Abstract
Alpha-actinins are an ancient family of actin-binding proteins that play structural and regulatory roles in cytoskeletal organization. In skeletal muscle, α-actinin-3 protein is an important structural component of the Z disc, where it anchors actin thin filaments, helping to maintain the myofibrillar array. A common nonsense polymorphism in codon 577 of the ACTN3 gene (R577X) results in α-actinin-3 deficiency in XX homozygotes. Based on knowledge about the role of ACTN3 R557X polymorphism in skeletal muscle function, we postulated that the genetic polymorphism of ACTN3 could also improve sprint and power ability.
We compared genotypic and allelic frequencies of the ACTN3 R557X polymorphism in two groups of men of the same Caucasian descent: 158 power-orientated athletes and 254 volunteers not involved in competitive sport.
The genotype distribution in the group of power-oriented athletes showed significant differences (P=0.008) compared to controls. However, among the investigated subgroups of athletes, only the difference of ACTN3 R577X genotype between sprinters and controls reached statistical significance (P=0.041). The frequencies of the ACTN3 577X allele (30.69% vs. 40.35%; P=0.005) were significantly different in all athletes compared to controls.
Our results support the hypothesis that the ACTN3 577XX allele may have some beneficial effect on sprint-power performance, because the ACTN3 XX genotype is significantly reduced in Polish power-oriented athletes compared to controls. This finding seems to be in agreement with previously reported case-control studies. However, ACTN3 polymorphism as a genetic marker for sport talent identification should be interpreted with great caution.
Keywords: α-actinin-3, genotype, power-orientated athletes
Introduction
Twin studies indicate that muscle function phenotypes are highly heritable (Arden and Spector, 1997; Spurway and Wackerhage, 2006) and various candidate genes have been studied to explain some of the high inter-individual variability observed in these phenotypes, e.g. myostatin gene (MSTN), angiotensin converting enzyme gene (ACE), peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), hypoxia-inducible factor-1 (HIF-1) and others (Buchner et al., 1993; Spurway and Wackerhage, 2006). Therefore, only a small number of genes containing single nucleotide polymorphisms (SNPs) have been identified as candidate genes for explaining this variability (Delmonico et al., 2008). One of them is alpha-actinin-3 gene (ACTN3), which encodes the protein a-actinin-3.
Alpha-actinins are an ancient family of actin-binding proteins (Mills et al., 2001) that play structural and regulatory roles in cytoskeletal organization. In skeletal muscle, two alpha-actinin proteins (α-actinin-2 and α+actinin-3) are an important structural component of the Z disc, where they anchor actin thin filaments, helping to maintain the myofibrillar array (Beggs et al., 1992; Blanchard et al., 1989). Besides their mechanical role, both sarcomeric alpha-actinins interact with proteins involved in numerous signalling and metabolic pathways (MacArthur and North, 2004; Mills at al., 2001). While α-actinin-2 is expressed in all types of muscle fibers, the expression of α-actinin-3 is almost exclusively restricted to fast, glycolytic type II fibers (Clarkson et al., 2005).
In 1999, North et al. (1999) identified a common polymorphism in ACTN3 R577X (dbSNP rs1815739) that results in absence of α-actinin-3 in more than one billion people worldwide, despite the ACTN3 gene being highly conserved during human evolution. Though this genetic variation is not associated with any known disease phenotype, the α-actinin-3-deficient XX genotype is believed to preclude top-level athletic performance in ‘pure’ power and sprint sports (North et al., 1999; Suminaga et al., 2000; Druzhevskaya et al., 2008).
The first evidence that a mononucleotide difference in DNA sequence was associated with power ability referred to the R577X polymorphism of the ACTN3 gene was indicated by Yang et al. (2003). The translation (C>T) at nucleotide position 1747 in the ACTN3 coding sequence converts an arginine (R) to a stop codon (X) at residue 577 (Mills et al., 2001). This variation creates two different versions of the ACTN3 gene, both of which are common in the general population: the 577R allele is the normal, functional version of the gene, whereas the 577X allele contains a sequence change that prevents completely production of functional α-actinin-3 protein (Ahmetov et al., 2008).
Till this moment, many reports suggested that the presence of ACTN3 may have a beneficial effect on skeletal muscle function (Niemi and Majamaa, 2005; Papadimitriou et al., 2007; Roth et al., 2008; Santiago et al., 2008), especially in the case of generating powerful contractions at high velocity (McCauley et al., 2009). As was mentioned above, the α-actinin-3 is a part of the sarcomeric α-actinins, which are major components of the Z line, where its function is twofold: to connect with actin filaments and sustain the order of myofilaments and coordinate myofilament contraction (Yang et al., 2003). The Z line is an important structure within the sarcomere and its function is to provide structural support for the transmission of force when the muscle fibres are activated (Wilmore et al., 2004). It is suspected that α-actinin-3 may be optimized to decrease the damage induced by eccentric muscular contraction (Yang et al., 2003).
Additionally, same reports indicated the positive association between the presence of the 577R allele and the capacity to perform high power muscle contractions (Clarson et al., 2005; Delmonico et al., 2007). Furthermore, Vincent et al. (2007) showed that the percentage surface and number of type IIx fibers (fast-twitch glycolytic, which are used predominantly in highly explosive events, such as the 100-m sprint) was greater in the RR than the XX genotype of young healthy men.
Based on knowledge about the role of ACTN3 R557X polymorphism in skeletal muscle function, we postulated that the genetic polymorphism of ACTN3 could also improve sprint and power ability.
The aim of this study was to perform preliminary studies to analyze the possible importance of the ACTN3 R577X polymorphism in Polish power-orientated athletes as well as in sedentary individuals representing possible relationships with the genotype and physical performance.
Material and methods
Ethics Committee
The Pomeranian Medical University Ethics Committee approved the study and written informed consent was obtained from each participant.
Subjects and controls
158 male power-orientated athletes of regional or national level with no less than 8 years training experience were recruited for this study (48 sprinters, 54 sprint swimmers, 56 weightlifters).
As a control group, samples were prepared from 254 unrelated volunteers (male students from the University of Szczecin). The athletes and controls were all Caucasian to ensure there was no ethnicity skew and to overcome any potential problems of population stratification.
Genotyping
The buccal cells donated by the subjects were collected in Resuspension Solution (Sigma, Germany) with use of Sterile Foam Tipped Applicators (Puritan, USA). DNA was extracted from the buccal cells using GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, Germany) according to the producer protocol.
The 290 bp fragment of exon 15 of the ACTN3 gene was amplified by PCR using the forward primer : 5′-CTGTTGCCTGTGGTAAGTGGG-3′ and the reverse primer : 5′-TGGTCACAGTATGCAGGAGGG-3′ as recommended by Mills et al. (2001). PCR reaction mix (total volume 10 μl) contained 1.5 mM MgCl2, 0.75 nM of each deoxynucleoside triphosphate (Novazym, Poland), 4 pM of each primer (Genomed, Poland), 0.5 U of Taq DNA polymerase (Sigma, Germany), and 1 μl (30–50 ng) of template DNA. After a first step consisting of 95°C for 5 min., 35 cycles of amplification were performed by using denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s and a final cycle at 72°C for 10 min (Lucia et al., 2006). The amplified PCR fragments were subsequently digested with Dde I endonuclease (Fermentas, Lithuania) in a condition recommended by the supplier (Mills et al., 2001). The alleles 577R and 577X were distinguished by the presence (577X) or absence (577R) of a Dde I restriction site. Digestion of PCR products of the 577X allele yields bands of 108, 97 and 86 bp, whereas digestion of PCR products of the 577R allele yields bands of 205 and 86 bp. The digested products were separated by 3% agarose gel electrophoresis, stained with ethidium bromide, and visualized in UV light.
Statistical analysis
Genotype distribution and allele frequencies between groups of athletes and controls were compared and significance was assessed by χ2 test using STATISTICA 8 statistical package. P values of < 0,05 were considered statistically significant.
Results
ACTN3 genotype distributions amongst subjects and controls were in Hardy-Weinberg equilibrium, making selection bias less likely. Genotype distribution results of the control group (RR-35,83%; RX-49,21%; XX-14,96%) were similar to those reported in previous studies on Caucasian populations (North et al., 1999; Yang et al., 2003; Ahmetov et al., 2008; Druzhevskaya et al., 2008). The distributions of the ACTN3 genotypes and alleles are given in Table 1. Genotype distribution in a whole cohort of athletes showed significant difference (P=0.008) compared to controls. However, among the investigated subgroups of athletes, only the difference of ACTN3 R577X genotype between sprinters and controls reached statistical significance (P=0.041)
Table 1.
ACTN3 genotype distribution of the athletes and controls (data is presented as relative values
| Group | n | Genotypes, % | P value | ||
|---|---|---|---|---|---|
| RR | RX | XX | |||
| All athletes | 158 | 44,94 | 48,73 | 6,33 | 0,008* |
| Sprinters | 48 | 50,00 | 45,83 | 4,17 | 0,041* |
| Sprint swimmers | 54 | 42,59 | 51,85 | 5,56 | 0,130 |
| Weightlifters | 56 | 42,86 | 50,00 | 7,14 | 0,205 |
| Control | 254 | 35,04 | 49,21 | 15,75 | - |
p<0.05 compared to control.
Comparison with controls was by χ2 test RR Wild-type homozygote, RX heterozygote, XX mutant homozygote
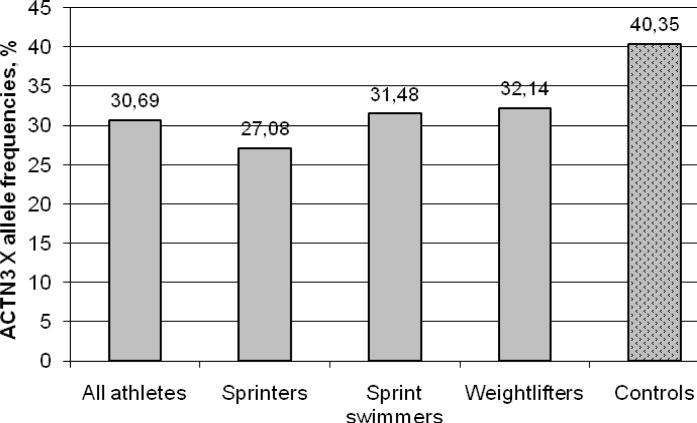
The frequencies of the ACTN3 577X allele (30.69% vs. 40.35%; P=0.005) were significantly different in all athletes group compared to controls. This trend was worse when comparing each subgroups of investigated athletes to controls separately (Figure 1).
Figure 1.
ACTN3 577X allele frequency amongst power-oriented athletes and controls is shown. X allele genotype frequency in controls was 40,35%. By comparison, it was 30,69% (P=0.005); 27.08% (P=0.014); 31.48% (P=0.085) and 32.14%% (P=0.106) for all athletes, sprinters, sprint swimmers and weightlifters.
Discussion
The first evidence for strong ACTN3 gene influence on athletic performance was first reported by Yang et al. (2003). The possible mechanism underlying the association of the ACTN3 R577X polymorphism with athletic performance was discussed in detail by MacArthur and North (2007) and MacArthur et al. (2007).
The presented report is the first to indicate that ACTN3 XX genotype is under-represented in Polish strength-power orientated athletes in comparison with controls. Here, we also show that the distribution of ACTN3 genotypes and alleles in Polish population is similar to these observed in several reported groups of Caucasian populations (Druzhevskaya et al., 2008; Ahmetov et al., 2008; Moran et al., 2007; North et al., 1999; Yang et al., 2003).
Our report suggests that the ACTN3 RR and RX genotypes are associated with predisposition to power-sprint sports, because the ACTN3 XX genotype is significantly reduced in Polish power-oriented athletes compared to controls. This finding seems to be in agreement with previously reported case-control studies.
The mentioned above report by Yang et al. (2003) indicated that only 6 % of elite Australian sprinters were homozygous for the 577X genotype. This finding seems to be supported by Druzhevskaya et al. (2003), who investigated 486 male and female Russian athletes of regional or national sports level. The mentioned study showed that variation in the ACTN3 gene was strongly associated with elite power athlete status in Russians. Also Eynon et al. (2009) found that the proportion of subjects homozygous for the 577R allele was significantly higher in sprinters (50 %) compared to endurance athletes and controls (19 % and 20 % respectively). Moreover, 88 % of the top-level Israel sprinters had at least one copy of the 577R allele, indicating the crucial role of this allele in the development of top-level sprint ability.
The association between the RR genotype of ACTN3 and elite athletic performance was also demonstrated in Finnish sprint athletes (Niemi and Majamaa, 2005). The comparison of endurance and sprint athletes has shown that the frequency of the XX genotype of ACTN3 was lower and that of RR higher among sprinters than among endurance runners (P=0.03). Furthermore, none of the top sprinters harboured the XX genotype.
Another study suggested that the presence of α-actinin-3 protein had a positive effect on power performance. A report by Papadimitriou et al. (2008) showed statistically significant differences in the frequencies of alleles (P=0.017) and genotypes (P=0.016) between elite power-orientated athletes and the control group (the power orientated athletes displayed a lesser frequency of the 577X allele than the control group). It is worth to mention that all of the investigated Olympic/European- level sprinters had at least one 577R allele of ACTN3.
However, the role of the ACTN3 R577X polymorphism in athletic performance has not yet been sufficiently clarified. Norman et al. (2009) indicated that R577X polymorphism in ACTN3 is not associated with differences in power output, fatigability, or force-velocity characteristics in physically active individuals. Moreover, Norman et al. (2009) suggested that alpha-actinins do not play any significant role in determining muscle fiber-type composition. Additionally, McCauley et al. (2009) indicated that ACTN3 R577X polymorphism did not influence absolute or relative torque at high velocities or the twitch response. This finding seems to be supported by Delmonico et al. (2008), who showed that in older women (64 years), knee extensor concentric peak power was found to be higher in 577X allele homozygotes compared with RR genotype individuals. Similarly, Clarkson et al. (2005) reported no association between ACTN3 R577X genotype and muscle phenotype in men when investigating isometric elbow flexor strength. Finally, Lucia et al. (2007) reported a case of a Spanish elite long jumper (two times Olympian) whose genotype for the ACTN3 gene is XX. The similar finding was made by Druzhevskaya et al. (2008), who observed one highly elite Russian hammer thrower (world record holder) with XX genotype.
Our study had some restrictions. The investigated group consisted mostly of regional or national level athletes. On the other hand, even in this group, the study proved that the ACTN3 R577X allele could be one of the factors influencing power-oriented sport disciplines. Such findings have important implications for understanding of molecular mechanisms underlying the predisposition to high power potential and support the hypothesis that the presence of α-actinin-3 has a beneficial effect on the function of skeletal muscle in generating forceful contractions at high velocity (Druzheyskaya et al., 2008).
It is worth noting that among all reported subgroups (i.e. sprinters, swimmers and weightlifters) statistically significant differences in genotype distributions were only demonstrated in sprinters. This fact can be explained by the specificity of performed movements and big loads, resulting in a greater risk of injury in sprinters than in swimmers or weightlifters. Presumably, that is the reason why having at least one ACTN3 R577 allele is a key factor in sprint training.
On the other hand, the differences in the genotype distribution between the subgroups were not statistically significant. This fact suggests that the ACTN3 R577 allele may also have a beneficial role in swimmers and weightlifters. Unfortunately, the relatively small size of subgroups in this study can cause some ambiguity in the obtained results. For example, the frequency of allele ACTN3 577X in swimmers (54) was 31.48 % and was statistically insignificant (p= 0.085), although, it was only 0.79 % lower than the frequency of this allele in all the examined athletes (158) which proved to be highly statistically significant (p= 0.005)). Therefore, our conclusions should be supported with more experimental studies on ACTN3 polymorphisms in elite athletes.
References
- Ahmetov II, Druzhevskaya AM, Astratenkova IV, Popov DV, Vinogradova OL, Rogozkin VA. The ACTN3 R577X polymorphism in Russian endurance athletes. Br J Sports Med. 2010;44:649–652. doi: 10.1136/bjsm.2008.051540. [DOI] [PubMed] [Google Scholar]
- Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Blanchard A, Ohanian V, Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989;10:280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Cress ME, de Lateur BJ, Wagner EH. Variability in the effect of strength training on skeletal muscle strength in older adults. Facts Res Gerontol. 1993;7:143–153. [Google Scholar]
- Clarkson PM, Hoffman EP, Zambraski E, Gordish-Dressman H, Kearns A, Hubal M, Harmon B, Devaney JM. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol. 2005;99:564–569. doi: 10.1152/japplphysiol.00130.2005. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Zmuda JM, Taylor BC, Cauley JA, Harris TB, Manini TM, Schwartz A, Li R, Roth SM, Hurley BF, Bauer DC, Ferrell RE, Newman AB. Association of the ACTN3 Genotype and Physical Functioning. With Age in Older Adults. J Gerontol A Biol Sci Med Sci. 2008;63:1227–1234. doi: 10.1093/gerona/63.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhevskaya AM, Ahmetov II, Astratenkova IV, Rogozkin VA. Association of the ACTN3 R577X polymorphism with power athlete status in Russians. Eur J Appl Physiol. 2008;103:631–634. doi: 10.1007/s00421-008-0763-1. [DOI] [PubMed] [Google Scholar]
- Eynon N, Duarte JA, Oliveira J, Sagiv M, Yamin C, Meckel Y, Sagiv M, Goldhammer E. ACTN3 R577X polymorphism and Israeli top-level athletes. Int J Sports Med. 2009;30:695–698. doi: 10.1055/s-0029-1220731. [DOI] [PubMed] [Google Scholar]
- Hanson ED, Ludlow AT, Sheaff AK, Park J, Roth SM. ACTN3 Genotype Does not Influence Muscle Power. Int J Sports Med. 2010;31:834–838. doi: 10.1055/s-0030-1263116. [DOI] [PubMed] [Google Scholar]
- Lucia A, Gómez-Gallego F, Santiago C, Bandrés F, Earnest G, Rabadán P, Alonso JM, Hoyos J, Córdova A, Villa G, Foster C. ACTN3 genotype in professional endurance cyclists. Int J Sports Med. 2006;27:880–884. doi: 10.1055/s-2006-923862. [DOI] [PubMed] [Google Scholar]
- McCauley T, Mastana S, Hossack J, MacDonald M, Folland JP. Human angiotensin-converting enzyme I/D and α-actinin 3 R577X genotypes and muscle functional and contractile properties. Exp Physiol. 2009;94:81–89. doi: 10.1113/expphysiol.2008.043075. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, North KN. A gene for speed? The evolution and function of a actinin 3. BioEssays. 2004;26:786–795. doi: 10.1002/bies.20061. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, North KN. ACTN3: A genetic influence on muscle function and athletic performance. Exerc Sport Sci Rev. 2007;35:30–34. doi: 10.1097/JES.0b013e31802d8874. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Seto JT, Raftery JM, Quinlan KG, Huttley GA, Hook JW, Lemckert FA, Kee AJ, Edwards MR, Berman Y, Hardeman EC, Gunning PW, Easteal S, Yang N, North KN. Loss of ACTN3 gene function alters mouse muscle metabolism and shows evidence of positive selection in humans. Nat Genet. 2007;39:1261–1265. doi: 10.1038/ng2122. [DOI] [PubMed] [Google Scholar]
- Mills M, Yang N, Weinberger R, Vander Woude DL, Beggs AH, Easteal S, North K. Differential expression of the actinbinding proteins, a-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum Mol Gen. 2001;10:1335–1346. doi: 10.1093/hmg/10.13.1335. [DOI] [PubMed] [Google Scholar]
- Moran CN, Yang N, Bailey M, Tsiokanos A, Jamurtas A, MacArthur DG, North K, Pitsiladis YP, Wilson RH. Association analysis of the ACTN3 R577X polymorphism and complex quantitative body composition and performance phenotypes in adolescent Greeks. Eur J Hum Genet. 2007;15:88–93. doi: 10.1038/sj.ejhg.5201724. [DOI] [PubMed] [Google Scholar]
- Niemi AK, Majamaa K. Mitochondrial DNA and ACTN3 genotypes in Finnish elite endurance and sprint athletes. Eur J Hum Genet. 2005;13:965–969. doi: 10.1038/sj.ejhg.5201438. [DOI] [PubMed] [Google Scholar]
- Norman B, Esbjörnsson M, Rundqvist H, Osterlund T, von Walden F, Tesch PA. Strength, power, fiber types and mRNA expression in trained men and women with different ACTN3 R577X genotypes. J Appl Physiol. 2009;106:959–965. doi: 10.1152/japplphysiol.91435.2008. [DOI] [PubMed] [Google Scholar]
- North KN, Yang N, Wattanasirichaigoo D, Mills M, Tong HQ, Easteal S, Beggs AH. A common nonsense mutation results in a-actinin-3 deficiency in the general population: Evidence for genetic redundancy in humans. Nat Genet. 1999;21:353–354. doi: 10.1038/7675. [DOI] [PubMed] [Google Scholar]
- Papadimitriou ID, Papadopoulos C, Kouvatsi A, Triantaphyllidis C. The ACTN3 Gene in Elite Greek Track and Field Athletes. Int J Sports Med. 2007;29:352–355. doi: 10.1055/s-2007-965339. [DOI] [PubMed] [Google Scholar]
- Roth SM, Walsh S, Liu D, Metter EJ, Ferrucci L, Hurley BF. The ACTN3 R577X nonsense allele is under-represented in elite-level strength athletes. Eur J Hum Genet. 2008;16:391–394. doi: 10.1038/sj.ejhg.5201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, González-Freire M, Serratosa L, Morate FJ, Meyer T, Gómez-Gallego F, Lucia A. ACTN3 genotype in professional soccer players. Br J Sports Med. 2008;42:71–73. doi: 10.1136/bjsm.2007.039172. [DOI] [PubMed] [Google Scholar]
- Spurway N, Wackerhage H. Advances in sport and exercise science series. Edinburgh: Elsevier; 2006. Genetics and molecular biology of muscle adaptation. [Google Scholar]
- Suminaga R, Matsuo M, Takeshima Y, Nakamura H, Wada H. Nonsense mutation of the alpha-actinin-3 gene is not associated with dystrophinopathy. Am J Med Genet. 2000;92:77–78. doi: 10.1002/(sici)1096-8628(20000501)92:1<77::aid-ajmg13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Costill DL. Physiology of Sport and Exercise. Champaign: Human Kinetics; 2004. [Google Scholar]
- Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, Hespel PJ, Thomis M. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol Genomics. 2007;32:58–63. doi: 10.1152/physiolgenomics.00173.2007. [DOI] [PubMed] [Google Scholar]
- Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. 2003;73:627–631. doi: 10.1086/377590. [DOI] [PMC free article] [PubMed] [Google Scholar]