Abstract
The genetic analysis of high-level mupirocin resistance (Hi-Mupr) in a Staphylococcus pseudintermedius isolate from a dog is presented. The Hi-Mupr ileS2 gene flanked by a novel rearrangement of directly repeated insertion sequence IS257 elements was located, together with the aminoglycoside resistance aacA-aphD determinant, on a conjugative plasmid related to the pSK41/pGO1 family plasmids.
TEXT
Staphylococcus pseudintermedius is one of the most common pathogens isolated from skin and ear infections in dogs. Although both methicillin-susceptible and methicillin-resistant S. pseudintermedius (MSSP and MRSP, respectively) have been isolated from serious human infections (1), the emergence and spread of MRSP are a major veterinary issue, with approximately 8% of all S. pseudintermedius isolates from diseased dogs and cats in Croatia being methicillin resistant (1, 2).
Mupirocin is a topical antibiotic frequently used for preoperative elimination of nasal carriage of methicillin-resistant S. aureus (MRSA) in humans (3). High-level mupirocin resistance (Hi-Mupr) in S. aureus is generally conferred by the plasmid-associated ileS2 gene, which encodes an alternate isoleucyl-tRNA synthetase that is not bound by mupirocin (4). ileS2 is frequently found on pSK41-like plasmids, where it is flanked by IS257 insertion elements (5). Recently, the determination of the organization of IS257-ileS2 spacer regions by PCR and sequencing has proven to be useful for typing ileS2-bearing plasmids (6).
Although mupirocin is used only sporadically in veterinary medicine, it has been recommended for treating recurrent interdigital furunculosis, callus pyoderma, and muzzle acne in dogs (7). In S. pseudintermedius, resistance to mupirocin has been reported occasionally, but its molecular basis has not been investigated (8, 9). The aim of the present study was to examine the occurrence of resistance to mupirocin in S. pseudintermedius strains isolated from infected sites of dogs and cats in Croatia and to reveal the molecular mechanism of mupirocin resistance.
In total, 106 clinical isolates previously identified as S. pseudintermedius by a multiplex PCR method (2, 10) were included in this study. They were obtained from 102 dogs and 4 cats in the period from April to September 2011. All isolates were associated with clinical disease and were recovered from the affected sites: skin (n = 40), external ear canal (n = 39), conjunctival sac (n = 8), nostrils (n = 7), infected wounds (n = 6), urine (n = 4), and female genital tract (n = 2).
MICs of mupirocin were determined by broth microdilution using doubling dilutions of mupirocin (0.03125 to 1,024 mg/liter) in Mueller-Hinton broth (Bio-Rad, France) by following the CLSI guidelines (11). The MIC of gentamicin was determined by Etest (AB bioMérieux, France).
Whole DNA was isolated using 2% Chelex-100 solution (Bio-Rad, USA) (2). The ileS2 gene fragment was amplified and sequenced using previously described primers (12). Amplification and sequencing of IS257-ileS2 segments were performed as described previously (6).
Primers ileS-F1 (5′-CGTGACCGTGGCGAATGGGT-3′) and ileS-R1 (5′-GTATGCGGAATGATTGGCG-3′) were designed based on sequences of two S. pseudintermedius strains deposited in GenBank (accession no. CP002439.1 and CP002478.1) and used for amplification and sequencing of a 956-bp ileS gene fragment in the single Hi-Mupr S. pseudintermedius strain detected in this study, designated HR547/11, and in three mupirocin-susceptible clinical isolates selected on the basis of their sequence types (STs): HR294/11 (MSSP ST117), HR23161 (MRSP ST106), and HR1084/08 (MRSP ST71, a dominant European MRSP clone). Sequences were aligned in CLC Genomics Workbench 4.9 (CLC bio, Denmark) and investigated for the presence of mutations conferring low-level mupirocin resistance in S. aureus (13).
Multilocus sequence typing (MLST) was performed according to the method of Bannoehr et al. (14), and sequence types were assigned using the key table kindly provided by the curator of the Staphylococcus intermedius group MLST database, Vincent Perreten.
Plasmid DNA was extracted with the QIAprep spin plasmid kit (Qiagen, Hilden, Germany), with the addition of lysostaphin (Sigma Chemical Co., St. Louis, MO), and digested separately with EcoRI and HindIII. Probes for ileS2, aacA-aphD, and traK were generated with the PCR digoxigenin (DIG) probe synthesis kit (Roche). Digested plasmid DNA was separated on 0.8% agarose and transferred to a Hybond-N+ membrane (Amersham). Southern blots were hybridized overnight at 68°C.
The MICs of mupirocin for the 105 susceptible strains ranged from ≤0.03125 to 0.25 mg/liter, while a single mupirocin-resistant/methicillin-susceptible isolate, designated HR547/11, exhibited a Hi-Mupr phenotype (MIC > 1,024 mg/liter). HR547/11 showed additional resistances to gentamicin (MIC = 8 mg/liter), kanamycin, tobramycin, and penicillin and belonged to ST44. It was isolated from an infected surgical wound after operative treatment of the anterior cruciate ligament injury in a 6-year-old Dachshund, which was seen by the Clinic for Surgery, Orthopedics and Ophthalmology at the Faculty of Veterinary Medicine, University of Zagreb. Antimicrobial history was obtained from the owner, a nurse working in a medical center. The dog received a combination of systemic (spiramycin and metronidazole) and topical (wound treatment with povidone-iodine and hydrogen peroxide) therapy. There was no prior exposure to mupirocin. Posttreatment swabs taken after 2 weeks failed to yield pathogenic bacteria.
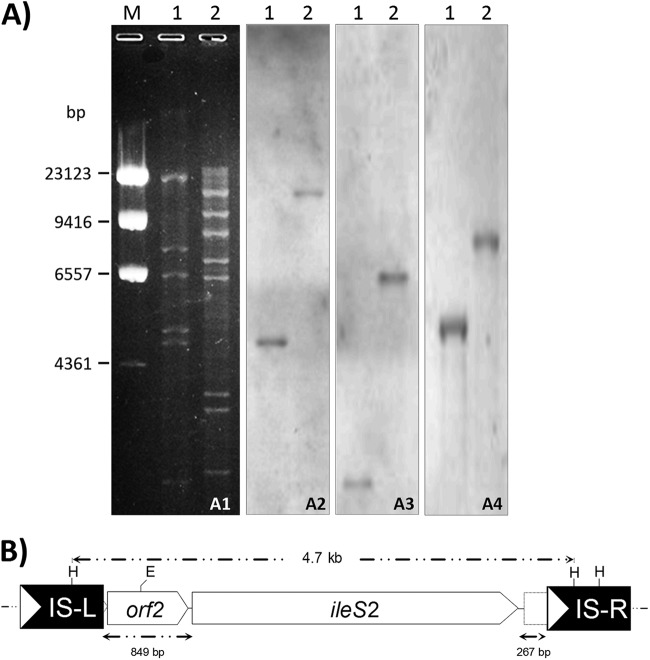
A specific ileS2 gene fragment was PCR amplified from HR547/11, and its DNA sequence had 100% similarity with the respective ileS2 gene sequences deposited in GenBank (accession no. HQ625435 to HQ625438). ileS2 was not detected in mupirocin-susceptible S. pseudintermedius strains (HR294/11, HR23161, and HR1084/08; mupirocin MICs, 0.25, 0.0625, and 0.0625 mg/liter, respectively). Analysis of the plasmid DNA content of the HR547/11 strain revealed the presence of a single high-molecular-weight conjugative plasmid called pKM01. Southern blotting confirmed that ileS2 and the aacA-aphD antibiotic resistance genes, as well as the conjugative-transfer-associated traK gene, were located on pKM01. These findings suggest that pKM01 belongs to the pSK41 family of conjugative plasmids (5, 15). Molecular analysis of IS257-ileS2 spacer regions on pKM01 demonstrated that the ileS2 gene is flanked by directly repeated IS257s showing an UpR849-DnR267 (upstream R849-downstream R267) rearrangement (Fig. 1).
Fig 1.
(A) Analysis of plasmid pKM01 by restriction endonuclease analysis and Southern hybridization. (A1) Restriction endonuclease analysis of pKM01. Lane M, lambda phage DNA digested with HindIII as a molecular size standard; lane 1, pKM01 digested with HindIII; lane 2, pKM01 digested with EcoRI. Numbers on the left correspond to the molecular sizes (in base pairs) of λ DNA HindIII restriction fragments. Southern hybridization was carried out with the ileS2-containing DNA as a probe (A2), an aacA-aphD gene fragment as a DNA probe (A3), and a traK gene fragment as a DNA probe (A4). The lane contents of panels A2 to A4 are the same as in panel A1. (B) Genetic organization of IS257s flanking the ileS2 gene on the pKM01 plasmid, encoding Hi-Mupr. Restriction endonuclease cleavage sites are abbreviated as follows: E, EcoRI, and H, HindIII. Upstream (IS-L) and downstream (IS-R) IS257 elements flanking the ileS2 gene are represented by solid boxes; the white arrows indicate the direction of IS257 transposase transcription. The ileS2 gene and the predicted open reading frames (ORFs) up- and downstream are represented as arrows, with the arrowhead indicating their orientation. Truncated ORFs are shown using a dotted line. The broken arrows above the diagram note the extent of the HindIII fragment hybridizing with the ileS2 probe in restriction fragment length polymorphism (RFLP) analyses. Below are shown the sizes (in bp) of sequences corresponding to up- and downstream IS257-ileS2 spacers.
ileS gene fragments amplified from HR547/11, HR294/11, HR23161, and HR1084/08 showed more than 99% sequence similarity between each other and with the sequences deposited in GenBank. All possessed a T-to-A silent mutation at position 1764 (T1764A) of the ileS gene (numbering is according to the ileS gene of HKU10-03, accession no. CP002439.1).
Only a few studies have reported S. pseudintermedius strains resistant to mupirocin, but the mechanism and the level of resistance were not investigated (8, 9). For instance, one mupirocin-resistant isolate was found among 8 MRSP strains obtained from dogs with superficial pyoderma in a study conducted in the United States, where mupirocin is licensed for use in dogs (9). The ileS2 gene is generally found on large staphylococcal plasmids but has also been detected rarely in the chromosome of S. aureus (16). It has been postulated previously that the members of the S. intermedius group prefer transposon-encoded resistance genes due to the large number of insertion sequences found in their chromosomal DNA (17). To investigate the location of ileS2 in HR547/11, we performed Southern blotting experiments, which confirmed that both the ileS2 and aacA-aphD genes were situated on a conjugative plasmid probably belonging to the pSK41/pGO1 family (5). To our knowledge, this is the first description of a plasmid related to the pSK41 family in S. pseudintermedius. Resistance genes, such as ileS2, are often integrated by the activities of IS257 insertion elements (15). The ileS2 gene of HR547/11 was also flanked by two copies of IS257, as previously found in mupirocin-resistant S. aureus (6). The IS257-ileS2 configuration (UpR849-DnR267) found on pKM01 has not been identified previously and represents a novel finding. The possibility of the presence of mutations within the native ileS gene and their influence on MICs was excluded by partial sequencing and comparison with the ileS gene sequences of mupirocin-susceptible strains. Interestingly, compared to S. aureus (GenBank accession no. X74219), all examined strains had a T1764A silent mutation in the ileS gene sequence.
Worryingly and taking into account the limitation of having collected a single mupirocin- and gentamicin-resistant S. pseudintermedius isolate, such strains could become more prevalent in the future. Thus, therapy with gentamicin, kanamycin, or tobramycin, which are more commonly used in veterinary medicine than mupirocin, might favor their coselection and plasmid maintenance.
In summary, we report a detailed genetic analysis of mupirocin resistance in an S. pseudintermedius isolate from a dog. The identification of the conjugative plasmid pKM01, bearing ileS2 and aacA-aphD resistance determinants in S. pseudintermedius, is worrisome and could lead to a future greater dissemination of such antibiotic resistances. Thus, veterinarians should be aware of this issue when prescribing mupirocin in pet animals. However, it will be necessary to carry out additional studies to have a complete picture of Hi-Mupr and associated resistances, as well as its impact on the epidemiology of the global antibiotic resistance of S. pseudintermedius.
Nucleotide sequence accession numbers.
The nucleotide sequences of the left and right IS257-ileS2 spacer regions (accession no. JX186508 and JX186509) and a partial sequence of ileS2 from mupirocin resistance plasmid pKM01 (accession no. JX186510) and partial sequences of the ileS gene from strains HR547/11, HR294/11, HR23161, and HR1084/08 (accession no. JX186511, JX186512, JX186513, and JX186514, respectively) were deposited in GenBank.
ACKNOWLEDGMENTS
This study was supported by a grant from the Ministry of Science, Education and Sports of the Republic of Croatia (053-0481153-1129). E.P.-R holds a Sara Borrel postdoctoral contract from the Instituto de Salud Carlos III (ISCIII), Spain.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Weese JS, Van Duijkeren E. 2010. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 140:418–429 [DOI] [PubMed] [Google Scholar]
- 2. Matanović K, Mekić S, Scaroneol B. 2012. Antimicrobial susceptibility of Staphylococcus pseudintermedius isolated from dogs and cats in Croatia during a six-month period. Vet. Arhiv. 82:505–517 [Google Scholar]
- 3. Hogue JS, Buttke P, Braun LE, Fairchok MP. 2010. Mupirocin resistance related to increasing mupirocin use in clinical isolates of methicillin-resistant Staphylococcus aureus in a pediatric population. J. Clin. Microbiol. 48:2599–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgson JE, Curnock SP, Dyke KG, Morris R, Sylvester DR, Gross MS. 1994. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob. Agents Chemother. 38:1205–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pérez-Roth E, López-Aguilar C, Alcoba-Florez J, Méndez-Alvarez S. 2006. High-level mupirocin resistance within methicillin-resistant Staphylococcus aureus pandemic lineages. Antimicrob. Agents Chemother. 50:3207–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez-Roth E, Armas-González E, Alcoba-Flórez J, Méndez-Alvarez S. 2011. PCR-based amplification of heterogeneous IS257-ileS2 junctions for molecular monitoring of high-level mupirocin resistance in staphylococci. J. Antimicrob. Chemother. 66:471–475 [DOI] [PubMed] [Google Scholar]
- 7. Werner AH, Russel DA. 1999. Mupirocin, fusidic acid and bacitracin: activity, action and clinical uses of three topical antibiotics. Vet. Dermatol. 10:225–240 [DOI] [PubMed] [Google Scholar]
- 8. Penna B, Varges R, Medeiros L, Martins GM, Martins RR, Lilenbaum W. 2010. Species distribution and antimicrobial susceptibility of staphylococci isolated from canine otitis externa. Vet. Dermatol. 21:292–296 [DOI] [PubMed] [Google Scholar]
- 9. Fulham KS, Lemarie SL, Hosgood G, Dick HL. 2011. In vitro susceptibility testing of meticillin-resistant and meticillin-susceptible staphylococci to mupirocin and novobiocin. Vet. Dermatol. 22:88–94 [DOI] [PubMed] [Google Scholar]
- 10. Sasaki T, Tsubakishita S, Tanaka Y, Sakusabe A, Ohtsuka M, Hirotaki S, Kawakami T, Fukata T, Hiramatsu K. 2010. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 48:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement M100-S20. CLSI, Wayne, PA [Google Scholar]
- 12. Anthony RM, Connor AM, Power EG, French GL. 1999. Use of the polymerase chain reaction for rapid detection of high-level mupirocin resistance in staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:30–34 [DOI] [PubMed] [Google Scholar]
- 13. Antonio M, McFerran N, Pallen MJ. 2002. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:438–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bannoehr J, Ben Zakour NL, Waller AS, Guardabassi L, Thoday KL, van den Broek AHM, Fitzgerald JR. 2007. Population genetic structure of the Staphylococcus intermedius group: insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 189:8685–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Firth N, Skurray RA. 2006. The Staphylococcus-genetics: accessory elements and genetic exchange, p 413–426 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI. (ed), Gram-positive pathogens, 2nd ed American Society for Microbiology, Washington, DC [Google Scholar]
- 16. Udo EE, Al-Sweih N, Noronha BC. 2003. A chromosomal location of the mupA gene in Staphylococcus aureus expressing high-level mupirocin resistance. J. Antimicrob. Chemother. 51:1283–1286 [DOI] [PubMed] [Google Scholar]
- 17. Hesselbarth J, Werckenthin C, Liebisch B, Schwarz S. 1995. Insertion elements in Staphylococcus intermedius. Lett. Appl. Microbiol. 20:180–183 [DOI] [PubMed] [Google Scholar]