Abstract
Recent studies of Toxoplasma gondii isolates from animals in Brazil have revealed high genetic diversity. Many of these isolates are virulent to mice. It is speculated that these isolates may also be virulent to humans. However, there is very limited data regarding T. gondii strains from human infection. Therefore, it is not clear whether there is any association between parasite genotypes and disease phenotypes. In this study, a total of 27 T. gondii strains were isolated from humans with congenital toxoplasmosis in Minas Gerais state, Brazil. The genetic variability was assessed by restricted fragment length polymorphism in 11 loci (SAG1, 5′ plus 3′ SAG2, alternative [alt.] SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, and Apico). Genetic analysis of 24 strains revealed 14 different genotypes, including 7 previously identified from animals and 7 new types. The widespread genotype BrII accounted for 29% (7/24) of the isolates and was the dominant genotype involved in this study. This is the first report of genotyping of T. gondii isolates obtained from blood samples from newborns with congenital toxoplasmosis. Genotypic characterization of these isolates suggests high genetic diversity of T. gondii in this human population in Brazil. Future studies are needed to determine the source of contamination of this human population.
INTRODUCTION
Toxoplasma gondii is a widely distributed Apicomplexan parasite of great medical importance. T. gondii infection in humans may lead to congenital, ocular, or encephalic toxoplasmosis. Congenital toxoplasmosis has been associated with abortion and stillbirth, as well as neonatal mortality and morbidity. The prevalence of congenital toxoplasmosis varies according to geographic location and is determined by social, cultural, and other factors, such as type of biological sample and diagnostic assay used (1). In Brazil, the prevalence of congenital toxoplasmosis ranges from 0.3/1,000 newborns in Ribeirão Preto, São Paulo (SP) (2) to 5.0/1,000 in Uberlândia, Minas Gerais (MG) (3). In Belo Horizonte, MG, Brazil, the prevalence of T. gondii infection in newborns is one in 1,540 live births (4). A more recent population-based study involving the entire state of Minas Gerais revealed one case of congenital toxoplasmosis in every 770 live births (1.3/1,000). Of the newborns with congenital toxoplasmosis, 79.8% had retinochoroidal lesions in at least one eye (5). Several factors can be related to the severity of congenital toxoplasmosis, including parasite strain and host genetic variability and immune response. Genetic diversity of T. gondii strains has been an interesting and important research topic. T. gondii was considered to have a clonal population genetic structure with three major lineages (types I, II, and III) in Europe and North America (6). In these regions, congenital toxoplasmosis was mainly associated with strains classified as type II (7, 8). However, recent studies using multilocus markers showed high genetic diversity in South America, which is absent in strains from North America and Europe (9, 10, 11). More recently, analysis of isolates from domestic animals in Brazil revealed 48 restriction fragment length polymorphism (RFLP) genotypes, with four of these isolates being considered to be common clonal lineages, designated types BrI, BrII, BrIII, and BrIV (12).
Genetic analysis of T. gondii infecting humans is important to understand epidemiology, transmission patterns, and mechanisms of the disease. However, the difficulty in obtaining T. gondii strains from humans is a limiting factor (11, 13, 14). In this study, we described the genetic and biological characteristics of T. gondii isolates from newborns with congenital toxoplasmosis in the state of Minas Gerais, Brazil.
MATERIALS AND METHODS
Study population.
This study was part of an investigation on neonatal screening for congenital toxoplasmosis conducted by a multidisciplinary research group (Universidade Federal de Minas Gerais [UFMG]-Brazilian Congenital Toxoplasmosis Group) in the Minas Gerais state in southeastern Brazil. From 1 November 2006 to 31 May 2007, a total of 146,307 children were tested for anti-T. gondii IgM antibodies in dried blood samples on filter paper using the Toxo IgM kit (Q-Preven; Symbiosis, Leme, Brazil). Confirmative serologic tests were carried out for 220 infants with positive or undetermined screening results. These infants were tested for anti-T. gondii IgG, IgA, and IgM by enzyme-linked fluorometric assay ELFA-VIDAS (bioMérieux SA, Lyon, France). Out of these 220 infants, 178 tested positive for the persistence of anti-T. gondii IgG antibodies in serum at the age of 12 months. IgM tests (Q-Preven and ELFA-VIDAS) showed a moderate level of discordance. However, this was expected, since the collection of blood samples on filter paper for the initial screening (Q-Preven) was conducted for children 7 to 10 days after birth. Confirmatory tests (ELFA-VIDAS) were performed after 31 to 86 days after birth (mean, 55.6 days). It is likely that, at this time, the levels of IgM previously initially detected by Q-Preven had decreased in some children and were no longer detected by ELFA-VIDAS. Ophthalmologic examinations were performed for all 220 children according to the method described previously (5). The protocols used in this study were approved by the local Human Research Ethics Committee (COEP-Federal University of Minas Gerais, protocol 298/06).
Toxoplasma gondii isolates.
Peripheral blood samples from children from 31 to 86 days after birth (average age of 55.6 ± 16.6 days) was collected. Heparinized peripheral blood samples (0.5 ml) were centrifuged at 2,000 × g for 15 min, and the blood cell sediments containing erythrocytes and leukocytes were resuspended in 0.2 ml of phosphate-buffered saline solution (PBS), pH 7.2. For parasite isolation, 0.1 ml of this cell suspension was inoculated intraperitoneally (i.p.) in each one of two 6- to 8-week-old female Swiss mice. Thirty days after inoculation, each surviving mouse was bled via retro-orbital plexus. The blood (0.1 ml) was centrifuged, and the plasma was used to perform enzyme-linked immunosorbent assay (ELISA) for anti-T. gondii IgG antibodies (15). Animals that died before 30 days postinoculation were examined for the presence of tachyzoites in the peritoneum or cysts in the brain. All surviving mice were sacrificed by cervical dislocation. The brains of ELISA seropositive mice were removed and macerated, and 1.0 ml of PBS, pH 7.2, was added. Ten-microliter samples of brain lysates were used to search for the presence of tissue cysts by light microscopy. To determine the virulence of T. gondii isolates in mice, tachyzoites were first produced from the peritoneal cavities of five Swiss mice i.p. inoculated with 500 to 1,000 brain cysts as described previously (16). Five to 7 days after inoculation, the parasites were collected and washed from the peritoneal cavity with PBS, pH 7.2, and used in the virulence assay (fresh tachyzoites) or stored as frozen pellets at −20°C, until genomic DNA extraction. In the case of virulent strains, the infected Swiss reservoirs were treated with sulfadiazine for 10 days after infection to obtain cysts (17). The protocols conducted in this study were approved by the local Animal Ethics Committee (CETEA-Federal University of Minas Gerais, protocol 013/2007).
Parasite virulence.
The same criteria adopted previously (18) were applied to determine the virulence of the isolates of T. gondii. Five female BALB/c mice were i.p. inoculated with 100, 101, 102, or 103 tachyzoites of each isolate in 0.2 ml of PBS (pH 7.2). Five animals inoculated i.p. with PBS were maintained as negative controls. Mice mortality was observed over a 30-day period. The survivors were bled via retro-orbital plexus, and the sera were tested by ELISA. The mice that did not seroconvert were excluded from the experiment. All the surviving mice were sacrificed by cervical dislocation to search for tissue cysts in the brain. For comparison, RH (virulent) and ME49 (nonvirulent) strains were used as references. Isolates killing 100% of the infected mice were classified as virulent. Isolates with 100% lethal dose (LD100) greater than 103 tachyzoites were classified as nonvirulent, and isolates with an intermediate pattern between the two extremes were classified as having intermediate virulence (6).
DNA extracts and multilocus PCR-RFLP genotyping of T. gondii.
DNA extraction of the tachyzoites was performed using the Promega Wizard genomic DNA purification kit following the manufacturer's instructions. The genotyping of isolates obtained from newborns with congenital toxoplasmosis was determined by PCR-RFLP patterns of 11 DNA segments, including SAG1, 3′ SAG2 plus 5′ SAG2, alternative (alt.) SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, and Apico, as described previously (19). However, we performed a PCR in a single stage using only the internal markers, without preamplification by multiplex PCR using external primers. The amplification reactions were carried out in a final volume of 10 μl, containing 2 μl of 5× green buffer (Promega), 25 mM MgCl2, 2.5 mM each deoxynucleotide (dATP/dTTP/dGTP/dCTP; Invitrogen), 5 U/μl of Taq DNA polymerase (Promega), 5 pmol of each primer, and 1 μl of DNA. A negative control, without DNA, was included in each reaction mixture. Strains RH88 (type I), ME49 (type II), and VEG (type III) were used as controls. The first amplification step consisted of 4 min of denaturation at 95°C, followed by 35 cycles, with 1 cycle consisting of denaturation at 94°C for 30 s, annealing at 60°C for 60 s, and extension at 72°C for 90 s. The extension step in the final cycle was extended to 5 min. The PCR products were visualized in 5% polyacrylamide gel stained with silver nitrate. The amplified products were digested using the appropriate restriction endonucleases by a previously published method (19). The digestions were conducted at a final volume of 10 μl, containing 3 μl of the PCR product, 1 μl of the corresponding buffer, and 2.5 U (0.25 μl) of the enzyme, at 37°C for 3 h, according to the manufacturer's instruction. The DNA of the digested products was purified by extraction with an equal volume of phenol-chloroform (1:1), submitted to polyacrylamide gel (5%) electrophoresis, stained with silver nitrate, and photographed.
Data analysis.
The profiles found after digestion with restriction endonucleases were compared with the profiles of the reference strains in a virtual database, the ToxoDB database (www.toxodb.org). The study database included serological results, demographic data (age, gender, place of birth) of each child, as well as bioassay, virulence, genotyping results, and clinical signs. To reveal the genetic relationship of all the parasite isolates, the composite data set of multilocus PCR-RFLP genotyping was analyzed by SplitsTree4 (20, 21). The composite data set was based on the most recent genotyping results from Brazil (22). The results are presented as a reticulated network to describe complex relationships of these T. gondii strains.
RESULTS
Strain isolation and virulence determination of the T. gondii isolates.
One hundred seventy-eight infants with anti-T. gondii IgG antibodies when they were 12 months old were selected for this study. We tested one blood sample from each child by bioassay. Twenty-seven isolates of T. gondii were obtained from 27 newborns with congenital toxoplasmosis, demonstrating parasitemia in 15.2% (27/178). The isolates were designated TgCTBr1 to TgCTBr27 (in the isolate designation TgCTBr, Tg stands for T. gondii, CT stands for congenital toxoplasmosis, and Br stands for Brazil, and the isolates were numbered according to the chronological order in which isolation was performed). Only one of two mice inoculated with blood from newborn infants was infected with T. gondii with one exception. Both mice inoculated with TgCTBr9 became infected. The TgCTBr6 isolate was lost before the production of tachyzoites and the DNA extraction and virulence experiment.
Table 1 presents data on 27 newborns from which T. gondii was isolated. It shows the age of the children at the time blood was collected for the bioassay, which ranged from 31 to 86 days (average age of 55.6 ± 16.6 days), gender, major clinical signs, and confirmative serologic results. The newborns from which T. gondii was isolated came from different regions of the state of Minas Gerais in Brazil (Fig. 1), with isolates being obtained from 10 out of the 12 regions in the state. The isolates were divided into three groups according to the virulence phenotype for BALB/c mice. The time between inoculation of blood from a newborn in two Swiss mice and isolation of T. gondii are in Table S1 in the supplemental material. Fourteen isolates (54%) were characterized as having intermediate virulence, 10 isolates (38%) were characterized as virulent, and only two isolates (8%) were characterized as nonvirulent (Table 1).
Table 1.
Toxoplasma gondii isolates from Minas Gerais, Brazil, by location, patient gender, age, occurrence of clinical signs, and mouse virulence
| Patient | Location | Patient gender | Age (days) | Clinical sign(s)a | IgM screeningb | ELFA-IgAc | ELFA-IgMc | ELFA-IgGc | Isolate | Virulence in mice | PCR-RFLP genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Norte de Minas | Male | 62 | ACRL | + | + | + | + | TgCTBr02 | Intermediate | 11 (BrII) |
| 2 | Triângulo Mineiro/Alto do Paranaíba | Male | 41 | ACRL | + | + | + | + | TgCTBr08 | Virulent | 11 (BrII) |
| 3 | Vale do Rio Doce | Female | 51 | ACRL, ICd | + | + | − | + | TgCTBr09 | Virulent | 11 (BrII) |
| 4 | Metropolitana de Belo Horizonte | Male | 78 | ARLe | + | ND | ND | + | TgCTBr11 | Virulent | 11 (BrII) |
| 5 | Noroeste de Minas | Male | 63 | ACRL | − | + | + | + | TgCTBr14 | Intermediate | 11 (BrII) |
| 6 | Sul/Sudoeste de Minas | Female | 73 | CRL | + | − | − | + | TgCTBr20 | Intermediate | 11 (BrII) |
| 7 | Triângulo Mineiro/Alto do Paranaíba | Male | 80 | No | + | − | − | + | TgCTBr27 | Intermediate | 11 (BrII) |
| 8 | Oeste de Minas | Female | 31 | No | + | − | + | + | TgCTBr05 | Avirulent | 8 (BrIII) |
| 9 | Zona da Mata | Male | 45 | CRL | + | − | + | + | TgCTBr01 | Virulent | 206 (new) |
| 10 | Vale do Jequitinhonha | Male | 52 | No | + | + | + | + | TgCTBr03 | Virulent | 206 (new) |
| 11 | Zona da Mata | Male | 64 | ACRL | + | − | − | + | TgCTBr25 | Virulent | 206 (new) |
| 12 | Oeste de Minas | Male | 86 | CRL | + | + | + | + | TgCTBr15 | Intermediate | 41 |
| 13 | Norte de Minas | Male | 65 | ACRL | + | − | + | + | TgCTBr23 | Virulent | 41 |
| 14 | Central Mineira | Male | 31 | ACRL | + | − | + | + | TgCTBr04 | Virulent | 108 |
| 15 | Zona da Mata | Male | 73 | ACRL | + | − | + | + | TgCTBr17 | Virulent | 108 |
| 16 | Triângulo Mineiro/Alto do Paranaíba | Female | 43 | ACRL | + | + | + | + | TgCTBr07 | Intermediate | 67 |
| 17 | Vale do Rio Doce | Male | 44 | ARL | + | − | + | + | TgCTBr10 | Avirulent | 207 (new) |
| 18 | Metropolitana de Belo Horizonte | Male | 61 | ACRL, IC | + | − | + | + | TgCTBr12 | Intermediate | 162 |
| 19 | Sul/Sudoeste de Minas | Female | 64 | No | + | − | + | + | TgCTBr13 | Virulent | 208 (new) |
| 20 | Vale do Jequitinhonha | Male | 61 | No | + | − | + | + | TgCTBr18 | Intermediate | 36 |
| 21 | Metropolitana de Belo Horizonte | Male | 63 | ACRL, IC | + | − | + | + | TgCTBr21 | Intermediate | 209 (new) |
| 22 | Vale do Jequitinhonha | Female | 69 | ACRL | + | − | − | + | TgCTBr22 | Intermediate | 210 (new) |
| 23 | Oeste de Minas | Male | 73 | CRL | + | − | + | + | TgCTBr24 | Intermediate | 211 (new) |
| 24 | Sul/Sudoeste de Minas | Male | 67 | ACRL | + | − | + | + | TgCTBr26 | Intermediate | 212 (new) |
| 25 | Metropolitana de Belo Horizonte | Female | 56 | No | + | + | + | + | TgCTBr19 | Intermediate | Mixed |
| 26 | Norte de Minas | Male | 60 | CRL | + | − | − | + | TgCTBr16 | Intermediate | No typing data |
| 27 | Noroeste de Minas | Female | 40 | No | + | + | + | + | TgCTBr06 | No data | No typing data |
ARL, active retinochoroidal lesions (unilateral or bilateral); CRL, cicatricial retinochoroidal lesions (unilateral or bilateral); ACRL, active and cicatricial retinochoroidal lesions; IC, intracranial calcifications.
Anti-T. gondii IgM antibodies present (+) or absent (−) in blood on filter paper using the TOXO IgM kit (Q-Preven; Symbiosis, Leme, Brazil).
Anti-T. gondii IgA, IgM and IgG present (+) or absent (−) by the enzyme-linked fluorometric assay ELFA-VIDAS (bioMérrieux SA, Lyon, France). ND, no data.
Dehydration, hypoactivity, and malnutrition. The child died at 9 months of age.
Hepatomegaly, splenomegaly, adenomegaly, and microphthalmia. The child died at 4 months of age.
Fig 1.

Origin of the T. gondii isolates from newborns in the 12 regions of Minas Gerais state, Brazil. The PCR-RFLP genotypes are indicated by numbers (e.g., #11 for genotype 11). *nd, genotyping not done; **mix, mixed genotype.
Genotyping analysis of T. gondii.
The complete genotype was obtained in 25/27 (92%) isolates (Table 2). It was not possible to carry out the complete genotyping in one sample (TgCTBr16) due to the nonamplification of some markers and the occurrence of extremely polymorphic digestion products. Isolate TgCTBr6 was lost before DNA was obtained. A representative result of PCR-RFLP analysis (BTUB marker) is shown in Fig. S2 in the supplemental material. Tables 1 and 2 present the isolates grouped according to the genotypes identified.
Table 2.
Multilocus genotyping of Toxoplasma gondii isolates from newborns in Minas Gerais state, Brazil
| Isolate(s) | Genotyping resulta of isolate of the following genetic marker: |
PCR-RFLP genotype | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAG1 | 5′-3′ SAG2 | Alt. SAG2 | SAG3 | BTUB | GRA6 | c22-8 | c29-2 | L358 | PK1 | Apico | |||
| RH88 | I | I | I | I | I | I | I | I | I | I | I | 10 | 10 |
| ME49 | II/ III | II | II | II | II | II | II | II | II | II | II | 1 | 10 |
| VEG | II/ III | III | III | III | III | III | III | III | III | III | III | 2 | 10 |
| TgCTBr01, -03, -25 (n = 3) | u-1 | I | II | III | III | III | II | III | I | III | I | 206 (new) | This study |
| TgCTBr02, -08, -09, -11, -14, -20, -27 (n = 7) | I | I | II | III | III | III | I | III | I | II | III | 11 (BrII) | 12 |
| TgCTBr04, -17 (n = 2) | I | I | II | III | III | III | II | I | I | III | I | 108 | 12 |
| TgCTBr15, -23 (n = 2) | I | I | I | III | I | II | I | I | I | I | I | 41 | 23 |
| TgCTBr05 (n = 1) | I | III | III | III | III | III | II | III | III | III | III | 8 (BrIII) | 12 |
| TgCTBr07 (n = 1) | I | III | III | III | I | III | I | III | III | u-1 | III | 67 | 12 |
| TgCTBr10 (n = 1) | I | I | u-1 | III | I | II | II | I | I | I | I | 207 (new) | This study |
| TgCTBr12 (n = 1) | I | III | III | III | III | III | II | I | I | III | I | 162 | 26 |
| TgCTBr13 (n = 1) | I | I | II | III | III | III | III | I | I | III | I | 208 (new) | This study |
| TgCTBr16 (n = 1) | I | ND | III | ND | III | III | II | ND | ND | ND | III | NDb | |
| TgCTBr18 (n = 1) | I | I | I | III | I | III | II | I | III | I | III | 36 | 23 |
| TgCTBr19 (n = 1) | I | I/III | I/III | III | I/III | III | II | I/III | III | I | III | Mixed | |
| TgCTBr21 (n = 1) | u-1 | I | II | I | III | II | I | I | I | I | III | 209 (new) | This study |
| TgCTBr22 (n = 1) | u-1 | I | II | III | III | III | II | III | I | III | III | 210 (new) | This study |
| TgCTBr24 (n = 1) | I | I | I | III | I | III | I | III | III | I | III | 211 (new) | This study |
| TgCTBr26 (n = 1) | I | I | I | III | III | III | I | III | I | II | III | 212 (new) | This study |
I, II, and III, clonal type I, II, and III alleles, respectively. II/III, clonal type II or III. u-1 is a new allele that is different from the clonal type I, II, and III alleles. ND, no data.
ND, no data.
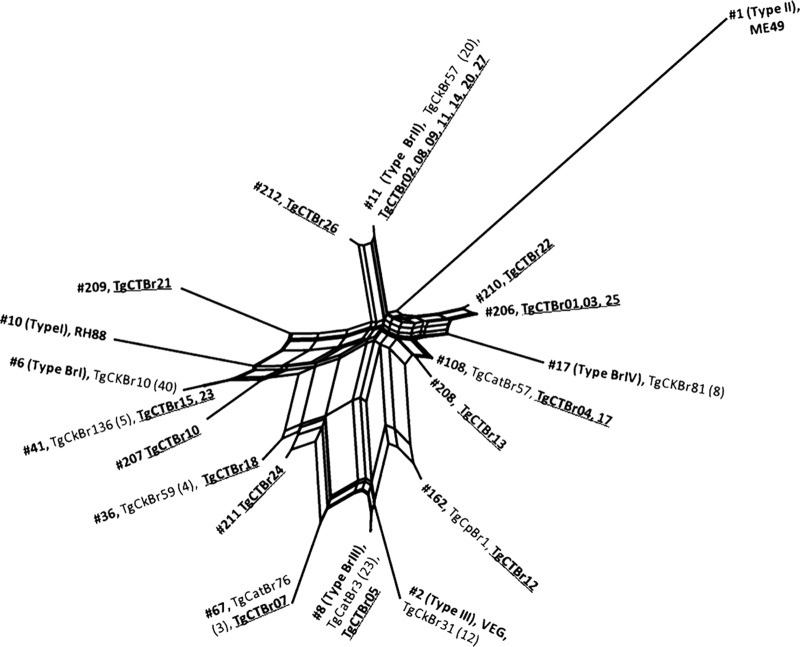
From the 25 isolates typed, 14 different genotypes were identified, and one isolate (TgCTBr19) had mixed infection, in which a combination of two alleles was observed in four of the 11 loci analyzed, i.e., 5′ plus 3′ SAG2, alt. SAG2, BTUB, and c29-2. Of the 14 genotypes found, 7 are considered new types that were not reported previously, and 7 (ToxoDB PCR-RFLP genotypes 8, 11, 36, 41, 67, 108, and 162) were previously identified (Tables 1 and 2). The 7 new genotypes were designated following the scheme of ToxoDB PCR-RFLP genotype numbers. The new genotypes are 206 (TgCTBr01, -03, and -25), 207 (TgCTBr10), 208 (TgCTBr13), 209 (TgCTBr21), 210 (TgCTBr22), 211 (TgCTBr24), and 212 (TgCTBr26). Seven isolates (TgCTBr02, -08, -09, -11, -14, -20, and -27) exhibited genotype 11 (also known as type BrII). This genotype was previously identified from domestic animals in southeastern Brazil (12, 23, 24, 25, 26, 27). The geographic distribution of genotype 11 in Minas Gerais, Brazil, was clearly widespread among 6 of 10 regions in which positive isolation of T. gondii from patients with congenital toxoplasmosis was achieved (Fig. 1). Isolate TgCTBr05 exhibited genotype 8 (also known as type BrIII), which was previously identified from domestic animals in other geographic regions of Brazil (12, 23, 25, 26, 28, 29). Isolate TgCTBr18 exhibited genotype 36, which was previously identified from chickens in Rio de Janeiro, Brazil (23). Isolates TgCTBr15 and TgCTBr23 exhibited genotype 41, which was previously identified from chickens in Amazon, Brazil (23) and capybaras in São Paulo state, Brazil (26). Isolate TgCTBr07 exhibited genotype 67, which was previously identified in dogs in São Paulo city (25) and cats in São Paulo state (12). Isolates TgCTBr04 and TgCTBr17 exhibited genotype 108, which was previously identified from cats in São Paulo state (12). Isolate TgCTBr12 exhibited genotype 162, which was previously identified from capybaras in Sao Paulo state (26). The genetic relationship of the 24 isolates of T. gondii obtained from newborns (excluding isolate TgCTBr19, due to its mixed infection origin), together with previously published data were compared using SplitsTree4 software (20, 21). The results are presented in Fig. 2. The 14 genotypes identified from the 24 congenital cases showed high diversity and are scattered in the network.
Fig 2.
NeighborNet phylogenetic network of Toxoplasma gondii isolates from Brazil. TgCTBr1 to TgCTBr27 are T. gondii isolates from newborns from Minas Gerais, Brazil.
Regarding virulence in mice, isolates with identical genotypes exhibited similar phenotypes. A descriptive analysis did not show any association between the genotypes of the isolates and retinochoroiditis in the newborns. Likewise, no association between the genotypes and the sites of origin of the isolates was observed.
DISCUSSION
Most of the isolates obtained and genotyped in Brazil are from domestic animals, including free-range chickens, cats, dogs, sheep, and goats; little is known about the genetics of T. gondii isolates from humans (22). To understand the epidemiology of toxoplasmosis, it is important to determine how and from which sources the parasite is transmitted from animals to humans. In the present study, we isolated and genotyped T. gondii from peripheral blood of newborns. The high positivity observed in the bioassay experiments confirms the presence of viable tachyzoites in the blood of these transplacentally infected infants, even after a period of 2 to 3 months. Isolation of T. gondii in infants up to 86 days old can be explained by the immunoimmaturity of the newborns, which allows parasitemia to occur longer than in immunocompetent adults (30). The 27 isolates were obtained from children from different regions in Minas Gerais, Brazil, showing that T. gondii is widely distributed in the state.
We observed variability in the virulence in mice of the T. gondii isolates from cases of congenital toxoplasmosis. Ninety-two percent (24/26) of the isolates are intermediately or highly virulent to mice. A previous study analyzed the virulence of T. gondii isolates obtained from domestic animals in the state of Minas Gerais in Brazil, showing a higher prevalence of samples with high/intermediate virulence phenotype (31). Several factors interfere in the virulence of T. gondii, including parasite stage, infection route, infective dose, inoculation mode, mouse lineage, and intrinsic characteristics of the isolate (32). Although few studies have been carried out with isolates of T. gondii derived from cases of congenital toxoplasmosis (33), it is known that, in general, the isolates obtained in Brazil are virulent (10). Of the 24 isolates completely genotyped in this study, 20 originated from newborns with retinochoroiditis. Comparing Brazilian and European infants with congenital toxoplasmosis, it was observed that T. gondii causes more severe ocular disease in the former than in the latter (34). These authors concluded that the differences in frequency, size, and multiplicity of the retinochoroidal lesions in the two populations may have been due to infections with more-virulent strains of the parasite that predominate in Brazil and are rarely found in Europe.
In this study, we completely genotyped 24 T. gondii isolates from peripheral blood from newborns with congenital toxoplasmosis. Analysis of the multilocus PCR-RFLP revealed 14 genotypes, suggesting high diversity of the parasite isolated from the human population in Minas Gerais, Brazil, similar to results reported by other authors in studies of parasites isolated from other host species (12, 23, 28). Of the 14 genotypes identified, four were identified in two or more isolates (genotypes 11, 41, 108, and 206), while the other 10 genotypes were found in only one isolate. Among the 14 genotypes, 7 were previously reported in animals and 7 were described for the first time in the literature (Table 2), with six found in only one isolate. This result reconfirmed the high diversity of T. gondii strains in Brazil (23, 28, 29).
Although the T. gondii population is highly diversified in Brazil, some clonal genotypes circulate in the hosts. The previously identified clonal isolates BrII (genotype 11) and BrIII (genotype 8) were found in this study. These genotypes had already been described in several hosts such as sheep, chicken, and cats (12, 23, 29), but this is the first report of these two genotypes in humans. The isolation of strains with identical genotypes in distinct geographic regions (genotypes 11 and 8) suggests the widespread distribution of clonal genotypes of T. gondii in Minas Gerais, Brazil.
In this study, the TgCTBr19 isolate presented mixed profiles in four of the 11 markers used. This mixed infection may have occurred due to simultaneous and sequential infections (reinfections), with parasites of different genotypes, acquired from oocysts directly from the environment or through ingestion of tissue cysts in the intermediate hosts infected with two distinct isolates of the parasite, possibilities already discussed in the literature (35, 36). Studies show evidence of mixed infections mainly associated with congenital cases (36). The occurrence of mixed genotypes in Brazil corroborates the hypothesis that several genotypes of T. gondii must be widely disseminated and circulating simultaneously (12, 23, 26, 28, 29). In Brazil, overall, isolates are recombinant and virulent (10, 12, 37). Isolate TgCTBr05, the only isolate classified as type BrIII, was shown to be nonvirulent to mice, corroborating a previous study (12). However, the seven isolates identified as genotype 11 (BrII) showed variable virulence. Biological differences between the isolates of the same genotype must not be neglected, and it may be possible that the genetic markers used in this study are incapable of reflecting possible phenotypic differences between the isolates under question (28). Thus, there is no clear correlation between the genotypes of the isolates of T. gondii obtained from newborns in the state of Minas Gerais in Brazil and virulence to mice. A recent study in Brazil has also not shown any concrete evidence of correlation between virulence of T. gondii in mice and different genotypes (38).
No association between the parasite genotype and retinochoroiditis in the newborns was observed, with further studies being necessary to define the role played by phenotypic and genotypic characteristics of T. gondii in the development of ocular lesions.
The results of this study indicate that a significant proportion of cases of congenital toxoplasmosis (62.5% [15/24]) were caused by T. gondii genotypes previously reported from animals (Table 2). In particular, 29% (7/24) cases were caused by the common T. gondii genotype 11 (BrII) that was previously reported from a variety of animals in Brazil (12, 23, 24, 25, 26, 27). Nine T. gondii isolates were grouped into 7 new genotypes that were not reported before, indicating high diversity of the parasite in the population. Further studies of sampling of T. gondii in animals from Minas Gerais, Brazil, are needed to better understand the population structure of T. gondii and transmission of the parasites among animals and humans. This is the first report of genotyping of isolates obtained from blood from newborns, providing important technical and scientific information on the epidemiology of congenital toxoplasmosis in Brazil.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Conselho Nacional de Desenvolvimento e Pesquisa (CNPq) (grants 301110/2009-3 and 471486/2010-8), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) (grant 00129-09), Secretaria de Saúde de Minas Gerais (SES-MG), and Núcleo de Ações e Pesquisa em Apoio Diagnóstico de Universidade Federal de Minas Gerais (NUPAD-UFMG). Ana Carolina Aguiar Vasconcelos Carneiro is the recipient of a scholarship from the CNPq.
Ana Carolina Aguiar Vasconcelos Carneiro, Gláucia Manzan Andrade, Daniel Vitor Vasconcelos-Santos, José Nélio Januário, and Ricardo Wagner Almeida Vitor are members of the UFMG Congenital Toxoplasmosis Brazilian Group.
We thank Rosalida Estevan Nazar Lopes for technical assistance. Technical support from the UFMG Congenital Toxoplasmosis Brazilian Group (UFMG-CTBG) is greatly appreciated: Ana Carolina Aguiar Vasconcelos Carneiro, Daniel Vitor Vasconcelos-Santos, Danuza O. Machado Azevedo, Ericka V. Machado Carellos, Fernando Oréfice, Gláucia Manzan Andrade, José Nélio Januário, Luciana Macedo Resende, Olindo Assis Martins-Filho, Ricardo Wagner Almeida Vitor, Roberta M. Castro Romanelli, Waleska Teixeira Caiaffa, and Wesley R. Campos.
Footnotes
Published ahead of print 2 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02502-12.
REFERENCES
- 1. Tenter AM, Heckeroth AR, Weiis ML. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carvalheiro CG, Mussi-Pinhata MM, Yamamoto AY, De Souza CB, Maciel LM. 2005. Incidence of congenital toxoplasmosis estimated by neonatal screening: relevance of diagnostic confirmation in asymptomatic newborn infants. Epidemiol. Infect. 133:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segundo GRS, Silva DAO, Mineo JR, Ferreira MS. 2004. A comparative study of congenital toxoplasmosis between public and private hospitals from Uberlândia, MG, Brazil. Mem. Inst. Oswaldo Cruz 99:13–17 [DOI] [PubMed] [Google Scholar]
- 4. Queiroz GM, Resende LM, Goulart EMA, Siqueira AL, Vitor RWA, Januário JN. 2008. Hearing loss in congenital toxoplasmosis detected by newborn screening. Rev. Bras. Otorrinolaringol. 74:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasconcelos-Santos DV, Machado DO, Campos WR, Oréfice F, Andrade GMQ, Carellos EVM, Romanelli RM, Januario JN, Resende LM, Martins-Filho O, Carneiro ACAV, Vitor RWA, Caiaffa WT. 2009. Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology 116:2199–2205 [DOI] [PubMed] [Google Scholar]
- 6. Howe DK, Sibley LD. 1995. Toxoplasma gondii comprises three clonal lineages, correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561–1566 [DOI] [PubMed] [Google Scholar]
- 7. Howe DK, Honore S, Derouin F, Sibley LD. 1997. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 35:1411–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dardé ML. 2008. Toxoplasma gondii, “new” genotypes and virulence. Parasite 15:366–371 [DOI] [PubMed] [Google Scholar]
- 9. Ajzenberg D, Bañuls AL, Su C, Dumètre A, Demar M, Carme B, Dardé ML. 2004. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int. J. Parasitol. 34:1185–1196 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira AM, Vitor RWA, Gazzinelli RT, Melo MN. 2006. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect. Genet. Evol. 6:22–31 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira IMR, Vidal JE, De Mattos CCB, De Mattos LC, Qu D, Su C, Pereira-Chioccola VL. 2011. Toxoplasma gondii isolates: multilocus RFLP PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp. Parasitol. 129:190–195 [DOI] [PubMed] [Google Scholar]
- 12. Pena HFJ, Gennari SM, Dubey JP, Su C. 2008. Population structure and mouse-virulence of Toxoplasma gondii. Int. J. Parasitol. 38:561–569 [DOI] [PubMed] [Google Scholar]
- 13. Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, Belfort JRR, Vitor RWA, Silveira CL, Sibley D. 2006. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg. Infect. Dis. 12:942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira IMR, Vidal JE, Costa-Silva TA, Meira CS, Hiramoto RM, Oliveira ACP, Pereira-Chioccola VL. 2008. Toxoplasma gondii: genotyping of strains from Brazilian AIDS patients with cerebral toxoplasmosis by multilocus PCR-RFLP markers. Exp. Parasitol. 118:221–227 [DOI] [PubMed] [Google Scholar]
- 15. Elsaid MM, Martins MS, Frézard F, Braga EM, Vitor RWA. 2001. Vertical toxoplasmosis in a murine model. Protection after immunization with antigens of Toxoplasma gondii incorporated into liposomes. Mem. Inst. Oswaldo Cruz 96:99–104 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira AM, Vitor RWA, Carneiro ACAV, Brandão GP, Melo MN. 2004. Genetic variability of Brazilian Toxoplasma gondii strains detected by random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) and simple sequence repeat anchored-PCR (SSR-PCR). Infect. Genet. Evol. 4:131–142 [DOI] [PubMed] [Google Scholar]
- 17. Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed, p 312–356 CRC Press, Boca Raton, FL [Google Scholar]
- 18. Ferreira AM, Martins MS, Vitor RWA. 2001. Virulence for BALB/c mice and antigenic diversity of eight Toxoplasma gondii strains isolated in Brazil. Parasite 8:99–105 [DOI] [PubMed] [Google Scholar]
- 19. Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. 2010. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 137:1–11 [DOI] [PubMed] [Google Scholar]
- 20. Huson DH. 1998. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14:68–73 [DOI] [PubMed] [Google Scholar]
- 21. Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254–267 [DOI] [PubMed] [Google Scholar]
- 22. Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. 2012. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139:1375–1424 [DOI] [PubMed] [Google Scholar]
- 23. Dubey JP, Velmurugan GV, Chockalingam A, Pena HFJ, Nunes de Oliveira L, Leifer CA, Gennari SM, Bahia-Oliveira LMG, Su C. 2008. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet. Parasitol. 157:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dubey JP, Passos LM, Rajendran C, Ferreira LR, Gennari SM, Su C. 2011. Isolation of viable Toxoplasma gondii from feral guinea fowl (Numida meleagris) and domestic rabbits (Oryctolagus cuniculus) from Brazil. J. Parasitol. 97:842–845 [DOI] [PubMed] [Google Scholar]
- 25. Dubey JP, Gennari SM, Sundar N, Vianna MC, Bandini LM, Kwok VH, Su C. 2007. Diverse and atypical genotypes identified in Toxoplasma gondii from dogs in São Paulo, Brazil. J. Parasitol. 93:60–64 [DOI] [PubMed] [Google Scholar]
- 26. Yai LEO, Ragozo AMA, Soares RM, Pena HFJ, Su C, Gennari SM. 2009. Genetic diversity among capybara (Hydrochaeris hydrochaeris) isolates of Toxoplasma gondii from Brazil. Vet. Parasitol. 162:332–337 [DOI] [PubMed] [Google Scholar]
- 27. da Silva RC, Langoni H, Su C, da Silva AV. 2011. Genotypic characterization of Toxoplasma gondii in sheep from Brazilian slaughterhouses: new atypical genotypes and the clonal type II strain identified. Vet. Parasitol. 175:173–177 [DOI] [PubMed] [Google Scholar]
- 28. Soares RM, Silveira LH, Silva AV, Ragozo A, Galli S, Lopes EG, Gennari SM, Pena HFJ. 2011. Genotyping of Toxoplasma gondii isolates from free range chickens in the Pantanal area of Brazil. Vet. Parasitol. 178:29–34 [DOI] [PubMed] [Google Scholar]
- 29. Ragozo AMA, Pena HFJ, Yai LEO, Su C, Gennari SM. 2010. Genetic diversity among Toxoplasma gondii isolates of small ruminants from Brazil: novel genotypes revealed. Vet. Parasitol. 170:307–312 [DOI] [PubMed] [Google Scholar]
- 30. McLeod R, Dowel M. 2000. Basic immunology: the fetus and the newborn, p 37–68 In Ambroise-Thomas P, Petersen E. (ed), Congenital toxoplasmosis: scientific background, clinical management and control. Springer-Verlag, Paris, France [Google Scholar]
- 31. Brandão GP, Ferreira AM, Melo MN, Vitor RWA. 2006. Characterization of Toxoplasma gondii from domestic animals from Minas Gerais. Parasite 13:143–149 [DOI] [PubMed] [Google Scholar]
- 32. Dubey JP, Graham DH, Blackston CR, Lehmann T, Gennari SM, Ragozo AMA, Shen SK, Kwok OCH, Hill DE, Thulliez P. 2002. Biological and genetic characterization of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int. J. Parasitol. 32:99–105 [DOI] [PubMed] [Google Scholar]
- 33. Rico-Torres CP, Figueroa-Damián R, López-Candiani C, Macías-Avilés HÁ, Cedillo-Peláez C, Canedo-Solares I, Luna-Pastén H, Tecuatl-Herrada BL, Correa D. 2012. Molecular diagnosis and genotyping of cases of perinatal toxoplasmosis in Mexico. Pediatr. Infect. Dis. J. 31:411–413 [DOI] [PubMed] [Google Scholar]
- 34. Gilbert R, Freeman K, Lago EG, Bahia-Oliveira L, Tan HK, Buffolano W, Petersen E. 2008. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl. Trop. Dis. 2:e277 doi:10.1371/journal.pntd.0000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aspinall TV, Guy EC, Roberts KE, Joynson DHM, Hyde JE, Sims PFG. 2003. Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: public health implications. Int. J. Parasitol. 33:97–103 [DOI] [PubMed] [Google Scholar]
- 36. Boughattas S, Ben-Abdallah RYM, Siala E, Souissi O, Aoun K, Bouratbine A. 2010. Direct genotypic characterization of Toxoplasma gondii strains associated with congenital toxoplasmosis in Tunisia (North Africa). Am. J. Trop. Med. Hyg. 82:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubey JP, Su C. 2009. Population biology of Toxoplasma gondii: what's out and where did they come from. Mem. Inst. Oswaldo Cruz 104:190–195 [DOI] [PubMed] [Google Scholar]
- 38. Frazão-Teixeira E, Sundar N, Dubey JP, Grigg ME, Oliveira FCR. 2011. Multi-locus DNA sequencing of Toxoplasma gondii isolated from Brazilian pigs identifies genetically divergent strains. Vet. Parasitol. 175:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.