Abstract
Filoviruses are the cause of severe hemorrhagic fever in human and nonhuman primates. The envelope glycoprotein (GP), responsible for both receptor binding and fusion of the virus envelope with the host cell membrane, has been demonstrated to interact with multiple molecules in order to enhance entry into host cells. Here we have demonstrated that filoviruses utilize glycosaminoglycans, and more specifically heparan sulfate proteoglycans, for their attachment to host cells. This interaction is mediated by GP and does not require the presence of the mucin domain. Both the degree of sulfation and the structure of the carbohydrate backbone play a role in the interaction with filovirus GPs. This new step of filovirus interaction with host cells can potentially be a new target for antiviral strategies. As such, we were able to inhibit filovirus GP-mediated infection using carrageenan, a broad-spectrum microbicide that mimics heparin, and also using the antiviral dendrimeric peptide SB105-A10, which interacts with heparan sulfate, antagonizing the binding of the virus to cells.
INTRODUCTION
Filoviruses are among the most virulent pathogens that affect humans, causing viral hemorrhagic fever (1). The family is comprised of the Marburgvirus (MARV) genus, with various strains of Lake Victoria MARV, and the Ebolavirus genus, with 5 species, Sudan ebolavirus (SUDV), Zaire ebolavirus (EBOV), Taï Forest ebolavirus (TAFV), Reston ebolavirus (RESTV), and Bundibugyo ebolavirus (BEBOV). More recently, a new genus has been proposed after the identification of the provisionally named Lloviu virus (LLOV) in dead insectivorous bats found in caves in France, Spain, and Portugal in 2002 (2). LLOV is genetically distinct, and pathogenicity in humans is not yet established. SUDV and EBOV, as well as MARV, have caused several large epidemics of severe hemorrhagic fever in humans, while TAFV has so far infected one person, who recovered. The most recently identified species, BEBOV, caused an outbreak in 2007 with 131 cases and a 37% case fatality rate. RESTV, however, appears to be nonpathogenic in humans, although it can cause lethal illness in nonhuman primates. Virus entry and attachment are mediated by a single envelope glycoprotein (GP) that contains the receptor-binding domain (RBD) and acts as a class I fusion protein (3, 4). The mucin-like (muc) domain within GP contains multiple sites of N- and O-linked glycosylation and has been linked to virus attachment to the human macrophage galactose- and N-acetylgalactosamine-specific C-type lectin (hMGL) (5) enhancing infection of target cells. This domain is responsible for loss of cell adherence in culture (6), and its removal has been shown to increase pseudotyped virus infectivity (3). Filoviruses infect and kill a wide range of host cells, and several cell surface proteins have been reported to be virus receptors or attachment factors, including C-type lectins (5, 7–9), β1 integrin adhesion receptors (10), and folate receptor alpha (FR-α) (11). T-cell immunoglobulin and mucin domain 1 (TIM-1) (12) has been shown to interact directly with the RBD at the cell surface, although this molecule is not present on all permissive cells. Taken together, these findings suggest that filoviruses utilize a combination of attachment and receptor molecules to enter cells, resulting in their broad host cell range. Following attachment, virus is internalized via macropinocytosis (13, 14) and translocated to cellular compartments, where GP is cleaved by endosomal proteases, predominantly cathepsin B (15). Subsequent to proteolysis, exposure of the RBD allows GP to specifically interact with Niemann-Pick C1 (NPC1), a cholesterol transporter present in late endosomal membranes (16–18).
Proteoglycans are widely distributed molecules, consisting of a transmembrane protein linked to sulfated glycosaminoglycans (GAGs). GAGs, typified by heparan sulfate (HS), are long (20 to 70 monosaccharides), unbranched, and negatively charged polysaccharides that are present on the surfaces of nearly all cells but are heterogeneous with respect to their compositions and quantities between different species, cell types, tissues, and developmental stages. The carbohydrate in cell surface proteoglycans is exploited by a number of viruses for cell surface attachment (19). Viruses using GAGs include the herpes simplex viruses (HSV) (20, 21), human papillomavirus (HPV) (22), cytomegalovirus (CMV) (23), vaccinia virus (24), Sindbis virus (25), respiratory syncytial virus (RSV) (26, 27), noroviruses (28), adeno-associated virus (29), dengue virus (30), hepatitis C virus (31), human immunodeficiency virus type 1 (HIV-1) (32), and human T-cell leukemia virus (33). In the majority of cases, virus-cell interactions use proteoglycans, mainly heparan sulfate proteoglycans (HSPG), as initial attachment factors for the viral envelope, although sometimes proteoglycans can act as fusion receptors. Often these virus-carbohydrate interactions are involved in directing specific tissue tropism.
In the present study, the possible role of GAGs in filovirus GP-mediated infection was examined. For this, we analyzed direct binding, inhibition by soluble GAGs and GAG analogs, and enzymatic removal of the GAGs from the cell surface, as well as inhibition by GAG-binding peptides.
MATERIALS AND METHODS
Cells and cell culture.
The adherent cell lines 293T, Vero, Huh7, and Huh7.5 were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (15 U/ml). Human endothelial cells (Clonetics, Hopkinton, MA) were cultured in Clonetics endothelial cell basal medium. Human primary monocyte-derived macrophages were isolated as previously described (34) and cultured in RPMI supplemented with 10% human serum and penicillin-streptomycin (15 U/ml).
Fetal livers were obtained from elective abortions with the approval of the Committee for Human Research at the University of California, San Francisco, and with the consent of the women undergoing the procedure. Midgestation specimens were used, with the gestational age of the tissues estimated based on the foot length of the fetus. Fetal liver parenchymal cells were isolated by enzymatic digestion and depletion of erythrocytes as previously described (35).
Plasmids.
Mammalian expression plasmids for EBOV, EBOV Δmuc, RESTV, TAFV, and SUDV GPs have been described previously (6). cDNA encoding the MARV Ravn GP was amplified by PCR utilizing the forward primer 5′ TAGAATTCACCATGAAGACCATATATTTTCTGATTAGTCTC3′, including EcoRI (in bold), the Kozac sequence (in italics), and ATG (in italics) and the reverse primer 5′TAGCTAGCTCATCCAATGTATTTAGTGAAGATACG3′, including NheI (in bold) and a stop codon, and subcloned into the mammalian expression plasmid pCAGGS (36) using EcoRI and NheI.
Pseudotype and VLP production.
Lentiviral pseudotypes harboring a heterologous GP were produced essentially as described previously (37) by using 10 μg of the HIV-based luciferase vector (pNL-luc, based on pNL3-4-R-E-) (38) and 30 μg of plasmid-encoding viral envelope. Virions were subjected to ultracentrifuge concentration at 28,000 rpm in an SW28 rotor (Beckman) through a 20% sucrose cushion for 1.5 h at 4°C. The pellets were resuspended overnight in Hanks balanced salt solution (HBSS) at 4°C. Virus-like particles (VLPs) were produced by CaCl2 transfection of 293T cells with a 2:1 ratio of Bla-VP40 and the plasmids encoding the viral envelope, as described in reference 9.
Heparin-Sepharose binding.
HiTrap heparin HP-coated Sepharose beads from 1-ml columns (mean particle size of 34 μm; GE Healthcare) were taken out of the column and washed twice in phosphate-buffered saline (PBS) and once in binding buffer (10 mM sodium phosphate, pH 7). Washed beads (50 μl) were then added to 20 μl of the different concentrated pNluc pseudotypes and incubated with mixing for 10 min. The beads were washed three times in binding buffer, and then 100 μl of elution buffer (10 mM sodium phosphate [pH 7] and 2 M NaCl) was added and incubated with mixing for 10 min. Supernatant was removed to another tube. Eight microliters of the same concentrated pNluc pseudotypes and 40 μl of the eluate supernatants were inactivated with 10 μl of 0.1% Triton in PBS. Sample buffer and reducing agent were added, and the samples were analyzed by SDS-PAGE using a 10% Bis-Tris gel (Invitrogen). The separated proteins were transferred to a nitrocellulose membrane and blocked with 2% powdered milk in Tris-buffered saline (TBS) for 30 min. at room temperature. The membrane was then incubated in TBS–0.05% Tween 20 with a 1:200 dilution of anti-p24 mouse sera to detect lentivirus-associated HIV p24 for 1 h at room temperature. After 3 washes, 10 min each, with TBS–0.05% Tween 20, the membrane was incubated with a 1:5,000 dilution of peroxidase-conjugated goat antimouse antibody (Thermo Scientific, Fremont, CA) for 1 h at room temperature. The membrane was then washed with TBS–0.05% Tween 20 three times for 10 min each, and immunoreactive proteins were visualized using an ImageQuant LAS 400 imager (GE Healthcare) after development with a Pierce ECL Plus substrate kit (Thermo Scientific).
Inhibition of infection by soluble glycosaminoglycans and by carrageenan.
Cells were seeded at 2 × 104 in 96-well plates 24 h prior to infection. Medium was removed, and the cells were incubated with the appropriate amounts of viral supernatant in 100 μl of medium containing cell-culture-grade heparin, chondroitin sulfate A, dermatan sulfate (also known as chondroitin sulfate B), heparan sulfate, or carrageenan type V (Sigma) for 2 h at 37°C. Supernatant was removed, and 200 μl of fresh medium was added to the cells, followed by incubation for 48 h at 37°C. Virus infection was analyzed by measuring the luciferase activity from the cell lysates as per the manufacturer's instructions (Promega, Sunnyvale, CA).
Heparinase treatment.
Cells were seeded at 2 × 104 in 96-well plates 24 h prior to infection. Medium was removed, and the cells were incubated with heparinase I from Flavobacterium heparinum (Sigma, Wisconsin) in 20 mM Tris-HCl (pH 7.5), 4 mM CaCl, 50 mM NaCl, and 0.01% bovine serum albumin (BSA) or heparinase III from Flavobacterium heparinum (Sigma) in 20 mM Tris-HCl (pH 7.5), 4 mM CaCl, and 0.01% BSA for 1 h at room temperature (RT) and then washed twice with PBS. To test the effect on HIV pseudotype infectivities, we then added the appropriate amounts of viral supernatant in 50 μl of medium and incubated for 2 h at 37°C. Supernatant was removed, and 200 μl of fresh medium was added to the cells and incubated for 48 h at 37°C. Virus infection was analyzed by measuring the luciferase activity from the cell lysates as per the manufacturer's instructions (Promega). To test the effect on Bla-VP40 VLP transduction efficiency, the cells were spin inoculated at 2,225 rpm for 90 min. at 21°C and then incubated for 3 h at 37°C. GP-mediated transfer of functional β-lactamase enzymatic activity to the cytoplasm of target cells was measured by labeling cells with CCF2-AM as per the manufacturer's instructions (GeneBLAzer detection kit; Life Technologies) and analyzed by fluorescence microscopy using a 405-nm excitation filter, a 425-nm dichroic filter, and a 435-nm long-pass emission filter to visualize green and blue cells simultaneously (Chroma Technologies).
Inhibition of infection by dendrimeric peptides.
Cells were seeded at 2 × 104 in 96-well plates 24 h prior to infection. Medium was removed, and the cells were incubated with the appropriate amounts of the dendrimeric peptides SB105-A10 (Spider Biotech, Colleretto Giacosa, Italy) or SB104 (CPC Scientific, Sunnyvale, CA) in 50 μl of medium for 2 h at 4°C. Then, the appropriate amounts of viral supernatant in 50 μl of medium were added and incubated for 2 h at 37°C. Supernatant was removed, and 200 μl of fresh medium was added to the cells and incubated for 48 h at 37°C. Virus infection was analyzed by measuring the luciferase activity from the cell lysates as per the manufacturer's instructions (Promega).
EBOV infectivity assay.
Zaire EBOV (kikwit) was kindly provided by Peter Jahrling. The filovirus stock was grown in Vero E6 cells in 150-mm tissue culture flasks with DMEM (Invitrogen) containing 4,500 mg/liter d-glucose, l-glutamine, and supplemented with 2% heat-inactivated FBS (DMEM-2) at 37°C, 5% CO2, and 85% humidity. At 8 days postinfection, supernatant was collected, and aliquots of the virus were stored at −80°C. EBOV is a risk group 4 (CDC) and category A (NIH) agent. All experiments with this virus were performed within the biosafety level 4 (BSL4) facility at the Texas Biomedical Research Institute (formerly Southwest Foundation for Biomedical Research) in San Antonio, TX. Personnel handling live virus wore appropriate protective equipment (positive-pressure biosafety suit). All virus manipulations were performed under BSL-4 maximum biocontainment within a biosafety laminar flow hood.
For the infectivity assay, Vero cells (3 × 105 cells/well) were seeded in a 6-well plate 24 h prior to infection in DMEM (Invitrogen) containing d-glucose (4,500 mg/liter) and l-glutamine and supplemented with 2% heat-inactivated FBS (DMEM-2). The cells were incubated for 2 h with compound before infection with EBOV for an additional 30 min at a multiplicity of infection (MOI) of 1. The inoculum was then removed, and fresh medium with the compound at the same concentration was added. At day 8 postinfection, supernatant was harvested and used in a conventional plaque assay. For the plaque assay, cells were seeded as described above in a 6-well plate 24 h prior to performing the assay. On the day of assay, samples were serially diluted in PBS and incubated for 1 h in duplicate wells of a 6-well plate. Following the 1-h adsorption period, samples were removed, and the monolayer was overlaid with DMEM-2 containing methylcellulose. The plates were then incubated for 10 days at 37°C ± 2°C. On day 10, the overlay was removed, and cells were stained with crystal violet. Plaques were enumerated by directly visualizing them on the monolayer using a dissecting microscope. Only plates with plaque numbers ranging from 25 to 250 were counted to avoid overlap or low representation.
RESULTS
Filovirus glycoprotein-dependent binding to heparin.
Viral pseudotypes have become an ideal tool to investigate cell entry of filoviruses without safety concerns (10, 39). In the present study, we initially wished to study whether filovirus GP could bind to glycosaminoglycans. Thus, binding of filovirus GP to immobilized heparin was tested. Heparin-Sepharose beads were used to capture and precipitate lentiviral particles pseudotyped with EBOV, RESTV, SUDV, and TAFV GP, as well as the GP of EBOV lacking a mucin-like domain (with deletion of amino acids at positions 311 to 462, termed “Δmuc”) and Lake Victoria MARV. Lentiviral pseudotypes bearing an unrelated GP from Lassa virus, as well as envelope GP-deficient pseudotypes, were used as controls. Heparin-bound particles were eluted and analyzed for the presence of HIV p24 antigen (Fig. 1). Heparin beads captured all of the particles bearing filovirus envelopes, including EBOV Δmuc, suggesting that the mucin domain is not absolutely required for interactions with heparin. The interaction is dependent on the presence of GP, since GP-deficient pseudotypes were unable to bind heparin, as were particles bearing Lassa virus GP. Addition of soluble heparin was able to elute bound filovirus pseudotypes from the heparin beads at a concentration of 50 mg/ml (data not shown), proving the specificity of this interaction. These results demonstrate that filovirus GPs, but not Lassa virus GP, possess the ability to bind heparin.
Fig 1.
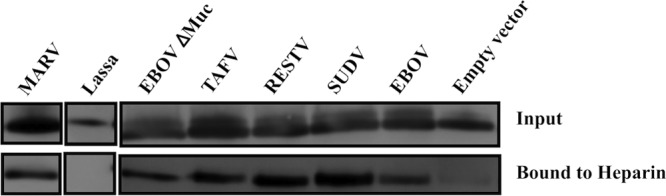
Binding of filovirus GP to immobilized heparin. Concentrated lentiviral pseudotypes bearing the different filovirus envelope glycoproteins, Lassa virus envelope glycoprotein, or no envelope were incubated with heparin-Sepharose beads. After several washes, bound virus was subsequently eluted with 2 M NaCl. Heparin-bound virus was detected by SDS-polyacrylamide gel electrophoresis and Western blotting for the p24 protein.
Soluble GAG inhibition of filovirus GP-mediated infection.
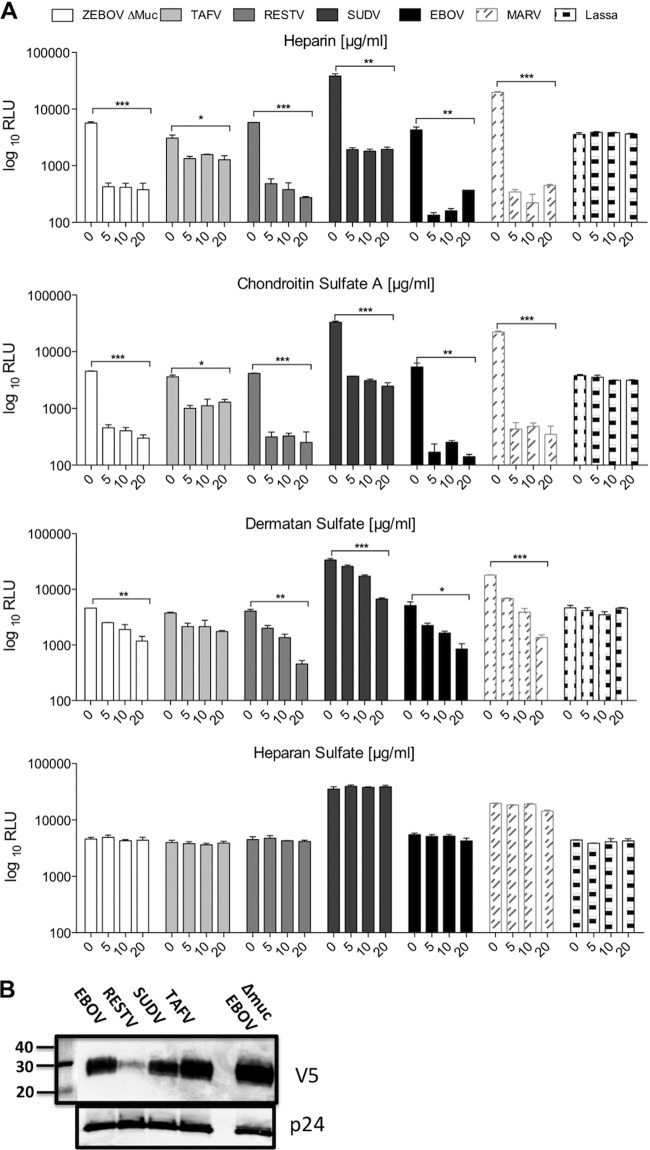
To determine whether the interaction with GAGs, such as heparin, might have an effect on filovirus GP-mediated infection, 293T cells were infected with infectivity-normalized pseudotypes bearing the different filoviral GPs and Old World arenavirus Lassa virus in the presence or absence of different concentrations of soluble heparin or the related ligands, chondroitin sulfate A (CSA), dermatan sulfate (DS), and heparan sulfate (HS) (Fig. 2A). Inhibition of infection was detected even with the lowest concentration of soluble GAGs for all the filovirus GPs, including EBOV Δmuc, but not for Lassa virus when utilizing heparin, CSA, and DS. Inhibition was similar for the different GPs, with the exception of TAFV, which, although significantly inhibited by HP, CSA, and DS at the highest concentrations of each (P < 0.05), was less affected by the presence of all the GAGs. This difference was statistically significant (P < 0.01 when comparing TAFV to EBOV). Although variations in the incorporation efficiency of GP are apparent, the differences in GAG inhibition were not merely a reflection of levels of expression, since EBOV, SUDV, and TAFV bearing V5 epitope tags on their C termini were expressed at broadly similar levels (Fig. 2B) despite the differences in inhibition that were observed (Fig. 2A). Inhibition of EBOV by heparin was further confirmed utilizing murine leukemia virus (MLV)-based pseudotypes, while MLV pseudotypes bearing Lassa virus GP and five different New World arenavirus GPs (Pichide, Flexal, Tacaribe, Guaranito and Carvallo strains) were not affected (data not shown). Inhibition of infection by DS was significantly lower than that by heparin and CSA (Fig. 2A), indicating that the inhibitory effect of GAGs on transduction is mediated by both electrostatic charge and the sequence of the carbohydrate moieties. No effect was observed with the less sulfated HS for any of the viruses tested, suggesting that the degree of sulfation is also an important factor in the interaction of the GAG with the filovirus GPs. While filovirus GP-mediated infection was almost completely abrogated, Lassa virus GP pseudotypes were not affected by any of the GAGs tested, even at the highest concentration. This confirms that infection mediated by Lassa virus GP is independent of GAGs. Similar results were obtained in Vero cells (data not shown), indicating that the effect is not cell type specific.
Fig 2.
Soluble GAGs inhibit filovirus GP-mediated infection of 293T cells. (A) 293T cells were infected with concentrated HIV pseudotypes bearing the different filovirus envelope glycoproteins and Lassa virus envelope glycoprotein in the presence or absence of different concentrations of soluble heparin, chondroitin sulfate A, dermatan sulfate, or heparan sulfate. Results are expressed as relative light units (RLU) and represent the means ± SD for duplicate wells. The results are representative of at least three different experiments. Statistics consisted of on one-way analysis of variance (ANOVA) followed by Bonferroni posttests. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) HIV pseudotypes were subjected to SDS–10% PAGE, transferred to nitrocellulose, and immunoblotted for the presence of the C-terminal V5 epitope using mouse anti-V5. Anti-p24 was used as a loading control.
Inhibition of filovirus GP-mediated infection in primary cells representing major targets of filovirus infection.
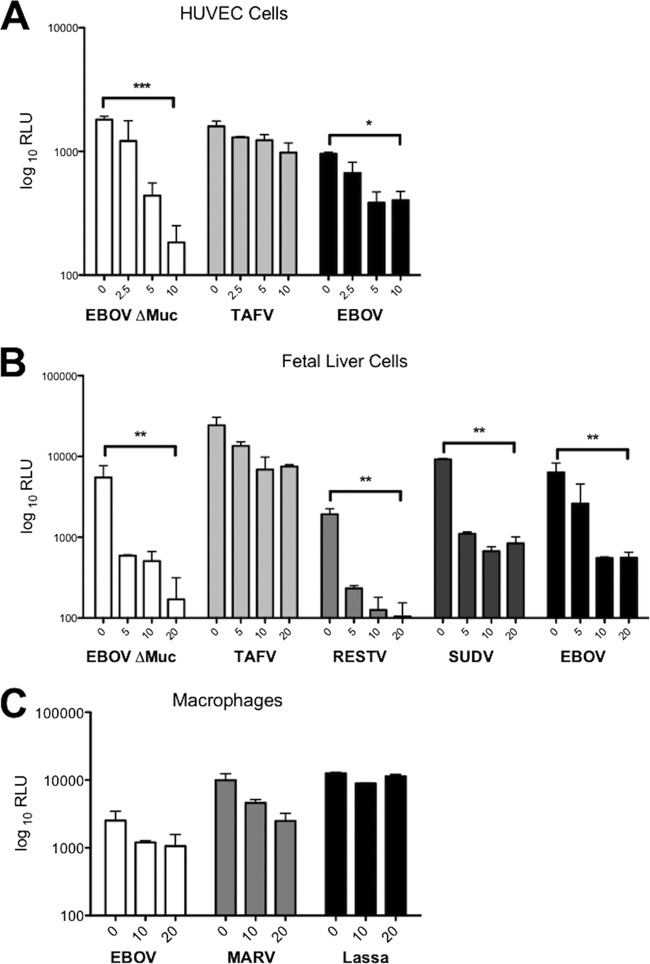
To further determine the relevance of GP/GAG interactions for infection, we decided to analyze the effect of soluble heparin in the infection of primary human endothelial and liver cells and macrophages, which represent some of the primary targets for in vivo filovirus infection. Heparin was able to inhibit infection mediated by EBOV GP in human umbilical vein endothelial cells (HUVEC) by up to 90% and TAFV GP by up to 30% (Fig. 3A). Similar results were obtained with human pulmonary artery endothelial cells (data not shown). Infection of human primary fetal liver cells by the different Ebola virus pseudotypes was also strongly affected (almost 100% for EBOV, RESTV, and SUDV and up to 60% for TAFV) by the addition of soluble heparin (Fig. 3B). Finally, both EBOV and MARV Ravn but not Lassa virus pseudotype infection of human macrophages was also inhibited by heparin in a concentration-dependent manner, with a more than 50% reduction in infectivity (Fig. 3C). These results confirm that targeting GAG binding by using soluble heparin inhibits the interaction of the filoviral GPs with its natural target cells.
Fig 3.
Soluble heparin inhibits EBOV GP-mediated infection of human primary umbilical vein endothelial cells (A), human fetal liver cells (B), and human macrophages (C), which represent main targets of filovirus natural infection. The cells were infected with concentrated HIV pseudotypes bearing the different filovirus envelope glycoproteins in the presence or absence of different concentrations of soluble heparin (μg/ml). Results are expressed as relative light units (RLU) and represent the means ± SD for duplicate wells. The results are representative of at least two different experiments. Statistics consisted of one-way ANOVA followed by Bonferroni posttests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Enzymatic removal of glycosaminoglycans decreases cell susceptibility to filovirus GP-mediated infection.
Heparinase digestion of cells was used to examine the effect of removal of HS (40). Infectivity was evaluated after digestion of 293T cells with heparinase I (which cleaves heparin and HS [41]) or heparinase III (which cleaves only HS [41]). Digestion of 293T cells with increasing concentrations of either heparinase I or III resulted in a maximum reduction of EBOV and MARV pseudotype infectivity of approximately 50% regardless of the enzyme used, while infectivity of Lassa virus pseudotypes remained unaffected (Fig. 4A). This effect was not cell type specific, since treatment of Huh7 cells with heparinase I also resulted in a dose-dependent reduction of up to 50% of filovirus pseudotype infectivity (Fig. 4A). The entry pathways of lentivirus pseudotypes are generally thought to faithfully mimic authentic virus entry. However, Ebola virus particles are filamentous, pleomorphic, and morphologically distinct from retrovirus particles, which are usually spherical. Thus, we decided to confirm the results with Bla-VP40 VLPs, which are morphologically similar to Ebola virus particles (9, 42). Consistent with the pseudovirus results, heparinase I treatment of primary HUVEC cells also resulted in a significant (P < 0.001) 50% reduction of EBOV VLP transduction (Fig. 4B). The fact that removal of heparin and/or HS significantly affected the ability of filovirus GP to mediate infectivity/fusion further indicates that cell surface GAGs contribute greatly to attachment of filoviruses to target cells.
Fig 4.
HSPGs mediate attachment of filovirus to the cells. 293T, Huh7, and HUVEC cells were pretreated or mock treated with different amounts of heparinase I or heparinase III and exposed to concentrated HIV pseudotypes bearing EBOV, MARV, or Lassa virus envelope glycoproteins (A) or EBOV and Lassa virus β-lactamase-based VLPs (B). Results are expressed as percentages of results with no heparinase and represent the means ± SD for duplicate wells. Heparinase concentrations are expressed in units. The results are representative of at least three different experiments.
Exploration of potential antivirals exploiting GP/GAG interactions.
Carrageenan, a type of sulfated polysaccharide extracted from red algae, has been reported to inhibit the infection of several human viral pathogens that utilize HS as an attachment factor, such as HPV, dengue virus (43), HSV, CMV, VSV, and HIV (44–48). Thus, the activity of carrageenan is most likely based on structural similarities with the HS residues of cell membrane proteoglycans. Like heparin, ι-carrageenan is more heavily sulfated than most tissue-derived heparan sulfate. Our data indicate that efficient filovirus GP-mediated infection requires GAGs and more specifically HS. Thus, to investigate the concept that ι-carrageenan chemically mimics HS and thereby can antagonize HS-dependent virus attachment, we tested its effectiveness for inhibiting filovirus pseudotype infectivity. As expected, a strong dose-dependent inhibition of pseudotypes bearing filovirus envelopes was observed in Huh7 and 293T cells (Fig. 5 and data not shown) in the presence of soluble ι-carrageenan, while infectivity of the HS-independent Lassa virus pseudotypes was not affected. Similar results were obtained with MLV-based pseudotypes in Vero cells, confirming that EBOV but not Lassa virus, or the majority of New World arenavirus envelopes, was strongly inhibited (data not shown).
Fig 5.
Sulfated polysaccharide ι-carrageenan inhibits filovirus GP-mediated infection. Huh7 cells were infected with concentrated HIV pseudotypes bearing EBOV, MARV, or Lassa virus envelope glycoproteins in the presence or absence of different concentrations of ι-carrageenan. Results are expressed as percentages of no ι-carrageenan and represent the means ±SD for duplicate wells. The results are representative of at least three different experiments. Similar results were observed with 293T cells (data not shown).
A peptide dendrimer, SB105-A10, has been developed as a potent inhibitor of several viruses, including HSV-1 and -2 (49), HPV (50), CMV (51,) and HIV-1 (A. Giuliani, S. Landolfo, D. Lembo, D. Gibellini, G. Pirri, L, Pizzuto, and G. Gribaudo, Italian patent application MI2009A001425). SB105-A10 exerts its inhibition by binding to HS on the cell surface and thus antagonizes virus attachment to the target cells (49–51). This novel peptide-derivatized dendrimer is based on the M6 prototype (52), a tetrabranched dendrimer synthesized on a lysine core, which tethers four 10-mer peptide chains in lysine alpha and epsilon positions. These functional units contain clusters of basic amino acids that bind to the negatively charged sulfate and carboxyl groups of HS.
Peptide-derivatized dendrimers have been shown to be highly resistant to blood proteases and to have low hemolytic activity and little cytotoxic effect against eukaryotic cells, making them promising candidates for the development of new antibacterial/antiviral drugs (52, 53). We tested SB105-A10 as a possible inhibitor for filovirus pseudotypes. Pretreatment of Huh7 cells with SB105-A10 1 h before infection produced a significant concentration-dependent inhibition of both MARV and EBOV pseudotypes (Fig. 6). This antiviral activity was not due to cytotoxicity, since infection by Lassa virus pseudotypes remained unaffected even at high peptide concentrations. The inhibitory effect is neither virus strain specific nor cell type specific, since similar effects were observed in 293T cell infection with three additional Ebola virus GPs (data not shown). Although they differ only in the specific amino acid sequence of the peptide surface groups attached to the dendrimer scaffold (49), dendrimeric peptide SB104 showed minimal inhibitory activity compared to that of SB105-A10 for CMV infection (51). When SB104 was used as a negative control in parallel with SB105-A10, it showed little inhibitory activity and only at high concentrations against MARV pseudotypes, indicating that, as with the other HS-dependent viruses, the inhibitory activity of the SB105-A10 dendrimeric peptide toward filovirus stems from the specific amino acid sequence of the peptides.
Fig 6.
Antiviral activity of dendrimeric peptide SB105-A10. Huh7 cells were treated with increasing concentrations of the dendrimer SB105-A10 or the SB104 negative control 1 h prior to and during the infection with concentrated HIV pseudotypes bearing EBOV, MARV, or Lassa virus envelope glycoproteins. Results are expressed as percentages of inhibition and represent the means ± SD for duplicate wells. The results are representative of at least three different experiments.
Inhibition by heparin and other polysaccharides is also effective against wild-type EBOV.
In order to further study the inhibitory properties against filoviruses of heparin and analog molecules, such as carrageenan, both compounds were evaluated as inhibitors of replication-competent EBOV in a plaque reduction assay with Vero cells (Fig. 7). Both heparin (50% inhibitory concentration [IC50] = 8) and carrageenan (IC50 = 3.162) inhibited EBOV replication in Vero E6 cells, with up to 1-log differences in PFU achieved even at the lowest concentration (1 μg/ml), indicating that the presence of these compounds inhibited cell entry. Prior studies demonstrated no cytotoxicity with either of these polysulfates in Vero cell monolayers (estimated 50% cytotoxic concentration [CC50], >1,000 μg/ml) (43, 54). Together with the results of pseudotype virus assays, these findings demonstrate that GAGs participate in cell entry of EBOV.
Fig 7.
Soluble polysaccharides inhibit infection of Vero cells by wild-type EBOV. EBOV was incubated with different concentrations of soluble heparin or carrageenan and then added to a monolayer of Vero cells for 2 h. Virus was removed, and the cells were cultured for 8 days in the presence of the compounds. Titers were determined by plaque assay. Results are expressed as PFU/ml and represent the means ± SD for duplicate wells.
DISCUSSION
The attachment to, entry into, and infection of target cells by filoviruses are the result of complex virus/cell interactions, including both nonspecific attachment to cell surface molecules and specific virus/receptor interactions. We conclude from these studies that filovirus GP attachment occurs at sites on the cell surface that include HS and possibly other GAG molecules. All filovirus GPs tested bound specifically to immobilized heparin. This interaction proved to be dependent on the filovirus GP but did not require the mucin domain, utilized for the interaction of GP with certain C-type lectins (5).
The importance of GAGs in filovirus interactions with target cells was further demonstrated with primary endothelial and liver cells and in primary macrophages by inhibition in the presence of heparin and importantly by the significant reduction of EBOV VLP transduction of primary endothelial cells after heparinase I treatment. Inhibition on macrophages was somewhat less impressive, which is not surprising given the numerous other potential attachment factors present on this particular cell type (5). However, overall, a role for GP/GAG interactions in filovirus infection and pathogenesis is likely since the cell types targeted represent many of the main targets for the virus in vivo. Our results where filovirus pseudotypes bound specifically to immobilized heparin and heparin blocked infection in both cell lines and primary cells suggest that proteoglycans and more specifically HSPGs act as attachment factors for these viruses. These findings are supported by the loss of infectivity in primary cells using filamentous VLPs following heparinase treatment cleaving heparin and/or HS side chains from HSPGs.
With the description of NPC1 as a true receptor for EBOV, able to bind the RBD of GP and apparently mediate membrane fusion (16–18), an important question remains as to how the filamentous virus particle reaches the endosomal compartments where NPC1 resides. Whether other specific receptors are required to be engaged to initiate this process or relatively nonspecific, lower-affinity interactions with attachment factors such as HS and other GAGs are sufficient is open to question. It is unlikely that GAGs are absolutely necessary for cellular infection, particularly since their removal with heparinases reduced infectivity only by approximately 50%. However, it is possible that HSPG is sufficient, in the absence of other cell surface factors, to shepherd filovirus particles to intracellular compartments for proteolysis and engagement of NPC1. Interestingly, cross-linking of HSPGs has been demonstrated to facilitate the trafficking of soluble proteins into cells via macropinocytosis (55, 56), the major route of ingress for EBOV (13, 14). EBOV uptake is promoted by the receptor tyrosine kinase Axl in the absence of direct interaction with the viral GP (57). Recently, drastic effects in virus-cell interactions caused by proteins involved in bridging virion envelope lipids to Axl have been described (58). Although the effect of heparinase treatment strongly points toward the involvement of the surface-associated GAGs in the entry process, it is a possibility that the high levels of inhibition achieved by soluble GAGs are the results of their involvement in similar bridging interactions that facilitate attachment of the viruses. Further studies into the relative contribution and role of GAGs in attachment and internalization are required.
The importance of sulfation levels and patterns for the attachment of other GAG-dependent viruses, such as dengue virus and RSV, has been described previously (30, 59). Similarly, adequate levels of sulfation appear to be important for filovirus/GAG interaction, since soluble HS, which closely resembles heparin but with a lower degree of sulfation (60), has no effect on infectivity. Therein, it could be argued that the interaction of filovirus GPs with GAGs is somewhat nonspecific and is based mostly on electrostatic effects due to the strong negative charge of the more sulfated GAGs. However, the significantly higher inhibition levels observed with CSA than with the closely related DS shows that not only the degree of sulfation but also the structure of the carbohydrate backbone is important for the interaction with the filovirus GPs. The fact that the interaction of filoviruses with the GAGs shows specific structure/sulfation requirements points out that the great heterogeneity of GAG expression level, structure, and sulfation known to occur between different cell types might have a strong effect in the attachment ability of these GPs and thus in virus tropism. Previous studies showed discrepancies between the entry mechanism of EBOV when utilizing different pseudotype systems (retroviral versus rhabdoviral) (13, 14), These studies suggest that rhabdovirus particles seem to mimic the EBOV virion more closely (13). With respect to the ability of heparin to inhibit filovirus GP-mediated infection, we have observed comparable results in 293T and Huh7 cell lines when the VSV-ΔG-GFP backbone was used instead of HIV to produce both EBOV and MARV pseudotypes (data not shown). Furthermore, we demonstrated that live EBOV infectivity in cell culture is also inhibited by the presence of low concentrations of both heparin and carrageenan polysaccharides. As for the effect of enzymatic removal of heparin and HS, a similar 50% reduction was observed for HIV pseudotypes and for filamentous EBOV VLPs, which closely resemble infectious Ebola virus particles, by electron microscopy (61, 62).
The reliance on GAGs for at least efficient initial attachment suggests that targeting these interactions could be an effective antiviral strategy. Indeed, in addition to soluble forms of the GAGs themselves, we demonstrated inhibition of GAG-dependent viruses by carrageenan. Inhibition most likely takes place by chemically mimicking HS, thereby competing against initial virion attachment to the cell surface GAGs. Since carrageenan is widely used in the food industry, it is being tested for use as a broad-spectrum microbicide (46). Novel peptidic antiviral drugs, and more specifically the peptide dendrimer SB105-A10, have emerged as potent inhibitors of many viruses that act via binding to HSPGs on the cell surface (49–51). As we expected, SB105-A10 was able to efficiently inhibit filoviral infections, and as in the previous studies, its antiviral activity was dependent on the specific amino acid sequences of the peptide surface groups attached to the dendrimer scaffold, since surface group sequence variants, such as SB104, reduced or completely abolished the antiviral action.
We conclude that filoviruses utilize HSPGs and possibly other cell surface GAGs for the initial attachment to their target cells. This step is not common to another hemorrhagic virus, Lassa virus, which interacts with cells in a GAG-independent manner. These findings not only contribute to furthering our understanding of the filovirus entry process but also reveal a new step in the virus cycle that can be the target for antiviral strategies that have already been proven effective for the inhibition of other viruses.
ACKNOWLEDGMENTS
We thank the staff and faculty at San Francisco General Hospital Women's Options Center for assistance in the collection of human fetal tissues, and we further thank Marina Fomin for her help in the isolation of fetal liver cells. We are grateful to Sean M. Amberg (Siga Technologies) for kindly providing the plasmids for the arenavirus GPs and Spider Biotech SRL, Turin, Italy, for the SB105-A10 peptide.
This work was supported by grant R01AI074986 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 9 January 2013
REFERENCES
- 1. Kuhn JH. 2008. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch. Virol. Suppl. 20:13–360 [PubMed] [Google Scholar]
- 2. Negredo A, Palacios G, Vazquez-Moron S, Gonzalez F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martinez M, Herrera JE, Pizarro M, Hutchison SK, Echevarria JE, Lipkin WI, Tenorio A. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7:e1002304 doi:10.1371/journal.ppat.1002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manicassamy B, Wang J, Jiang H, Rong L. 2005. Comprehensive analysis of Ebola virus GP1 in viral entry. J. Virol. 79:4793–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manicassamy B, Wang J, Rumschlag E, Tymen S, Volchkova V, Volchkov V, Rong L. 2007. Characterization of Marburg virus glycoprotein in viral entry. Virology 358:79–88 [DOI] [PubMed] [Google Scholar]
- 5. Takada A, Fujioka K, Tsuiji M, Morikawa A, Higashi N, Ebihara H, Kobasa D, Feldmann H, Irimura T, Kawaoka Y. 2004. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78:2943–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker S, Spiess M, Klenk HD. 1995. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J. Gen. Virol. 76(Part 2):393–399 [DOI] [PubMed] [Google Scholar]
- 9. Simmons G, Rennekamp AJ, Chai N, Vandenberghe LH, Riley JL, Bates P. 2003. Folate receptor alpha and caveolae are not required for Ebola virus glycoprotein-mediated viral infection. J. Virol. 77:13433–13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 94:14764–14769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan SY, Empig CJ, Welte FJ, Speck RF, Schmaljohn A, Kreisberg JF, Goldsmith MA. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117–126 [DOI] [PubMed] [Google Scholar]
- 12. Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr, Chiorini J, Maury W. 2011. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. U. S. A. 108:8426–8431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, Halfmann P, Kawaoka Y. 2010. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 6:e1001121 doi:10.1371/journal.ppat.1001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. 2010. Cellular entry of Ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 6:e1001110 doi:10.1371/journal.ppat.1001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477:340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cote M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. 2011. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477:344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, Ndungo E, Sandesara RG, Carette JE, Kuehne AI, Ruthel G, Pfeffer SR, Dye JM, Whelan SP, Brummelkamp TR, Chandran K. 2012. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 31:1947–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spillmann D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811–817 [DOI] [PubMed] [Google Scholar]
- 20. Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WuDunn D, Spear PG. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Compton T, Nowlin DM, Cooper NR. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834–841 [DOI] [PubMed] [Google Scholar]
- 24. Chung CS, Hsiao JC, Chang YS, Chang W. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72:1577–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Byrnes AP, Griffin DE. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feldman SA, Audet S, Beeler JA. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 74:6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hallak LK, Kwilas SA, Peeples ME. 2007. Interaction between respiratory syncytial virus and glycosaminoglycans, including heparan sulfate. Methods Mol. Biol. 379:15–34 [DOI] [PubMed] [Google Scholar]
- 28. Tamura M, Natori K, Kobayashi M, Miyamura T, Takeda N. 2004. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 78:3817–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Summerford C, Samulski RJ. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866–871 [DOI] [PubMed] [Google Scholar]
- 31. Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, Van Kuppevelt TH, Depla E, Von Weizsacker F, Blum HE, Baumert TF. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003–41012 [DOI] [PubMed] [Google Scholar]
- 32. Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retroviruses 9:167–174 [DOI] [PubMed] [Google Scholar]
- 33. Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. 2005. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 79:12692–12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simmons G, McKnight A, Takeuchi Y, Hoshino H, Clapham PR. 1995. Cell-to-cell fusion, but not virus entry in macrophages by T-cell line tropic HIV-1 strains: a V3 loop-determined restriction. Virology 209:696–700 [DOI] [PubMed] [Google Scholar]
- 35. Fomin ME, Tai LK, Barcena A, Muench MO. 2011. Coexpression of CD14 and CD326 discriminate hepatic precursors in the human fetal liver. Stem Cells Dev. 20:1247–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- 37. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. 2004. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 101:4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944 [DOI] [PubMed] [Google Scholar]
- 39. Wool-Lewis RJ, Bates P. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rostand KS, Esko JD. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Desai UR, Wang HM, Linhardt RJ. 1993. Specificity studies on the heparin lyases from Flavobacterium heparinum. Biochemistry 32:8140–8145 [DOI] [PubMed] [Google Scholar]
- 42. Manicassamy B, Rong L. 2009. Expression of Ebolavirus glycoprotein on the target cells enhances viral entry. Virol. J. 6:75 doi:10.1186/1743-422X-6-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Talarico LB, Pujol CA, Zibetti RG, Faria PC, Noseda MD, Duarte ME, Damonte EB. 2005. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res. 66:103–110 [DOI] [PubMed] [Google Scholar]
- 44. Baba M, Snoeck R, Pauwels R, de Clercq E. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandez-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, Begay O, Seidor S, Ford BE, Gil PI, Peters J, Katz D, Robbiani M, Zydowsky TM. 2012. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob. Agents Chemother. 56:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gonzalez ME, Carrasco L. 1987. Animal viruses promote the entry of polysaccharides with antiviral activity into cells. Biochem. Biophys. Res. Commun. 146:1303–1310 [DOI] [PubMed] [Google Scholar]
- 47. Hamasuna R, Eizuru Y, Minamishima Y. 1994. Inhibition by iota-carrageenan of the spread of murine cytomegalovirus from the peritoneal cavity to the blood plasma. J. Gen. Virol. 75(Part 1):111–116 [DOI] [PubMed] [Google Scholar]
- 48. Konlee M. 1998. Sulfated polysaccharides (chondroitin sulfate and carrageenan) plus glucosamine sulfate are potent inhibitors of HIV. Posit. Health News 1998:4–7 [PubMed] [Google Scholar]
- 49. Luganini A, Nicoletto SF, Pizzuto L, Pirri G, Giuliani A, Landolfo S, Gribaudo G. 2011. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob. Agents Chemother. 55:3231–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Donalisio M, Rusnati M, Civra A, Bugatti A, Allemand D, Pirri G, Giuliani A, Landolfo S, Lembo D. 2010. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob. Agents Chemother. 54:4290–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luganini A, Giuliani A, Pirri G, Pizzuto L, Landolfo S, Gribaudo G. 2010. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate. Antiviral Res. 85:532–540 [DOI] [PubMed] [Google Scholar]
- 52. Pini A, Giuliani A, Falciani C, Runci Y, Ricci C, Lelli B, Malossi M, Neri P, Rossolini GM, Bracci L. 2005. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 49:2665–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pini A, Giuliani A, Falciani C, Fabbrini M, Pileri S, Lelli B, Bracci L. 2007. Characterization of the branched antimicrobial peptide M6 by analyzing its mechanism of action and in vivo toxicity. J. Pept. Sci. 13:393–399 [DOI] [PubMed] [Google Scholar]
- 54. Talarico LB, Noseda MD, Ducatti DR, Duarte ME, Damonte EB. 2011. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 92:1332–1342 [DOI] [PubMed] [Google Scholar]
- 55. Chang XZ, Wang ZM, Yu JM, Tian FG, Jin W, Zhang Y, Yu J, Li LF, Liu XF, Li ZW, Shao ZM. 2007. Isolation of a human gallbladder cancer cell clone with high invasive phenotype in vitro and metastatic potential in orthotopic model and inhibition of its invasiveness by heparanase antisense oligodeoxynucleotides. Clin. Exp. Metastasis 24:25–38 [DOI] [PubMed] [Google Scholar]
- 56. Imamura J, Suzuki Y, Gonda K, Roy CN, Gatanaga H, Ohuchi N, Higuchi H. 2011. Single particle tracking confirms that multivalent Tat protein transduction domain-induced heparan sulfate proteoglycan cross-linkage activates Rac1 for internalization. J. Biol. Chem. 286:10581–10592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brindley MA, Hunt CL, Kondratowicz AS, Bowman J, Sinn PL, McCray PB, Jr, Quinn K, Weller ML, Chiorini JA, Maury W. 2011. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology 415:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen IS. 2011. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe 9:286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martinez I, Melero JA. 2000. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J. Gen. Virol. 81:2715–2722 [DOI] [PubMed] [Google Scholar]
- 60. Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156–167 [DOI] [PubMed] [Google Scholar]
- 61. Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]