Abstract
Influenza A virus is an important pathogenic virus known to induce host cell cycle arrest in G0/G1 phase and create beneficial conditions for viral replication. However, how the virus achieves arrest remains unclear. We investigated the mechanisms underlying this process and found that the nonstructural protein 1 (NS1) is required. Based on this finding, we generated a viable influenza A virus (H1N1) lacking the entire NS1 gene to study the function of this protein in cell cycle regulation. In addition to some cell cycle regulators that were changed, the concentration and activity of RhoA protein, which is thought to be pivotal for G1/S phase transition, were also decreased with overexpressing NS1. And in the meantime, the phosphorylation level of cell cycle regulator pRb, downstream of RhoA kinase, was decreased in an NS1-dependent manner. These findings indicate that the NS1 protein induces G0/G1 cell cycle arrest mainly through interfering with the RhoA/pRb signaling cascade, thus providing favorable conditions for viral protein accumulation and replication. We further investigated the NS1 protein of avian influenza virus (H5N1) and found that it can also decrease the expression and activity of RhoA, suggesting that the H5N1 virus may affect the cell cycle through the same mechanism. The NS1/RhoA/pRb cascade, which can induce the G0/G1 cell cycle arrest identified here, provides a unified explanation for the seemingly different NS1 functions involved in viral replication events. Our findings shed light on the mechanism of influenza virus replication and open new avenues for understanding the interaction between pathogens and hosts.
INTRODUCTION
Manipulating the cell cycle is a common strategy used by DNA and RNA viruses to achieve favorable cellular environments and facilitate their own replication (1–4). By interacting with cellular proteins, viruses can utilize a number of mechanisms to subvert the cell cycle (5, 6). Among the RNA viruses, it is well known that the influenza A viruses, such as the current pandemic swine-origin influenza virus (S-OIV), continue to pose a worldwide threat (7) and that the highly pathogenic avian influenza virus H5N1 still retains considerable pandemic potential (8, 9). Although several studies have provided evidence that influenza viruses can cause G0/G1 cell cycle arrest (10, 11), the mechanism remains less obvious. Intensive research into this little-known aspect of the influenza virus life cycle will promote better understanding of the viral replication process and give insights into antiviral interventions.
Influenza A viruses within the Orthomyxoviridae family contain a single-stranded, negative-sense, segmented RNA genome consisting of eight segments of viral RNA (vRNA) encoding 11 to 13 known proteins (12–14). The nonstructural protein 1 (NS1) of influenza A viruses is a nonessential viral protein that has multiple accessory functions during viral infection (15, 16). The key functions of NS1 protein include regulating viral protein synthesis through mRNA splicing and translation (17–19), interfering with host restriction factors (20–22), and inhibiting the antiviral type 1 interferon (IFN) response (23–25). Evidence shows that the NS1 proteins of many viruses, such as the latest reported human respiratory syncytial virus (26) and the autonomous parvovirus minute virus (27, 28), could control cellular processes, perhaps in part, by promoting cell cycle arrest to facilitate viral replication (29, 30).
Cell cycle transition represents a series of complex and tightly regulated processes that control how a single cell divides into two cells. The G1/S cell cycle checkpoint controls the first gap phase (31, 32). In this switch, two-cell-cycle kinesis, involving CDK4/6-cyclin D and CDK2-cyclin E, along with the transcription complex, including Rb and E2F, is pivotal in controlling this checkpoint (33, 34). The Ras homolog gene family member A (RhoA) is a small GTPase that controls many cellular functions, including gene transcription, actin polymerization, cell cycle progression, and cell transformation (35–38). RhoA has two states, and the phosphorylation of Ser188 is important for its function in cell cycle transition (39–41). In the cell cycle, RhoA activation can affect G1/S progression by at least three signaling pathways. One is the suppression of the RhoA-ROCK pathway leading to the accumulation of INK4 family proteins and the competitive binding of CDK4 and CDK6 (42, 43). The second involves the downregulation of mDIA to increase the expression of p21Waf1/Cip1 and p27Kip1. The third pathway involves the substantial effect of RhoA on extracellular signal-regulated kinase (ERK) activity to decrease the level of cyclin D1 (44, 45). Together, these three pathways reduce the levels of Rb phosphorylation, thus inducing G1/S cell cycle arrest.
In this study, we generated a viable influenza A virus (H1N1) lacking the entire NS1 gene in order to study the function of this protein in cell cycle regulation. We show that NS1 can downregulate the expression of RhoA in an NF-κB-dependent manner and inhibit RhoA activity by direct binding. In addition, we found that the NS1 protein of avian influenza virus (H5N1) can also decrease RhoA expression and activity, suggesting that the H5N1 virus may use the same mechanism to arrest the cell cycle. The NS1/RhoA/pRb cascade, which can induce the G0/G1 cell cycle arrest identified here, provides new features for understanding influenza virus-host interaction.
MATERIALS AND METHODS
Cell culture and transient transfection.
A549 cells (human airway epithelial cell line), 293T cells (human embryonic kidney cell line), MDCK cells (Madin-Darby canine kidney cell line), and Vero cells (African green monkey kidney epithelial cell line) were cultured in Dulbecco modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Paisley, United Kingdom). Plasmid transfection was performed using the Effectene transfection reagent (Qiagen, Germany) according to the manufacturer's instructions.
Plasmids and antibodies.
The cDNA of H1N1 NS1 was derived from strain A/WSN/33 and subcloned into the prokaryotic expression vector pGEX4T-1, the eukaryotic expression vector pcDNA3.0-Flag, and pEGFP-N1. The truncated constructs GST-NS1-R (N terminal, 1 to 73 amino acids [aa]) and GST-NS1-E (C terminal, 73 to 230 aa) of H1N1 NS1 were created using PCR with specific primers and cloned into the prokaryotic expression vector pGEX4T-1. The NS1 gene of the H5N1 virus (A/Bar-headed Goose/Qinghai/12/05) was amplified from the pGEM-T-NS1 plasmid (kindly provided by George F. Gao, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) using PCR. The product was cloned into the prokaryotic expression vector pGEX4T-1, the eukaryotic expression vector pcDNA3.0-Flag, and pEGFP-N1.
Using published sequences, we obtained full-length human RhoA cDNA by reverse transcription-PCR (RT-PCR). The following constructs were obtained and sequenced: pcDNA4.0-myc-RhoA, pcDNA4.0-myc-RhoA97 (N terminal, 1 to 97 aa), pcDNA4.0-myc-RhoA194 (C terminal, 98 to 194 aa), pcDNA4.0-myc-RhoAS188A (RhoA S188 site mutated to A), and pcDNA4.0-myc-RhoAS188E (RhoA S188 site mutated to E). The shRhoA1-resistant RhoA (KDR-RhoA) was constructed by PCR-based mutagenesis, which changed six nucleotides of the RhoA sequence and cloned them into pcDNA4.0-myc vector (the mutant sites are indicated in Table 1). The pEZH2 was constructed in our laboratory. The pEGFP-H2b plasmid was provided by Xin Ye (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China). The pNF-κB-Luc plasmid was a gift from Linbai Ye (College of Life Science, Wuhan University, Wuhan, China). The pTRAF6 was a gift from Feng Shao (National Institute of Biological Sciences, Beijing, China).
Table 1.
Mutantsequences for the shRhoA1-resistant plasmid
| Primer, orientationa | Sequence (5′–3′)b |
|---|---|
| shRhoA1-resistant mutant, F | AGGAAGATTATGACAGACTACGACCCCTCT |
| shRhoA1-resistant mutant, R | AGAGGGGTCGTAGTCTGTCATAATCTTCCT |
F, forward; R, reverse.
Mutations are indicated by underscoring.
Mouse anti-flag (M2; F3165) antibody was purchased from Sigma (St. Louis, MO). Mouse anti-RhoA (26C4; sc-418), anti-myc (9E10; sc-40), anti-p21 (187; sc-817), anti-cyclin D1 (A-12; sc-8396), anti-CDK6 (B-10; sc-7961), anti-E2F-1 (KH95; sc-251), anti-DP-1 (TFD-11; sc-56657), anti-β-actin (C4; sc-47778), anti-influenza virus A NP (sc-101352), and anti-influenza virus A NS1 (sc-130568) antibodies and rabbit anti-CDK2 (M2; sc-163) and anti-CDK4(C-22; sc-260) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-Rb (4H1; catalog no. 9309) and rabbit anti-Phospho-Rb (Ser795; catalog no. 9301) antibodies were purchased from Cell Signaling Technology, Inc. Mouse anti-p16INK4a antibody (2D9A12; ab54210) was purchased from Abcam (Cambridge, MA). Fluorescein isothiocyanate (FITC)/TRITC (tetramethyl rhodamine isothiocyanate)-conjugated anti-mouse/rabbit IgG antibody was purchased from Zhongshan Golden Bridge Biotechnology (Beijing, China).
Virus generation and infection.
Influenza virus A/WSN/33 (H1N1) was generated using reverse genetics and propagated in 10-day-old embryonated eggs. Virus titers for infection were calculated by plaque assay titration on MDCK cells (46). The 12-plasmid IAV reverse genetics system, which included eight vRNA expression plasmids (pPolI-PB2, pPolI-PB1, pPolI-PA, pPolI-HA, pPolI-NP, pPolI-NA, pPolI-M, and pPolI-NS) and four protein expression plasmids (pcDNA-PB2, pcDNA-PB1, pcDNA-PA, and pCAGGS-NP), was kindly provided by George F. Gao (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China).
Influenza virus A/WSN/33 virus lacking the NS1 gene (NS1− H1N1 virus) was generated using plasmid-based reverse genetics (47, 48). Vero or 293T cells transfected with 1 μg of each of the eight vRNA expression plasmids (we used pPolI-NS2 to change pPolI-NS), 1 μg of each of the four protein expression plasmids, and pcDNA-NS1. At 24 h after transfection, the medium was replaced with DMEM supplemented with 1% FBS (49). Three days later, the supernatants were collected and inoculated into NS1-expressing MDCK cells. After 72 h, rescue of the NS1− H1N1 virus was confirmed using hemagglutination, and the viral NS segments were amplified using RT-PCR for sequence confirmation. The virus titer was measured by indirect immunofluorescence microscopy with an anti-NP antibody in the MDCK cells (21, 49).
For cell cycle analysis, cells were mock infected or infected with virus at different multiplicities of infection (MOIs). After 1 h of virus adsorption, the cells were treated with medium containing 10% FBS and harvested at various times postinfection for cell cycle and Western blot analysis.
Immunofluorescence staining and cell cycle analysis.
To determine the rate of viral infection, 106 cells were collected after infection and fixed in 4% paraformaldehyde for 15 min at room temperature. The cells were then permeabilized with 0.2% Triton X-100 for 5 min. Influenza A virus nucleoprotein (NP) was first stained using an anti-NP polyclonal antibody. Goat anti-mouse antibody conjugated to FITC was used as a secondary antibody. For plasmid transfection, we used pEGFP-H2b as an internal control (the control plasmid was only 10% of the research plasmid). The nuclear DNA content was measured using propidium iodide (PI) staining (50 μg of PI [Sigma]/ml, 50 μg of RNase/ml, 0.1% sodium citrate, 0.2% Triton X-100). We then used fluorescence-activated cell sorting (FACS) to select green fluorescence 10-fold more intensely than wild-type cells, when excited at 488 nm. At least 20,000 cells were counted per sample. The data analysis was performed using ModFit LT version 2.0 to determine the percentages of cells in each phase of the cell cycle.
Quantitative RT-PCR analysis.
The total RNA was isolated from the transfected cells using TRIzol (TianGen Biotech Co., Ltd., Beijing, China). Aliquots (2 μg) of RNA were used to synthesize first-strand cDNA using Moloney murine leukemia virus reverse transcriptase (Promega). The amplification of specific PCR products was detected using SYBR Premix Ex Taq II (TaKaRa). The cycling conditions for real-time PCR were as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The GAPDH primer sequences were as follows: forward, 5′-GGAGAAACCTGCCAAGTATG-3′, and reverse, 5′-TTACTCCTTGGAGGCCATGTAG-3′. The RhoA primer sequences were as follows: forward, 5′-GAAGAAACTGGTGATT-3′, and reverse, 5′-GATGTTATACTGATGTGT-3′. A melting curve and standard controls were run to evaluate amplification specificity and efficiency, respectively. Real-time PCR was conducted using the ABI Prism 7300 sequence detection system.
miRNA expression vectors.
psiSTRIKE vectors (Promega) were used to construct shRhoA expression vectors according to the manufacturer's protocol. The sequences of the control shRNA and shRhoA primers were designed according to the instructions provided and are shown in Table 2. The primers were annealed to form double-stranded DNA and then inserted into the psiSTRIKE vectors. Bacteria were transformed with the resulting constructs, and a reasonable number of colonies was obtained. The resulting shRNA expression vectors were confirmed by digestion with the restriction enzyme PstI according to the manufacturer's protocol. To assess the function of the shRNA expression vectors, shRNA expression was analyzed by Western blotting. Caenorhabditis elegans miR239b was used as a negative control.
Table 2.
Sequencesfor shRNA primers
| Primer | Orientationa | Sequence (5′–3′) |
|---|---|---|
| shRNA (control) | F | ACCGCATTCCGGAATTCCGGCACCTTCCTGTCA GTGCCGGAATTCCGGAATGCTTTTTC |
| R | TGCAGAAAAAGCATTCCGGAATTCCGGCACTGACAGGAAGGTGCCGGAATTCCGGAATG | |
| shRhoA1 | F | ACCGATTATGATCGCCTGAGGCCTTCCTGTCAGCCTCAGGCGATCATAATCTTTTTC |
| R | TGCAAAAAAGATTATGATCGCCTGAGGCTGACAGGAAGGCCTCAGGCGATCATAAT | |
| shRhoA2 | F | ACCGGAAGAAACTGGTGATTGTCTTCCTGTCAACAATCACCAGTTTCTTCTTTTTC |
| R | TGCAGAAAAAGAAGAAACTGGTGATTGTTGACAGGAAGACAATCACCAGTTTCTTC | |
| shRhoA3 | F | ACCGGATGTTATACTGATGTGTCTTCCTGTCAACACATCAGTATAACATCTTTTTC |
| R | TGCAGAAAAAGATGTTATACTGATGTGTTGACAGGAAGACACATCAGTATAACATC |
F, forward; R, reverse.
Luciferase assay.
To measure the activation of the RhoA promoter, the luciferase expression vector pRL-TK (Promega) was used as a parent vector for RhoA promoter reporter analysis experiments. The bp −2000 and bp −1900 promoters were amplified by PCR using four pairs of primers and then inserted into a pRL-TK vector (pRL-TK-RhoA promoter). The luciferase activity was measured using a dual-luciferase reporter assay system (Promega) according to the manufacturer's recommendations. Each set of assays was performed in triplicate. The primer sequences for the RhoA promoter are provided in Table 3.
Table 3.
Primersequences for the RhoA promoter
| Promoter site | Primer |
|
|---|---|---|
| Orientationa | Sequence (5′–3′) | |
| 34505–34524 | F | CCGCTCGAGCAGTGTCTCACTGGGGTTCC |
| 35024–35005 | R | CCCAAGCTTGTGAGCCGAGATCATGCCAT |
| 34985–35004 | F | CCGCTCGAGGTTGCCCAGGCTGGAGTGCA |
| 35524–35505 | R | CCCAAGCTTGAGACAGAGCTTGCAGTGAG |
| 35665–35684 | F | CCGCTCGAGGTGCAGTGGCCCGATCTTGG |
| 36024–36005 | R | CCCAAGCTTACCTCAGGTGATCCACCTGC |
| 35985–36004 | F | CCGCTCGAGCAGCACTTTGGGAGGCTGAG |
| 36524–36505 | R | CCCAAGCTTTTCTTCCGGATGGCAGCCAT |
F, forward; R, reverse.
RhoA activation and the Rhotekin-pulldown assay.
RhoA activation was determined using the Rho-binding reagent Rhotekin-pulldown assay. Transfected A549 cells were grown to confluence in 100-mm dishes and then placed in serum-free medium for 24 h. The cells were stimulated with 10% FBS for 15 min. Simvastatin and farnesylpyrophosphate (FPP) or geranylgeranylpyrophosphate (GGPP) were added 24 h before stimulation. After stimulation by FBS, the cells were washed with ice-cold phosphate-buffered saline (PBS). The GTP-RhoA-enriched lysates were incubated with 20 μl of the Rho assay reagent (Rho binding domain of the Rho effector protein, Rhotekin), which only binds RhoA in its GTP-bound form (GST-Rhotekin); glutathione S-transferase (GST) was used as a control. The assay therefore provides a simple means of quantitating RhoA activation in cells. The amount of activated RhoA was determined by Western blotting with a RhoA-specific antibody.
GST-pulldown assays and coimmunoprecipitation.
GST and GST fusion protein were purified from Escherichia coli BL21 cells (DE3) using glutathione-Sepharose 4B beads (Amersham Biosciences, Uppsala, Sweden). An equal amount of either GST or GST fusion protein bound to the beads was incubated with the lysates from transiently transfected 293T cells in NP-40 lysis buffer for 4 h at 4°C. The beads were then washed five times with PBS containing 0.1% Triton X-100. The bound proteins were eluted by boiling in 2× sodium dodecyl sulfate (SDS) loading buffer and analyzed by Western blotting.
For the coimmunoprecipitation experiments, total cell lysates from transfected 293T cells in lysis buffer (1% Triton X-100, 150 mM NaCl, 20 mM HEPES [pH 7.5], 10% glycerol, 1 mM EDTA, protease inhibitors) were incubated with antibody at 4°C for 2 h. Protein G-agarose beads (Sigma) were then added, followed by incubation at 4°C overnight. The beads were washed three times with lysis buffer and boiled in 2× SDS loading buffer for 5 min. They were then analyzed by Western blotting.
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed according to the manufacturer's protocol (P2078; Beyotime Co.) with slight modifications. The soluble chromatin was incubated with 1 μg of rabbit IgG, E2F1 (KH95; sc-251, Santa Cruz), or DP-1 (TFD-11; sc-56657, Santa Cruz) antibodies. PCR was performed using published primers specific for the E2F binding regions in the EZH2 promoter (5′-CGCCGGTTCCCGCCAAGAG-3′ and 5′-GTTCGCTGTAAGGGACGCCA-3′) (50). PCR products were separated on a 2% agarose gel containing ethidium bromide.
Subcellular localization and immunofluorescence assay.
To determine the localization of RhoA in NS1-expressing cells, A549 cells were transfected with pEGFP or pEGFP-NS1. After 24 h, the cells were washed three times with PBS, fixed in 4% paraformaldehyde for 15 min at room temperature, and permeabilized with 0.2% Triton X-100 for 5 min. After blocking in 5% bovine serum albumin for 30 min, the cells were incubated for 1 h with an anti-RhoA monoclonal antibody at room temperature. After a washing step with PBS, the cells were incubated for 1 h with a TRITC-conjugated anti-mouse IgG antibody. The cells were observed under an Olympus confocal microscope.
Statistical analysis.
Western blots are shown for one of three independent experiments. The expression of protein was quantified using ImageJ software, and the results are presented as means ± the standard deviations (SD) of experiments performed in triplicate. The data were evaluated using a Student t test with SPSS 11.5 software (SSPS, Inc., Chicago, IL); a P value of <0.05 was considered statistically significant.
RESULTS
NS1 induces host cell cycle arrest in the G0/G1 phase.
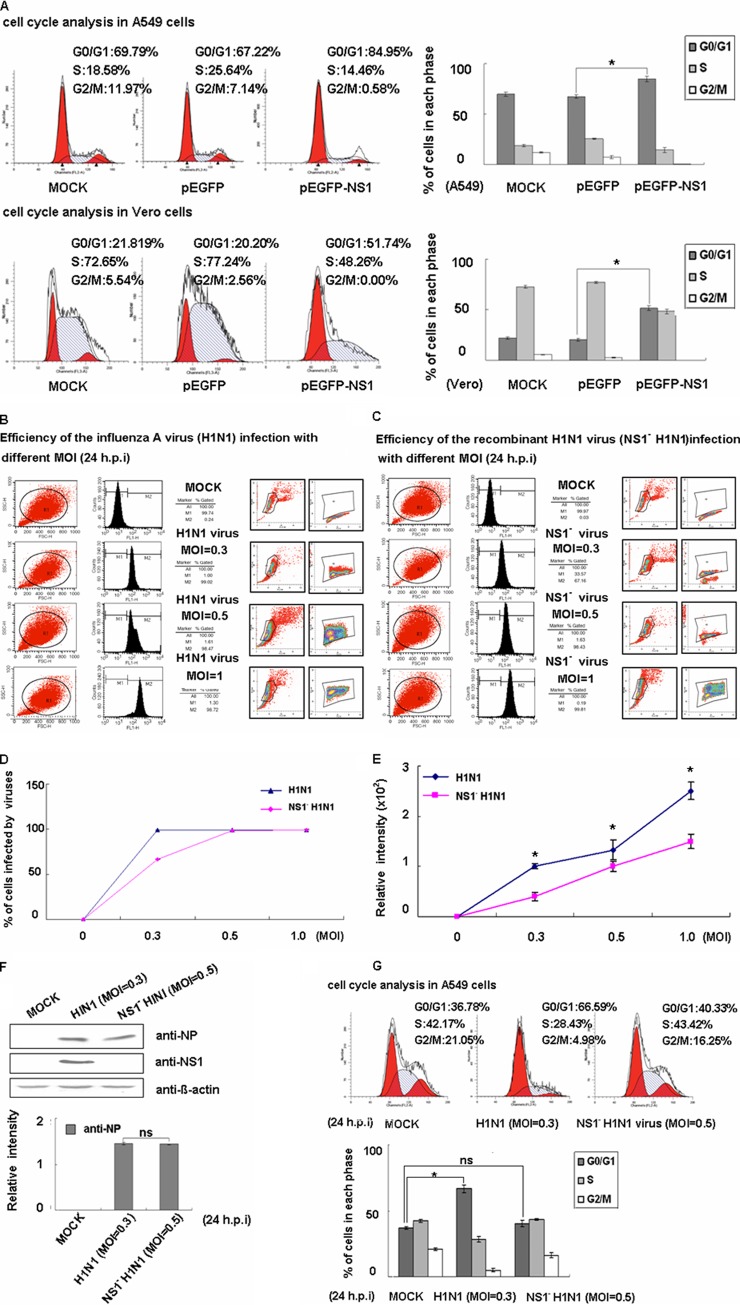
H1N1 viruses can arrest the cell cycle in G0/G1 phase and create favorable conditions for viral protein accumulation and viral production (10). We therefore sought to determine which protein plays the key role in this process. Since several studies have reported that the NS1 protein of other viruses could control the cell cycle, we were interested in determining whether the influenza virus NS1 protein could account for the inhibition of the cell cycle (26, 51). We therefore transfected A549 cells with pEGFP or pEGFP-NS1 plasmids used flow cytometry to select for green fluorescent protein (GFP)-positive cells. We found that compared to pEGFP transfection, the EGFP-NS1 fusion protein could arrest the cell cycle in G0/G1 phase according to PI analysis (Fig. 1A). Because previous studies had shown that NS1 is a negative regulator of alpha/beta interferon (IFN-α/β) signaling (52), we sought to determine whether NS1 induces cell cycle arrest independently of the IFN pathway (53, 54). We performed the same experiments using Vero cells, which lack the genes for IFN-α and IFN-β, and found that pEGFP-NS1 transfection could also induce cell cycle arrest (Fig. 1A). The results suggest that NS1 can induce cell cycle arrest in an IFN pathway-independent manner.
Fig 1.
The NS1 protein of influenza virus A/WSN/33 induces host cell cycle arrest in the G0/G1 phase. (A) A549 and Vero cells were transfected with the pEGFP and pEGFP-NS1 plasmids, respectively. The cells were incubated with 10% FBS-containing medium for 24 h, treated by serum starvation for 24 h, and cultured for 18 h in FBS-containing medium. The cells were harvested and mixed with PI for flow cytometry to determine cell cycle populations. EGFP-positive cells were selected. The results are presented as the means ± the SD of three independent experiments (*, P < 0.05 relative to control group). (B and C) A549 cells were mock infected (mock) or infected with the A/WSN/33 (H1N1) virus (H1N1 virus) or the recombinant H1N1 virus with the nonstructural protein NS1 deletion (NS1− H1N1 virus) at different MOIs. Virus-infected cells were stained with anti-NP antibody, and the infection efficiency was evaluated based on the percentage of NP-positive cells (M2) relative to gated cells (M1). (D) Ratios of virus-infected cells with different MOIs. The results are presented as means ± the SD of three experiments. (E) The relative intensity of NP was analyzed by the ModFit LT program after being monitored by FACS. The results are presented as means ± the SD of three experiments (*, P < 0.05 relative to the control group). (F) Equal amounts (80 μg) of total virus-infected cell lysate fractionated on SDS–12% PAGE gels and immunoblotted with MAbs against the NP and NS1 proteins. The results are presented as means ± the SD of three experiments (ns, no significant difference). (G) Mock-infected A549 cells and NP-positive virus-infected A549 cells were both stained with PI. Cell cycle profiles determined at 24 h postinfection are shown. The results are presented as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group; ns, no significant difference).
To investigate whether the NS1 protein is necessary for cell cycle arrest, we constructed an NS1-deficient virus. We then adjusted the MOI values to ensure that equal amounts of wild-type or NS1-deficient viruses were used. We detected the presence of intracellular NP by flow cytometry across an MOI dose-response curve as a readout of viral replication in cells, and the infection efficiency was evaluated based on the percentage of NP-positive cells (M2; Fig. 1B and C). As shown in Fig. 1D and E, we performed the experiments using A/WSN/33 virus (H1N1) at an MOI of 0.3 and the recombinant H1N1 virus that lacked NS1 (NS1− H1N1) at an MOI of 0.5. Western blotting detected the NP expression level shown in Fig. 1F. As predicted, we found that the NS1-deficient virus lost the ability to induce the G0/G1 cell cycle arrest (Fig. 1G). The data illustrate that the NS1 protein is necessary for influenza A virus-induced G0/G1 cell cycle arrest.
NS1 suppressed the RhoA/pRb signaling pathway.
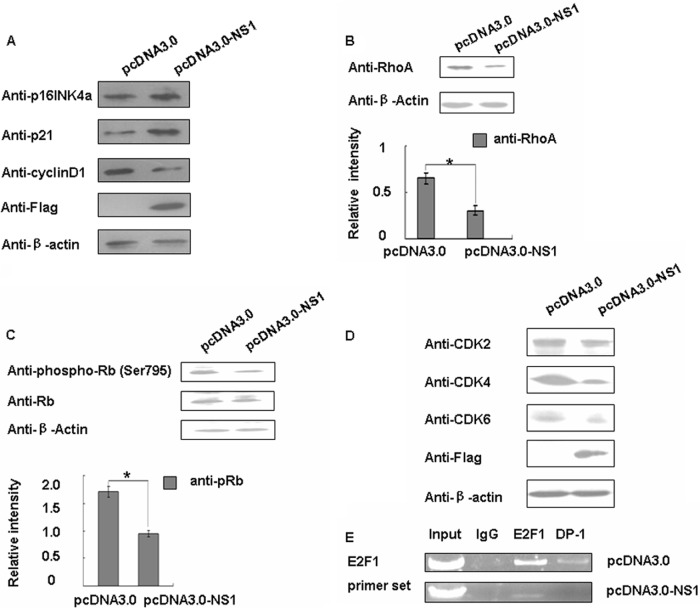
In order to determine the molecular mechanism of NS1-induced cell cycle arrest, we used Western blotting to detect the expression of several G1/S regulators. We also found that p16INK4a, which belongs to the INK4 family, was increased. p21, the cyclin-dependent kinase (CDK) inhibitor 1, was also increased when NS1 was overexpressed, whereas cyclin D1 was decreased (Fig. 2A). Interestingly, they were all involved in the RhoA pathway. Thus, we detected RhoA and its downstream mediator pRb's phosphorylation level (retinoblastoma [Rb], which was identified over a decade ago as the first tumor suppressor). As predicted, we observed that RhoA and the pRb level were both decreased (Fig. 2B and C).
Fig 2.
NS1 suppressed of RhoA/pRb pathway. (A) A549 cells were transfected with pcDNA3.0 and pcDNA3.0-NS1 for 48 h and then analyzed for p21, cyclin D1, and p16INK4a protein expression levels by Western blotting. (B) Western blotting was used to detect the RhoA protein expression level. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (C) Western blotting for Rb and pRb protein. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (D) A549 cells were transfected with pcDNA3.0 and pcDNA3.0-NS1 for 48 h and then analyzed for CDK2, CDK4, and CDK6 protein expression levels by Western blotting. (E) A549 cells were cotransfected with pEZH2 and pcDNA3.0 or pcDNA3.0-NS1 for 48 h and then harvested. Chromatin proteins cross-linked to DNA were immunoprecipitated with IgG, E2F1, and DP-1. DNA that was pulled down was analyzed by PCR using primers that amplified the E2F/DP-1 binding region in the EZH2 promoter.
Since the phosphorylation of Rb is catalyzed by CDK, we assessed CDK2, CDK4, and CDK6 for further validation. We found that CDK4 and CDK6 were both reduced (Fig. 2D). It is known that Rb function depends, at least in part, on interactions with the E2F family of DNA-binding transcription factors (E2F) (55, 56), so we used a ChIP assay to confirm E2F1 and DP-1 binding ability. As shown in Fig. 2E, when we overexpressed the NS1 protein, E2F1 and DP-1 binding ability to its downstream gene was decreased. All of these data confirmed that the NS1 can downregulate RhoA and the pRb expression level and inhibit the RhoA/pRb pathway.
NS1 arrests the cell cycle through inhibition of RhoA.
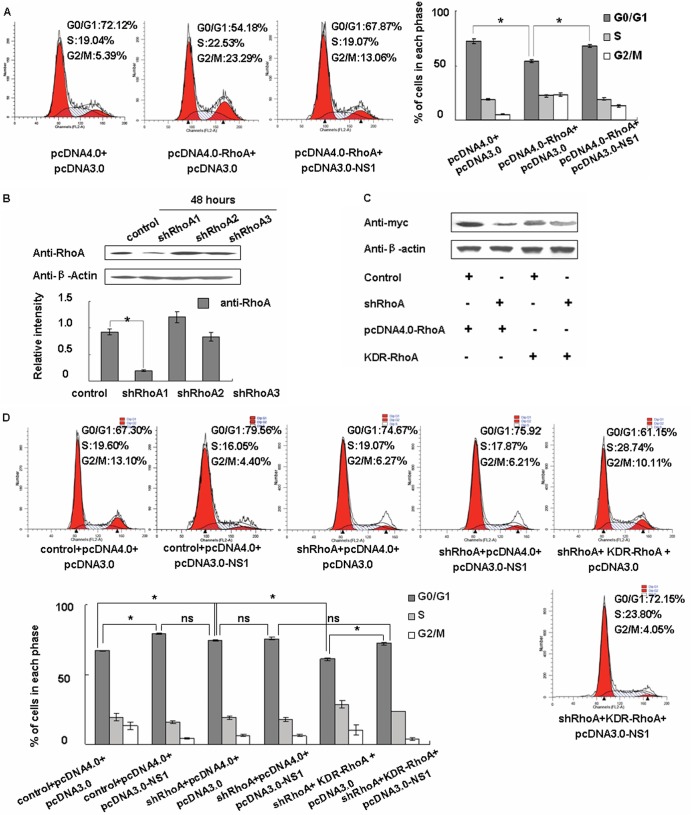
To further determine whether RhoA is the key regulator mediating NS1-induced cell cycle arrest, we compared cotransfection for pcDNA4.0-RhoA and pcDNA3.0 to cotransfection for pcDNA4.0-RhoA and pcDNA3.0-NS1. As shown in Fig. 3A, we found that RhoA can induce G1/S progress and that the G0/G1-phase cells decreased to 54.18%. However, overexpressing NS1 simultaneously can inhibit this progress so that the G0/G1-phase cells only decrease to 67.87%. We then designed shRhoA sequences and, according to the knockdown efficiencies, we chose shRhoA1 as an RhoA interfering sequence for the following experiments (Fig. 3B). After that, we constructed an shRhoA-resistant expression plasmid (KDR-RhoA) which can still be expressed while the shRhoA is active (Fig. 3C).
Fig 3.
NS1 arrests the cell cycle through the RhoA pathway. (A) A549 cells were cotransfected with either pcDNA4.0 or pcDNA4.0-RhoA and either pcDNA3.0 or pcDNA3.0-NS1 as indicated, along with pGFP-H2b as an internal control (the quantity of pGFP-H2b was one-tenth that of each of the test plasmids). The transected cells were incubated with 10% FBS-containing medium for 24 h, treated by serum starvation for 24 h, and cultured for 18 h in FBS-containing medium. The cells were then harvested and mixed with PI for flow cytometry analyses to determine the cell cycle populations. EGFP-positive cells were selected. The data are shown as mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (B) Western blotting was used to select effective shRhoA plasmids. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (C) A549 cells were cotransfected with shRNA control or shRhoA and pcDNA4.0-RhoA or KDR-RhoA as indicated. After 48 h, we used Western blotting to detect the RhoA expression level. (D) A549 cells were cotransfected with shRNA control or shRhoA, pcDNA4.0 or pcDNA4.0-RhoA, and pcDNA3.0 or pcDNA3.0-NS1 as indicated, along with pGFP-H2b as an internal control (the quantity of pGFP-H2b was one-tenth that of each of the test plasmids). The transected cells were incubated with 10% FBS-containing medium for 24 h, treated by serum starvation for 24 h, and cultured for 18 h in FBS-containing medium. The cells were then harvested and mixed with PI for flow cytometry analyses to determine the cell cycle populations. EGFP-positive cells were selected. The data are shown as mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to control group; ns, no significant difference).
We then used shRhoA and KDR-RhoA for further validation. We found that knockdown RhoA can lead to an increase in G0/G1-phase cells (G0/G1, 74.67%) more like that of cells transfected with pcDNA3.0-NS1 (G0/G1, 79.56%). Moreover, cotransfected pcDNA3.0-NS1 with shRhoA cannot increase the percentage of G0/G1-phase cells compared to transfected shRhoA alone (Fig. 3D). After cotransfection of shRhoA and KDR-RhoA, the G0/G1-phase cells can still decrease to 61.15%, which means RhoA rescue will allow the G0/G1 cell cycle to partially recover (Fig. 3D). However, adding pcDNA3.0-NS1 will increase the percentage of G0/G1-phase cells to 72.15% (Fig. 3D). In summary, these results suggest that the viral NS1 protein is able to arrest cells in G0/G1 phase through the inhibition of RhoA.
NS1 can downregulate RhoA expression via inhibition of NF-κB activity.
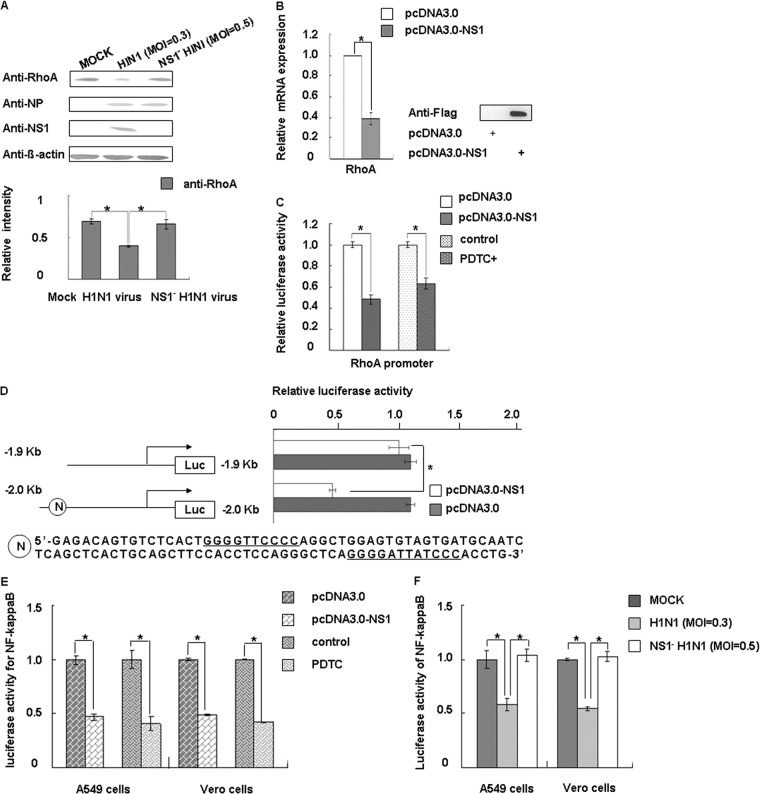
Because RhoA was decreased in cells overexpressing NS1 (Fig. 2B), we infected the cells with viruses for further confirmation. The results were consistent with plasmid transfection (Fig. 4A). To test whether NS1 participates in regulating RhoA mRNA expression, we used real-time PCR to detect the mRNA levels of RhoA. Clearly, ectopically expressed NS1 could decrease RhoA expression (Fig. 4B). We then cloned the 5′-promoter region of human RhoA from bp −2000 upstream of the transcription initiation site for the luciferase assay, and the results indicated that NS1 inhibited RhoA promoter activity (Fig. 4C). Since the region from bp −2000 to bp −1900 contains two putative NF-κB binding sequences, we used the specific NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC; Sigma-Aldrich) to investigate whether NF-κB activity can influence the RhoA promoter (57, 58). We found that that inhibiting NF-κB can downregulate RhoA promoter activity (Fig. 4C).
Fig 4.
NS1 downregulates RhoA protein expression by inhibiting NF-κB activation. (A) A549 cells were mock infected (mock) or infected with virus and, after 24 h postinfection, the cells were collected and analyzed by Western blotting with antibodies as indicated. The data represent mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (B) The expression level of RhoA mRNAs in transfected A549 cells was assessed using real-time PCR after transfection of the relevant plasmids for 24 h. GAPDH mRNA values were used for normalization. The relative mRNA expression of pcDNA3.0 set as 1. The data represent the mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). The expression of Flag-NS1 was monitored by Western blotting with an anti-Flag antibody. (C) Cells were transfected with plasmids or treated with reagents as indicated. pRL-TK-RhoA promoter activity was measured using a dual-luciferase reporter assay system (Promega) after 24 h. hRluc luciferase reporter vector pGL3.0 (Promega) was used as an internal control for transfection efficiency. (D) RhoA promoter and NF-κB binding site deletion sequences were both cloned upstream of luciferase. The candidate NF-κB binding site sequence is shown at the bottom. The cells were harvested at 24 h posttransfection and assayed for luciferase activity. The data represent the mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (E) The A549 and Vero cells were cotransfected with pNF-κB-Luc, pRL-tk (internal control), and the plasmid or reagent as indicated. At 30 h posttransfection, the cells were treated with TNF-α (10 ng/ml) for 4 h, and the luciferase activity was assayed. The data represent the mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (F) A549 cells and Vero cells were cotransfected with pNF-κB-Luc or pRL-tk (internal control) and infected with virus as indicated. Luciferase activity was assayed 24 h later. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group).
We then performed a new luciferase assay by generating another luciferase reporter plasmid from the bp −1900 upstream transcription initiation site of RhoA. Figure 4D shows that NS1 inhibited RhoA promoter activity mainly through the NF-κB sites. In subsequent experiments, we tested the effect of NS1 protein on NF-κB activation using pNF-κB-Luc, a commercial reporter plasmid specifically designed to measure the activation of NF-κB. PDTC was used as a positive control. The NS1 protein decreased the luciferase activity of pNF-κB-Luc in A549, and these results were verified using plasmid transfection and virus infection (Fig. 4E and F). We then conducted experiments with Vero cells, since the IFN antagonistic activity of NS1 cannot be ignored; the results are shown in Fig. 4E and F. We therefore conclude that the decrease in RhoA mRNA levels was mainly caused by downregulation of the NF-κB activity by NS1.
NS1 can downregulate RhoA activity by interacting with RhoA.
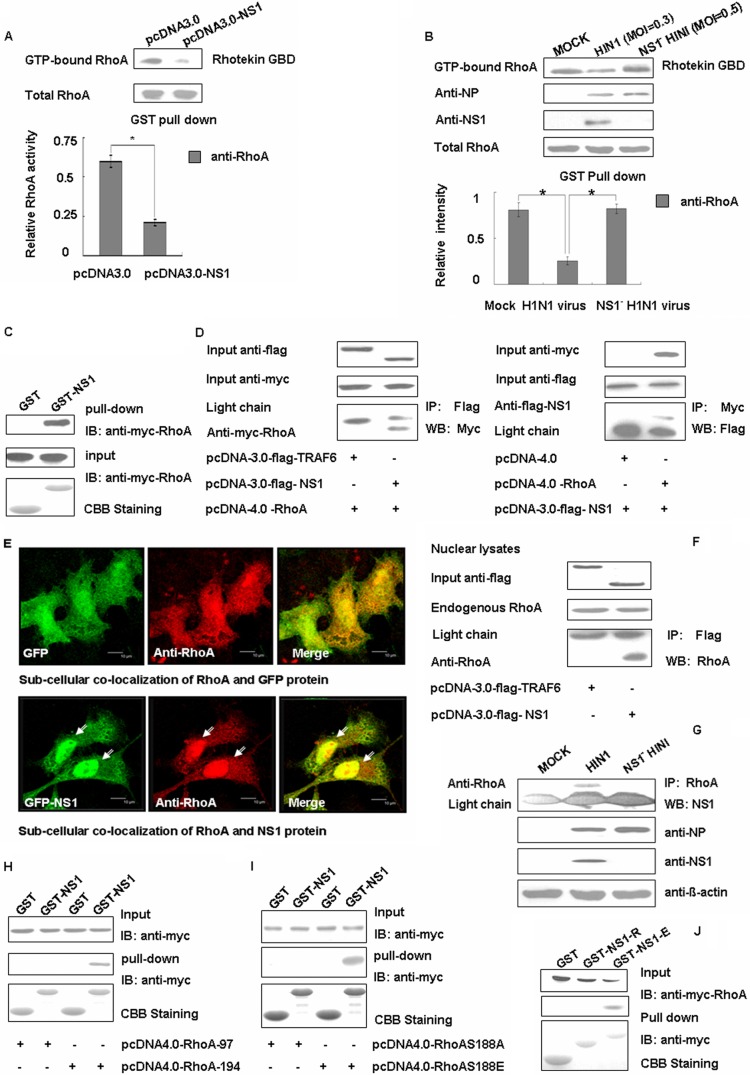
In addition to the regulation of RhoA expression levels, we sought to determine whether NS1 also interfered with the function of RhoA. We were able to observe an obvious decrease in the GTP-bound RhoA in the presence of NS1 using a Rhotekin-pulldown assay (Fig. 5A). This phenomenon was also observed during viral infection (Fig. 5B). The results suggest that the NS1 protein of influenza virus A not only represses the expression of RhoA but also inhibits its activity. As shown by the GST-pulldown assay (Fig. 5C), the NS1 protein was able to directly bind to RhoA, and this was further confirmed in transfected cells using coimmunoprecipitation (Fig. 5D). Several studies have indicated that RhoA interferes with the cell cycle in the nucleus, so we used immunofluorescence and immunoprecipitation experiments to determine whether NS1 and RhoA can interact in the nucleus. In Fig. 5E, we can see that after transfection with pGFP, the RhoA protein was uniformly distributed. However, when transfected with pGFP-NS1, the RhoA protein is highly expressed in the nucleus and overlaps with the NS1 protein. This may partially mean that the NS1 protein can recruit more RhoA protein in the nucleus. An immunoprecipitation experiment was performed and further confirmed this speculation (Fig. 5F). To determine whether these proteins interacted during actual influenza A virus infection, we mimicked true infection by infecting A549 cells with A/WSN/33 and found that the NS1 protein was clearly detectable in RhoA antibody immunoprecipitation complexes. This finding indicated that the NS1 protein interacts with RhoA even during influenza A virus infection (Fig. 5G).
Fig 5.
NS1 can downregulate RhoA activity by direct interaction with RhoA protein. (A) Transfected A549 cell lysates were incubated with equal amounts of GST-Rhotekin bound to glutathione-Sepharose 4B beads. After washing, the bound proteins were analyzed by Western blotting with an anti-RhoA antibody. The data represent the mean fold changes ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (B) A549 cells were mock infected (mock) or infected with virus for 24 h, and then the cells were collected and analyzed by Western blotting with antibodies as indicated. At the same time, the cell lysates were incubated with equal amounts of GST-Rhotekin bound to glutathione-Sepharose 4B beads. After washing, the bound proteins were analyzed by Western blotting with an anti-RhoA antibody. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (C) Whole 293T cell lysates transfected with Myc-tagged RhoA were subjected to GST pulldown, followed by immunoblotting (IB) with the Myc antibody. CBB, Coomassie brilliant blue staining. (D) The 293T cells were cotransfected with Myc-tagged RhoA and either pcDNA3.0-TRAF6 or pcDNA3.0-NS1 and with Flag-tagged NS1 and either pcDNA4.0 or pcDNA4.0-RhoA. Flag antibodies were used with the cell lysates for immunoprecipitation (IP), followed by Western blotting (WB) with Myc, or for immunoprecipitation with Myc, followed by Western blotting with Flag. (E) A549 cells were transfected with pEGFP-N1 and pEGFPN1-NS1 for 24 h and then fixed, permeabilized, and stained for RhoA (red). Yellow indicates overlap. Scale bar, 10 μm. (F) The 293T cells were transfected with Flag-tagged TRAF6 and Flag-tagged NS1, separately. Then, the cell nuclear lysates were subjected to immunoprecipitation with Flag antibody, followed by Western blotting with RhoA antibody. (G) A549 cells were mock infected (mock) or infected with virus for 24 h, and the cells were subjected to immunoprecipitation with NS1 antibody, followed by Western blotting with RhoA. (H) Whole 293T cell lysates transfected with Myc-tagged RhoA97 and RhoA194 were subjected to GST pulldown, followed by immunoblotting with Myc antibody. (I) Whole 293T cell lysates transfected with Myc-tagged RhoAS188A and RhoAS188E were subjected to GST pulldown, followed by immunoblotting with Myc antibody. (J) Myc-tagged RhoA-transfected 293T cell lysates were incubated with equal amounts of GST, GST-NS1-R (N terminal, 1 to 73 aa), or GST-NS1-E (C terminal, 73 to 230 aa). The bound proteins were analyzed using Myc antibody.
To determine how NS1 and RhoA directly interact, we further characterized the domains on NS1 required for NS1-RhoA interaction. GST fusion constructs of the NS1 protein and its deletion mutants GST-NS1-R (N terminal, 1 to 73 aa) and GST-NS1-E (C terminal, 73 to 230 aa) were generated for the pulldown assays. We found that NS1 interacted with RhoA via its C-terminal domain (Fig. 5J). To understand the corresponding binding sites on RhoA, we also constructed mutants containing RhoA97 ((N terminal, RhoA, aa 1 to 97) and RhoA194 (C terminal, RhoA, aa 98 to 194). When we found that the C-terminal domain of RhoA can bind the NS1 directly (Fig. 5H), we assayed two mutants of the RhoA Ser188 constructs (RhoA S188A [RhoA S188 site mutated to A] and RhoA S188E [RhoA S188 site mutated to E]) since the phosphorylation on Ser188 of RhoA is important for cell cycle transition. We found that NS1 cannot combine with RhoA S188A, which indicates that position 188 is crucial for the binding of RhoA and NS1. Mutation of position 188 in RhoA from S back to E restored the binding of RhoA to NS1 (Fig. 5I). Thus, our data showed that the key site for RhoA binding of NS1 was S188. Collectively, our results indicate that the C-terminal effector domain of NS1 can physically interact with RhoA at its S188 site.
NS1 promotes viral protein accumulation in G0/G1-phase synchronized cells that correlates with enhanced viral replication.
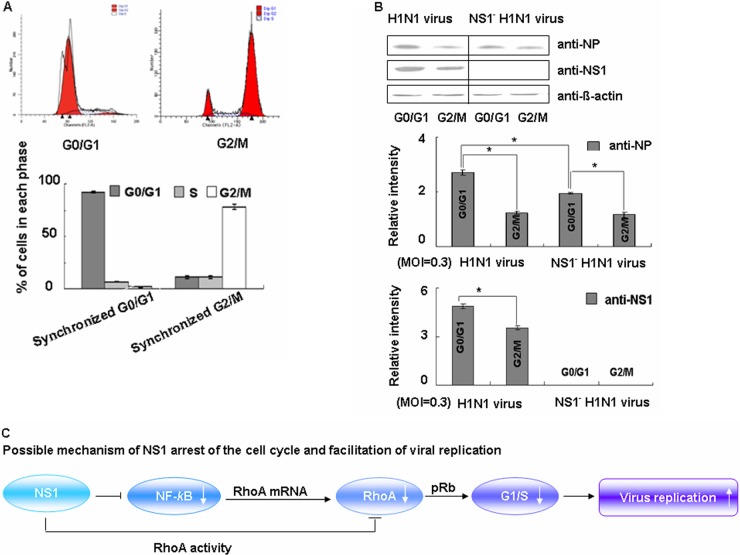
To determine whether G0/G1-phase arrest will benefit the viral replication, we next compared the expression levels of viral NP and NS1 proteins in G0/G1-phase and G2/M-phase synchronized cells. As shown in Fig. 6A, 92.7% of the cells were successfully blocked in G0/G1 phase, whereas 88.95% of the cells were in the G2/M phase. All cells were infected at the same time with the wild-type or NS1 mutant viruses at a low MOI of 0.3. At 24 h after infection, the infected cell lysates were harvested individually, and the expression levels of NP and NS1 were evaluated by Western blotting.
Fig 6.
NS1 promotes viral protein accumulation in G0/G1-phase synchronized cells. (A) Synchronized A549 cells were treated by serum starvation for 48 h or with nocodazole for 16 h and detected by FACS analysis. Cell cycle profiles before infection were determined by FACS analysis. (B) The synchronized cells were infected with wild-type virus or NS1-deficient virus at an MOI of 0.3 for 1 h. After infection, the medium was restored to maintain cell cycle synchronization, and 24 h later the cells were harvested and analyzed by Western blotting. The data are from one of three independent experiments. The results are presented as means ± the SD of three experiments (*, P < 0.05 relative to the control group). (C) Our proposed model showing how NS1 arrests the host cell cycle in G0/G1 via inhibition of RhoA.
Upon comparing the expression levels of both viral proteins between the G0/G1 and G2/M phases in wild-type and NS1-deficient virus-infected cells, we found that the expression levels of both viral proteins were significantly higher in G0/G1-phase synchronized cells than in G2/M-phase synchronized cells (Fig. 6B). Upon comparing the levels viral NP in the G0/G1 phase between wild-type and NS1-deficient cells, we found that NS1-deficient cells had a significantly lower percentage of accumulated NP. These results suggested that viral proteins preferentially accumulated in the G0/G1 phase of the cell cycle and that this accumulation was at least in part dependent on the presence of virus-encoded NS1. Our experiments indicate that the NS1 protein arrests the host cell cycle at G0/G1 via inhibition of RhoA, therefore facilitating viral replication (Fig. 6C).
The NS1 protein of avian influenza virus H5N1 also negatively regulates RhoA.
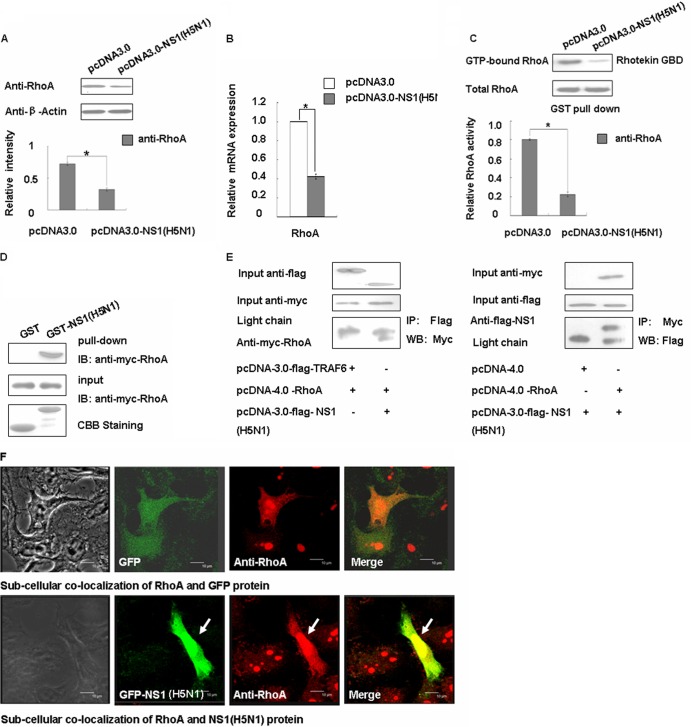
We sought to determine whether any other influenza virus NS1 proteins could arrest the cell cycle using the same mechanism as the laboratory strain of WSN, and thus we repeated the experiment using the NS1 protein of H5N1 virus. As predicted, the NS1 protein of the H5N1 can downregulate the RhoA protein and inhibit its activity (Fig. 7A and C). We then determined the internal mechanisms behind these changes using RT-PCR, GST-pulldown, and coimmunoprecipitation methods (Fig. 7B, D, and E). We found that H5N1 NS1 could decrease RhoA mRNA expression levels and interact with RhoA directly. We then used immunofluorescence experiments to further confirm their localization. As shown in Fig. 7F, we can conclude that the RhoA protein was colocalized with the NS1 protein and mainly concentrated in the nucleus as H1N1 NS1 protein, suggesting that the H5N1 virus may affect the cell cycle through the same mechanism.
Fig 7.
The NS1 protein of influenza virus H5N1 can negatively regulate RhoA. (A) A549 cells were transfected with pcDNA3.0 and pcDNA3.0-NS1 (H5N1) for 48 h and then analyzed for RhoA expression levels by Western blotting. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (B) The mRNA levels of transfected A549 cells were assessed using real-time PCR after transfection with the relative plasmids for 24 h. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (C) Transfected A549 cell lysates were incubated with equal amounts of GST-Rhotekin bound to glutathione-Sepharose 4B beads. After washing, the bound proteins were analyzed by Western blotting with an anti-RhoA antibody. The data are shown as means ± the SD of three independent experiments (*, P < 0.05 relative to the control group). (D) Whole 293T cell lysates transfected with Myc-tagged RhoA were subjected to GST pulldown, followed by immunoblotting with Myc antibody. (E) 293T cells were cotransfected with Myc-tagged RhoA and either pcDNA3.0-TRAF6 or pcDNA3.0-NS1 (H5N1) and with Flag-tagged NS1 (H5N1) and either pcDNA4.0 or pcDNA4.0-RhoA. The cell lysates were subjected to immunoprecipitation with the Flag antibody, followed by Western blotting with Myc, or to immunoprecipitation with Myc, followed by Western blotting with Flag. (F) A549 cells were transfected with pEGFP and pEGFP-NS1 (H5N1) for 24 h and then fixed, permeabilized, and stained for RhoA (red). Yellow indicates overlap. Scale bar, 10 μm.
DISCUSSION
Several influenza virus strains, including H1N1, H3N2, and H9N2 viruses, have been shown to cause infected cells to accumulate in G0/G1 phase to different degrees (10). However, the corresponding viral protein and downstream signaling pathway responsible for this process has been unclear. In the present study, we used influenza virus A/WSN/33 (H1N1) to determine this mechanism and found that the NS1 protein of WSN and the cell cycle regulator RhoA are necessary for host cell cycle arrest.
First, we used viral plasmids to transfect cells and found that the NS1 protein can cause cell cycle arrest in G0/G1. Next, we used reverse genetic techniques to construct an NS1-deficient virus for further verification, and the results were consistent. We showed that NS1 arrested the cell cycle by inhibiting the RhoA/pRb pathway through the downregulation of RhoA protein expression and direct interaction. Then, since some researchers have reported that the G0/G1 cell cycle position is presumably favorable for viral DNA replication (59, 60), we synchronized the cell cycle in the G0/G1 phase and the G2/M phase for comparison. The results showed that the NP protein of both kinds of virus exhibited a high expression level in G0/G1 phase. Finally, we know that a number of NS1 proteins from avian influenza viruses, together with those of all human, swine, and equine influenza viruses, are described as allele A NS1 proteins, whereas those of allele B are exclusively from avian viruses. The level of homology within each allele is 93 to 100%, and between alleles it can be as little as 62%. However, the majority of highly pathogenic avian influenza viruses isolated from humans have contained an allele A NS1 protein (16). Based on this, we assessed the H5N1 NS1 protein and found that it also could decrease RhoA expression and activity, suggesting that the H5N1 virus may affect the cell cycle through the same mechanism.
In our study, since type I IFN signaling leads to cell cycle arrest (20, 21), an indirect effect due to NS1-dependent inhibition of type I IFN needed to be assessed. Thus, we used Vero cells, which lack the IFN-α and IFN-β genes, as a control. The ability of NS1 to prevent normal cell cycle progression and to promote cell cycle arrest in G0/G1 was not fully dependent on the IFN pathway, which expands the role of viral NS1 in creating optimal host conditions for the survival and replication of the influenza virus by subverting normal mechanisms.
We detected the expression of several regulators of the G1/S cell cycle transition and found they were all involved in the RhoA pathway. Thus we examined the phosphorylation levels of RhoA and its downstream mediator pRb. As predicted, we observed that RhoA and pRb levels were both decreased in cells that overexpressed NS1. It is known that the inhibition of RhoA results in the decrease of CDK and the accumulation of the CDK inhibitor, which in turn causes G1/S cell cycle arrest (61). Our experiments showed that the CDK4 and CDK6 were both decreased and that the CDK inhibitor p16INK4a was in creased. At the same time, ChIP assays also confirmed a decrease in pRb. In addition, we demonstrated that the NS1 can recruit more RhoA protein in the nucleus and overlap in the nucleus, as well as in the cytoplasm. There is evidence that RhoA and some of the proteins involved in the RhoA signaling pathways, including RhoA-GAP DLC1, p190 RhoGAP, and the RhoA effectors ROCK II/ROKα, LIMK, and mDia2, are present in the nucleus (62, 63). Moreover, RhoA exists in the nucleus in a biologically active GTP-bound form (64). These phenomena reaffirm our observation that NS1 can interact with RhoA in either the cytoplasm or the nucleus and that their interaction in the nucleus may have special functions during cell cycle arrest.
Consistent with our previous observations, we uncovered a signaling pathway linking the NS1 protein to cell cycle arrest in the G0/G1 phase. This NS1/RhoA/pRb pathway offers new insights that could provide a unified explanation for the seemingly different NS1 functions involved in viral replication events. Our discovery provides avenues for further study of viral replication mechanisms and demonstrates the importance of the NS1 protein in regulating the host cell response.
ACKNOWLEDGMENTS
This study was supported by grants from the National Basic Research Program of China (973 Program; grants 2012CB518900, 2011CB504706, 2011CB504805, and 2010CB912201), the National Natural Science Foundation of China (grants 30900759, 30973448, and 81171572), and the Guangdong Innovative Research Team Program (grant 2009010058).
Footnotes
Published ahead of print 2 January 2013
REFERENCES
- 1. Chen AY, Qiu J. 2010. Parvovirus infection-induced cell death and cell cycle arrest. Future Virol. 5:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katz RA, Greger JG, Skalka AM. 2005. Effects of cell cycle status on early events in retroviral replication. J. Cell. Biochem. 94:880–889 [DOI] [PubMed] [Google Scholar]
- 3. Song B, Liu JJ, Yeh KC, Knipe DM. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326–334 [DOI] [PubMed] [Google Scholar]
- 4. Yuan X, Yao Z, Wu J, Zhou Y, Shan Y, Dong B, Zhao Z, Hua P, Chen J, Cong Y. 2007. G1 phase cell cycle arrest induced by SARS-CoV 3a protein via the cyclin D3/pRb pathway. Am. J. Respir. Cell Mol. Biol. 37:9–19 [DOI] [PubMed] [Google Scholar]
- 5. Castillo JP, Kowalik TF. 2004. HCMV infection: modulating the cell cycle and cell death. Int. Rev. Immunol. 23:113–139 [DOI] [PubMed] [Google Scholar]
- 6. Kalejta RF, Shenk T. 2003. The human cytomegalovirus UL82 gene product (pp71) accelerates progression through the G1 phase of the cell cycle. J. Virol. 77:3451–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rappole JH, Hubalek Z. 2006. Birds and influenza H5NI virus movement to and within North America. Emerg. Infect. Dis. 12:1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Webster RG. 2004. Wet markets: a continuing source of severe acute respiratory syndrome and influenza? Lancet 363:234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He Y, Xu K, Keiner B, Zhou J, Czudai V, Li T, Chen Z, Liu J, Klenk HD, Shu YL, Sun B. 2010. Influenza A virus replication induces cell cycle arrest in G0/G1 phase. J. Virol. 84:12832–12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terrier O, Josset L, Textoris J, Marcel V, Cartet G, Ferraris O, N′Guyen C, Lina B, Diaz JJ, Bourdon JC, Rosa-Calatrava M. 2011. Cellular transcriptional profiling in human lung epithelial cells infected by different subtypes of influenza A viruses reveals an overall downregulation of the host p53 pathway. Virol. J. 8:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fitzgerald DA. 2009. Human swine influenza A [H1N1]: practical advice for clinicians early in the pandemic. Paediatr. Respir. Rev. 10:154–158 [DOI] [PubMed] [Google Scholar]
- 13. Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw ML, Stone KL, Colangelo CM, Gulcicek EE, Palese P. 2008. Cellular proteins in influenza virus particles. PLoS Pathog. 4:e1000085 doi:10.1371/journal.ppat.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birch-Machin I, Rowan A, Pick J, Mumford J, Binns M. 1997. Expression of the nonstructural protein NS1 of equine influenza A virus: detection of anti-NS1 antibody in postinfection equine sera. J. Virol. Methods 65:255–263 [DOI] [PubMed] [Google Scholar]
- 16. Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89:2359–2376 [DOI] [PubMed] [Google Scholar]
- 17. Chien CY, Tejero R, Huang Y, Zimmerman DE, Rios CB, Krug RM, Montelione GT. 1997. A novel RNA-binding motif in influenza A virus nonstructural protein 1. Nat. Struct. Biol. 4:891–895 [DOI] [PubMed] [Google Scholar]
- 18. Qian XY, Alonso-Caplen F, Krug RM. 1994. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J. Virol. 68:2433–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Das P, Schmolke M, Manicassamy B, Wang Y, Deng X, Cai L, Tu BP, Forst CV, Roth MG, Levy DE, Garcia-Sastre A, de Brabander J, Phillips MA, Fontoura BM. 2012. Inhibition of pyrimidine synthesis reverses viral virulence factor-mediated block of mRNA nuclear export. J. Cell Biol. 196:315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80:6295–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geiss GK, Salvatore M, Tumpey TM, Carter VS, Wang X, Basler CF, Taubenberger JK, Bumgarner RE, Palese P, Katze MG, Garcia-Sastre A. 2002. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl. Acad. Sci. U. S. A. 99:10736–10741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayman A, Comely S, Lackenby A, Hartgroves LC, Goodbourn S, McCauley JW, Barclay WS. 2007. NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response. J. Virol. 81:2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kochs G, Garcia-Sastre A, Martinez-Sobrido L. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 81:7011–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu W, Tran KC, Teng MN, Heesom KJ, Matthews DA, Barr JN, Hiscox JA. 2012. The interactome of the human respiratory syncytial virus NS1 protein highlights multiple effects on host cell biology. J. Virol. 86:7777–7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Op De Beeck A, Caillet-Fauquet P. 1997. The NS1 protein of the autonomous parvovirus minute virus of mice blocks cellular DNA replication: a consequence of lesions to the chromatin? J. Virol. 71:5323–5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietenpol JA, Stewart ZA. 2002. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 181–182:475–481 [DOI] [PubMed] [Google Scholar]
- 29. Alcami A. 2010. The interaction of viruses with host immune defenses. Curr. Opin. Microbiol. 13:501–502 [DOI] [PubMed] [Google Scholar]
- 30. Randow F, Lehner PJ. 2009. Viral avoidance and exploitation of the ubiquitin system. Nat. Cell Biol. 11:527–534 [DOI] [PubMed] [Google Scholar]
- 31. Park MT, Lee SJ. 2003. Cell cycle and cancer. J. Biochem. Mol. Biol. 36:60–65 [DOI] [PubMed] [Google Scholar]
- 32. Sa G, Das T. 2008. Anti-cancer effects of curcumin: cycle of life and death. Cell Div. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartek J, Lukas J. 2001. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 13:738–747 [DOI] [PubMed] [Google Scholar]
- 34. Johnson DG, Walker CL. 1999. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 39:295–312 [DOI] [PubMed] [Google Scholar]
- 35. Burridge K, Wennerberg K. 2004. Rho and Rac take center stage. Cell 116:167–179 [DOI] [PubMed] [Google Scholar]
- 36. Cachero TG, Morielli AD, Peralta EG. 1998. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell 93:1077–1085 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F. 2009. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol. Cell 35:841–855 [DOI] [PubMed] [Google Scholar]
- 38. Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M, Liao Z, Li Z, Luo D, Shi F, Zheng Y, Bi F. 2009. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol. Cancer Res. 7:570–580 [DOI] [PubMed] [Google Scholar]
- 39. Ellerbroek SM, Wennerberg K, Burridge K. 2003. Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 278:19023–19031 [DOI] [PubMed] [Google Scholar]
- 40. Nusser N, Gosmanova E, Makarova N, Fujiwara Y, Yang L, Guo F, Luo Y, Zheng Y, Tigyi G. 2006. Serine phosphorylation differentially affects RhoA binding to effectors: implications to NGF-induced neurite outgrowth. Cell Signal. 18:704–714 [DOI] [PubMed] [Google Scholar]
- 41. Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P. 2005. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ. Res. 96:1152–1160 [DOI] [PubMed] [Google Scholar]
- 42. Croft DR, Olson MF. 2006. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol. Cell. Biol. 26:4612–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. 1998. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 18:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H, Ung CY, Ma XH, Li BW, Low BC, Cao ZW, Chen YZ. 2009. Simulation of crosstalk between small GTPase RhoA and EGFR-ERK signaling pathway via MEKK1. Bioinformatics 25:358–364 [DOI] [PubMed] [Google Scholar]
- 45. Weber JD, Hu W, Jefcoat SC, Jr, Raben DM, Baldassare JJ. 1997. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J. Biol. Chem. 272:32966–32971 [DOI] [PubMed] [Google Scholar]
- 46. Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U. S. A. 96:9345–9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao S, Song L, Li J, Zhang Z, Peng H, Jiang W, Wang Q, Kang T, Chen S, Huang W. 2012. Influenza A virus-encoded NS1 virulence factor protein inhibits innate immune response by targeting IKK. Cell Microbiol. 14:1849–1866 [DOI] [PubMed] [Google Scholar]
- 48. Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330 [DOI] [PubMed] [Google Scholar]
- 49. Bourmakina SV, Garcia-Sastre A. 2005. The morphology and composition of influenza A virus particles are not affected by low levels of M1 and M2 proteins in infected cells. J. Virol. 79:7926–7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bohrer LR, Chen S, Hallstrom TC, Huang H. 2010. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology 151:5136–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Op De Beeck A, Sobczak-Thepot J, Sirma H, Bourgain F, Brechot C, Caillet-Fauquet P. 2001. NS1- and minute virus of mice-induced cell cycle arrest: involvement of p53 and p21Cip1. J. Virol. 75:11071–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jia D, Rahbar R, Chan RW, Lee SM, Chan MC, Wang BX, Baker DP, Sun B, Peiris JS, Nicholls JM, Fish EN. 2010. Influenza virus nonstructural protein 1 (NS1) disrupts interferon signaling. PLoS One 5:e13927 doi:10.1371/journal.pone.0013927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balkwill F, Taylor-Papadimitriou J. 1978. Interferon affects both G1 and S+G2 in cells stimulated from quiescence to growth. Nature 274:798–800 [DOI] [PubMed] [Google Scholar]
- 54. Creasey AA, Bartholomew JC, Merigan TC. 1980. Role of G0-G1 arrest in the inhibition of tumor cell growth by interferon. Proc. Natl. Acad. Sci. U. S. A. 77:1471–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. 1991. The E2F transcription factor is a cellular target for the RB protein. Cell 65:1053–1061 [DOI] [PubMed] [Google Scholar]
- 56. Dyson N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245–2262 [DOI] [PubMed] [Google Scholar]
- 57. Bessho R, Matsubara K, Kubota M, Kuwakado K, Hirota H, Wakazono Y, Lin YW, Okuda A, Kawai M, Nishikomori R. 1994. Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor κB (NF-κB) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem. Pharmacol. 48:1883–1889 [DOI] [PubMed] [Google Scholar]
- 58. Ziegler-Heitbrock HW, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, Schreck R, Bauerle P, Strobel M. 1993. Pyrrolidine dithiocarbamate inhibits NF-κB mobilization and TNF production in human monocytes. J. Immunol. 151:6986–6993 [PubMed] [Google Scholar]
- 59. Kalejta RF, Shenk T. 2002. Manipulation of the cell cycle by human cytomegalovirus. Front. Biosci. 7:d295–d306 [DOI] [PubMed] [Google Scholar]
- 60. Kalejta RF, Shenk T. 2003. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. U. S. A. 100:3263–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Coleman ML, Marshall CJ, Olson MF. 2004. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell. Biol. 5:355–366 [DOI] [PubMed] [Google Scholar]
- 62. Dubash AD, Guilluy C, Srougi MC, Boulter E, Burridge K, Garcia-Mata R. 2011. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS One 6:e17380 doi:10.1371/journal.pone.0017380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morin P, Flors C, Olson MF. 2009. Constitutively active RhoA inhibits proliferation by retarding G1 to S phase cell cycle progression and impairing cytokinesis. Eur. J. Cell Biol. 88:495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li Y, Chen Y, Tao Y, Xu J, Chen M. 2010. RhoA protein is generally distributed in the nuclei of cancer cells. Oncol. Rep. 24:1005–1009 [DOI] [PubMed] [Google Scholar]