Spain (40).
The major complications of autochthonous HEV infection in Europe are fatal outcome, which occurred mostly in patients with underlying liver diseases, and progression to chronicity and cirrhosis (14, 17, 20, 32, 41–43). HIV-infected patients are at particular risk of both severe outcomes due to frequent liver injury related to hepatitis virus coinfections or antiretroviral-induced toxicity and to HIV-induced immunosuppression (11, 15). The present observation questioned if concurrent acute HEV-HBV infections could be associated with more severe histopathology than acute HEV or HBV monoinfection, as observed for concurrent acute infections with Delta agent and HBV (7), and if reciprocal inhibition of HEV and HBV replication, as suspected during co-primary HBV-HCV infection, may occur (6). Then introduction of antiviral therapy for both viruses prevented speculation on these issues. Of note, HBV DNA clearance only occurred after 14 months on tenofovir-emtricitabine, while HEV RNA clearance occurred within 1 month under ribavirin. Regarding progression toward chronic hepatitis E in HIV-infected patients, its occurrence was previously observed in patients exhibiting a CD4 count of <200/mm3 (15, 21). Here, ribavirin therapy was administered to avoid potential severe outcome due to concurrent HBV infection and progression toward chronicity because the patient's CD4 count was around 200/mm3. No treatment is currently recommended for acute hepatitis E, but ribavirin led to HEV clearance within 2 to 12 weeks in chronically infected solid organ transplant recipients (44–46) and was associated with rapid HEV RNA negativation in nonimmunocompromised patients exhibiting severe acute hepatitis E (47, 48).
Overall, the present case prompts to test systematically for HEV in HIV-infected patients with acute hepatitis regardless of whether primary infection with another hepatitis virus has been diagnosed. The indication for ribavirin therapy in similar contexts remains to be clarified.
Footnotes
Published ahead of print 9 January 2013
REFERENCES
- 1. Henry M, Tourres C, Colson P, Ravaux I, Poizot-Martin I, Tamalet C. 2006. Coexistence of the K65R/L74V and/or K65R/T215Y mutations on the same HIV-1 genome. J. Clin. Virol. 37:227–230 [DOI] [PubMed] [Google Scholar]
- 2. Motte A, Blanc J, Minodier P, Colson P. 2009. Acute hepatitis A in a pregnant woman at delivery. Int. J. Infect. Dis. 13:e49–e51 [DOI] [PubMed] [Google Scholar]
- 3. Panassie L, Borentain P, Nafati C, Bernardin G, Doudier B, Thibault V, Gerolami R, Colson P. 2012. Fatal fulminant primary hepatitis B virus infections with G1896A precore viral mutants in southeastern France. Clin. Res. Hepatol. Gastroenterol. 36:e1–e8 [DOI] [PubMed] [Google Scholar]
- 4. Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. 2010. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 202:825–834 [DOI] [PubMed] [Google Scholar]
- 5. Naha K, Karanth S, Prabhu M, Sidhu MS. 2012. Dual infection with hepatitis A and E virus presenting with aseptic meningitis: a case report. Asia Pac. J. Trop. Med. 5:587–588 [DOI] [PubMed] [Google Scholar]
- 6. Coppola N, Marrocco C, Di Caprio D, Coviello G, Scolastico C, Filippini P, Sagnelli E. 2003. Acute hepatitis B and C virus coinfection: a virological and clinical study of 3 cases. Clin. Infect. Dis. 36:528–532 [DOI] [PubMed] [Google Scholar]
- 7. Wedemeyer H, Manns MP. 2010. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 7:31–40 [DOI] [PubMed] [Google Scholar]
- 8. Yurdaydin C, Idilman R, Bozkaya H, Bozdayi AM. 2010. Natural history and treatment of chronic delta hepatitis. J. Viral Hepat. 17:749–756 [DOI] [PubMed] [Google Scholar]
- 9. Colson P, Gerolami R, Moreau J, Borentain P, Brouqui P. 2010. Concurrent autochthonous acute hepatitis E and hepatitis B reverse seroconversion in an HIV-1-infected patient: one virus may hide another. Int. J. Infect. Dis. 14:e357 doi:10.1016/j.ijid.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 10. Larsen C, Pialoux G, Salmon D, Antona D, Le SY, Piroth L, Pol S, Rosenthal E, Neau D, Semaille C, Delarocque AE. 2008. Prevalence of hepatitis C and hepatitis B infection in the HIV-infected population of France, 2004. Euro Surveill. 13:18888 http://www.eurosurveillance.org/ViewArticle.aspx?Articleid=18888 [PubMed] [Google Scholar]
- 11. Sulkowski MS. 2008. Viral hepatitis and HIV coinfection. J. Hepatol. 48:353–367 [DOI] [PubMed] [Google Scholar]
- 12. Sulkowski MS, Thomas DL. 2003. Hepatitis C in the HIV-infected person. Ann. Intern. Med. 138:197–207 [DOI] [PubMed] [Google Scholar]
- 13. Thio CL. 2003. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin. Liver Dis. 23:125–136 [DOI] [PubMed] [Google Scholar]
- 14. Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. 2012. Hepatitis E. Lancet 379:2477–2488 [DOI] [PubMed] [Google Scholar]
- 15. Colson P, Dhiver C, Poizot-Martin I, Tamalet C, Gerolami R. 2010. Acute and chronic hepatitis E in patients infected with human immunodeficiency virus. J. Viral Hepat. 17:807–815 [DOI] [PubMed] [Google Scholar]
- 16. Crum-Cianflone NF, Curry J, Drobeniuc J, Weintrob A, Landrum M, Ganesan A, Bradley W, Agan BK, Kamili S. 2012. Hepatitis E virus infection in HIV-infected persons. Emerg. Infect. Dis. 18:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jagjit Singh GK, Ijaz S, Rockwood N, Farnworth SP, Devitt E, Atkins M, Tedder R, Nelson M. 2013. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J. Infect. 66:103–106 [DOI] [PubMed] [Google Scholar]
- 18. Kaba M, Richet H, Ravaux I, Moreau J, Poizot-Martin I, Motte A, Nicolino-Brunet C, Dignat-George F, Menard A, Dhiver C, Brouqui P, Colson P. 2011. Hepatitis E virus infection in patients infected with the human immunodeficiency virus. J. Med. Virol. 83:1704–1716 [DOI] [PubMed] [Google Scholar]
- 19. Keane F, Gompels M, Bendall R, Drayton R, Jennings L, Black J, Baragwanath G, Lin N, Henley W, Ngui SL, Ijaz S, Dalton H. 2012. Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 13:83–88 [DOI] [PubMed] [Google Scholar]
- 20. Jardi R, Crespo M, Homs M, van den Eynde E, Girones R, Rodriguez-Manzano J, Caballero A, Buti M, Esteban R, Rodriguez-Frias F. 2012. HIV, HEV and cirrhosis: evidence of a possible link from eastern Spain. HIV Med. 13:379–383 [DOI] [PubMed] [Google Scholar]
- 21. Kenfak-Foguena A, Schöni-Affolter F, Bürgisser P, Witteck A, Darling KEA, Kovari H, Kaiser L, Evison JM, Elzi L, Gurter-De La Fuente V, Jost J, Moradpour D, Abravanel F, Izopet J, Cavassini M, Swiss HIV Cohort Study 2011. Hepatitis E virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg. Infect. Dis. 17:1074–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouquet J, Tesse S, Lunazzi A, Eloit M, Rose N, Nicand E, Pavio N. 2011. Close similarity between sequences of hepatitis E virus recovered from humans and swine, France, 2008–2009. Emerg. Infect. Dis. 17:2018–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Legrand-Abravanel F, Mansuy JM, Dubois M, Kamar N, Peron JM, Rostaing L, Izopet J. 2009. Hepatitis E virus genotype 3 diversity, France. Emerg. Infect. Dis. 15:110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Legrand-Abravanel F, Kamar N, Sandres-Saune K, Garrouste C, Dubois M, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Izopet J. 2010. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J. Infect. Dis. 202:835–844 [DOI] [PubMed] [Google Scholar]
- 25. Mansuy JM, Bendall R, Legrand-Abravanel F, Saune K, Miedouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. 2011. Hepatitis E virus antibodies in blood donors, France. Emerg. Infect. Dis. 17:2309–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Renou C, Lafeuillade A, Cadranel JF, Pavio N, Pariente A, Allegre T, Poggi C, Penaranda G, Cordier F, Nicand E. 2010. Hepatitis E virus in HIV-infected patients. AIDS 24:1493–1499 [DOI] [PubMed] [Google Scholar]
- 27. Renou C, Moreau X, Pariente A, Cadranel JF, Maringe E, Morin T, Causse X, Payen JL, Izopet J, Nicand E, Bourliere M, Penaranda G, Hardwigsen J, Gerolami R, Peron JM, Pavio N. 2008. A national survey of acute hepatitis E in France. Aliment. Pharmacol. Ther. 27:1086–1093 [DOI] [PubMed] [Google Scholar]
- 28. Montella F, Rezza G, Di Sora F, Pezzotti P, Recchia O. 1994. Association between hepatitis E virus and HIV infection in homosexual men. Lancet 344:1433 doi:10.1016/S0140-6736(94)90598-3 [DOI] [PubMed] [Google Scholar]
- 29. Bordi L, Rozera G, Scognamiglio P, Minosse C, Loffredo M, Antinori A, Narciso P, Ippolito G, Girardi E, Capobianchi MR. 2012. Monophyletic outbreak of hepatitis A involving HIV-infected men who have sex with men, Rome, Italy 2008–2009. J. Clin. Virol. 54:26–29 [DOI] [PubMed] [Google Scholar]
- 30. Urbanus AT, van Houdt R, van de Laar TJ, Coutinho RA. 2009. Viral hepatitis among men who have sex with men, epidemiology and public health consequences. Euro Surveill. 14:19421 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19421 [DOI] [PubMed] [Google Scholar]
- 31. van de Laar TJ, van der Bij AK, Prins M, Bruisten SM, Brinkman K, Ruys TA, van der Meer JT, de Vries HJ, Mulder JW, van Agtmael M, Jurriaans S, Wolthers KC, Coutinho RA. 2007. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J. Infect. Dis. 196:230–238 [DOI] [PubMed] [Google Scholar]
- 32. Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. 2009. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 361:1025–1027 [DOI] [PubMed] [Google Scholar]
- 33. Sellier P, Mazeron MC, Tesse S, Badsi E, Evans J, Magnier JD, Sanson-Le-Pors MJ, Bergmann JF, Nicand E. 2011. Hepatitis E virus infection in HIV-infected patients with elevated serum transaminases levels. Virol. J. 8:171 doi:10.1186/1743-422X-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dao DY, Balko J, Attar N, Neak E, Yuan HJ, Lee WM, Jain MK. 2011. Hepatitis B virus genotype G: prevalence and impact in patients co-infected with human immunodeficiency virus. J. Med. Virol. 83:1551–1558 [DOI] [PubMed] [Google Scholar]
- 35. Desire N, Sanchis T, Ben MF, Stitou H, Katlama C, Thibault V. 2011. Development and validation of a specific method for relative HBV-genotype G (G-HBV) quantification in the context of co-infection with other genotypes. Pathol. Biol. (Paris) 59:e13–e19 (In French.) [DOI] [PubMed] [Google Scholar]
- 36. Mata Marin JA, Arroyo Anduiza CI, Calderon GM, Cazares RS, Fuentes Allen JL, Arias FR, Gaytan MJ. 2012. Prevalence and resistance pattern of genotype G and H in chronic hepatitis B and HIV co-infected patients in Mexico. Ann. Hepatol. 11:47–51 [PubMed] [Google Scholar]
- 37. Bottecchia M, Souto FJ, O KM, Amendola M, Brandao CE, Niel C, Gomes SA. 2008. Hepatitis B virus genotypes and resistance mutations in patients under long term lamivudine therapy: characterization of genotype G in Brazil. BMC Microbiol. 8:11 doi:1471-2180-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chu CJ, Keeffe EB, Han SH, Perrillo RP, Min AD, Soldevila-Pico C, Carey W, Brown RS, Jr, Luketic VA, Terrault N, Lok AS. 2003. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 125:444–451 [DOI] [PubMed] [Google Scholar]
- 39. Sanchez LV, Tanaka Y, Maldonado M, Mizokami M, Panduro A. 2007. Difference of hepatitis B virus genotype distribution in two groups of Mexican patients with different risk factors. High prevalence of genotype H and G. Intervirology 50:9–15 [DOI] [PubMed] [Google Scholar]
- 40. Perez-Sautu U, Costafreda MI, Cayla J, Tortajada C, Lite J, Bosch A, Pinto RM. 2011. Hepatitis a virus vaccine escape variants and potential new serotype emergence. Emerg. Infect. Dis. 17:734–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colson P, Kaba M, Moreau J, Brouqui P. 2009. Hepatitis E in an HIV-infected patient. J. Clin. Virol. 45:269–271 [DOI] [PubMed] [Google Scholar]
- 42. Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. 2007. Locally acquired hepatitis E in chronic liver disease. Lancet 369:1260 doi:10.1016/S0140-6736(07)60596-0 [DOI] [PubMed] [Google Scholar]
- 43. Peron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. 2007. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J. Viral Hepat. 14:298–303 [DOI] [PubMed] [Google Scholar]
- 44. Chaillon A, Sirinelli A, De Muret A, Nicand E, d'Alteroche L, Goudeau A. 2011. Sustained virologic response with ribavirin in chronic hepatitis E virus infection in heart transplantation. J. Heart Lung Transplant. 30:841–843 [DOI] [PubMed] [Google Scholar]
- 45. Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Esposito L, Basse G, Cointault O, Ribes D, Nogier MB, Alric L, Peron JM, Izopet J. 2010. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology 139:1612–1618 [DOI] [PubMed] [Google Scholar]
- 46. Mallet V, Nicand E, Sultanik P, Chakvetadze C, Tesse S, Thervet E, Mouthon L, Sogni P, Pol S. 2010. Brief communication: case reports of ribavirin treatment for chronic hepatitis E. Ann. Intern. Med. 153:85–89 [DOI] [PubMed] [Google Scholar]
- 47. Gerolami R, Borentain P, Raissouni F, Motte A, Solas C, Colson P. 2011. Treatment of severe acute hepatitis E by ribavirin. J. Clin. Virol. 52:60–62 [DOI] [PubMed] [Google Scholar]
- 48. Peron JM, Dalton H, Izopet J, Kamar N. 2011. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J. Hepatol. 54:1323–1324 [DOI] [PubMed] [Google Scholar]