Abstract
Retroviral Gag proteins direct virus particle assembly from the plasma membrane (PM). Phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] plays a role in PM targeting of several retroviral Gag proteins. Here we report that depletion of intracellular PI(4,5)P2 and phosphatidylinositol-(3,4,5)-triphosphate [PI(3,4,5)P3] levels impaired Rous sarcoma virus (RSV) Gag PM localization. Gag mutants deficient in nuclear trafficking were less sensitive to reduction of intracellular PI(4,5)P2 and PI(3,4,5)P3, suggesting a possible connection between Gag nuclear trafficking and phosphoinositide-dependent PM targeting.
TEXT
The retroviral Gag polyprotein orchestrates the assembly of virus particles, which are released from the plasma membrane (PM) of infected cells. The major domains of Gag include matrix (MA), capsid (CA), and nucleocapsid (NC), with MA containing the PM-targeting signal (1–9). Studies to determine how MA directs membrane targeting of Gag revealed that interactions with phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] and other negatively charged lipid molecules are required for proper localization of several retroviral Gag proteins, including human immunodeficiency viruses type 1 and type 2 (HIV-1 [10–13] and HIV-2 [12]), murine leukemia virus (MLV) (1, 14), and Mason-Pfizer monkey virus (MPMV) (3, 7). In the case of HIV-1, Gag is directed to the PM by a bipartite signal in MA consisting of N-terminal myristic acid modification and a cluster of basic amino acids (9). Stable association of HIV-1 Gag with the PM is achieved when PI(4,5)P2 is bound by this basic cleft in MA, leading to insertion of the myristoyl chain into the PM (10, 15). Similarly, MLV MA is myristoylated and binds PI(4,5)P2 in the presence of phosphatidylserine but lacks an obvious PI(4,5)P2 binding cleft (1). The MPMV MA protein must be myristoylated to bind PI(4,5)P2, which it binds with lower affinity than does HIV-1 or HIV-2 MA (3). In contrast, equine infectious anemia virus and human T-lymphotropic virus type 1 are less dependent than HIV-1 on specific binding of PI(4,5)P2 for Gag recruitment to the PM and particle release (16–18).
Rous sarcoma virus (RSV) Gag, which is acetylated (19, 20) but not myristoylated, undergoes nuclear trafficking prior to PM localization owing to nuclear localization signals (NLS) in the MA and NC domains. Nuclear export is mediated by a nuclear export signal (NES) in p10, an RSV-specific sequence upstream of the CA domain (21–23). The NLS in MA overlaps the membrane-binding domain (MBD), suggesting the need for an ordered sequence of trafficking events from the nucleus to the plasma membrane (8). However, the mechanism by which RSV Gag is targeted to the PM after it exits the nucleus is not well understood.
The RSV Gag MBD spans the first 86 amino acids of MA and contains 11 basic residues that form a basic patch on a surface-exposed region of the protein, similarly to other retroviral MBDs (8, 24, 25). The structural similarity between the RSV MBD and those of other retroviruses suggests that RSV Gag may interact with acidic phospholipids via electrostatic interactions (24, 26). A previous study of RSV Gag membrane targeting reported that PM localization in cells was unaffected by PI(4,5)P2 and phosphatidylinositol-(3,4,5)-triphosphate [PI(3,4,5)P3] depletion, although the authors found that both of these phosphoinositides enhanced Gag binding to liposomes in vitro (27). In contrast, we report that expression of the enzyme inositol polyphosphate 5-phosphatase type IV (5ptaseIV), which depletes intracellular levels of PI(4,5)P2 and PI(3,4,5)P3 by dephosphorylation of position 5 (28), significantly reduced RSV Gag PM localization and virus-like particle (VLP) assembly. Interestingly, we found that Gag mutants varied in their sensitivity to 5ptaseIV activity, and mutants we tested that are impaired in nuclear trafficking were less dependent on PI(4,5)P2 and PI(3,4,5)P3 for PM binding.
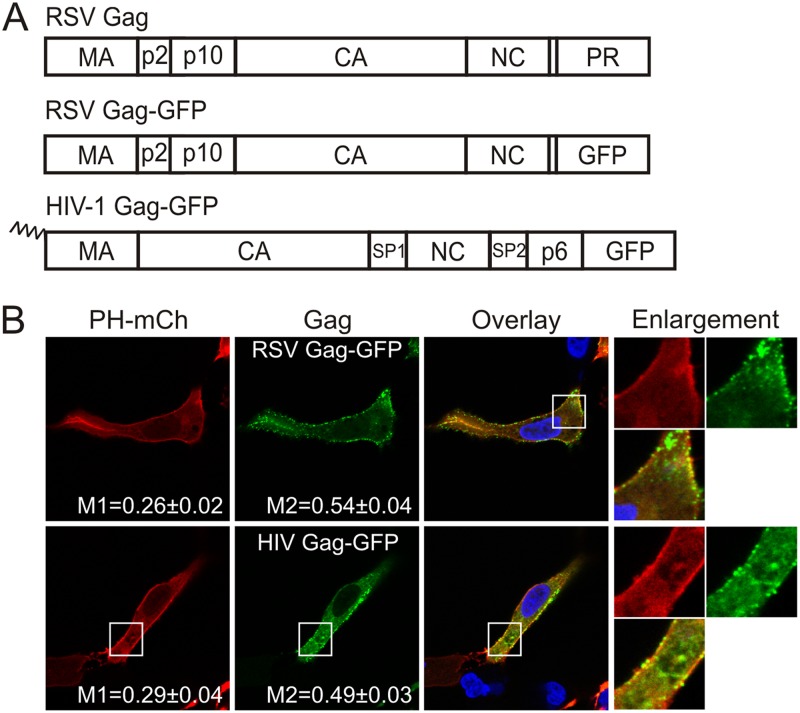
To examine the possibility that the wild-type RSV Gag protein might bind to regions of the PM similar to the pleckstrin homology (PH) domain from phospholipase Cδ, which specifically binds PI(4,5)P2 on the inner leaflet of the PM (17, 29, 30), we examined the membrane localization of RSV Gag compared to that of the isolated PH domain. We fused mCherry to the PH domain (pPH-mCherry) by PCR amplifying the mCherry sequence from pRSET8-mCherry (a gift from R. Tsien, University of California at San Diego) (31) and exchanging the sequence with green fluorescent protein (GFP) in pGFP-PHPLCδ (a gift from C. Carter, Stony Brook University) (17, 29) using AgeI-BsrGI. The fusion proteins were expressed in QT6 quail fibroblast cells, which are a natural host for RSV. QT6 cells were transfected with 0.5 μg of each plasmid DNA using the calcium phosphate method (32, 33). At 15 h posttransfection, cells were fixed in 2% paraformaldehyde, permeabilized with methanol at −20°C, immunostained (if needed), mounted on slides using SlowFade reagent (Invitrogen), and imaged through the center of the nuclear plane using a Leica SP2 laser-scanning confocal microscope (Leica Microsystems). RSV Gag-GFP and PH-mCherry both localized to the PM of QT6 cells, with 0.26 ± 0.02 of the PH-mCherry signal overlapping with Gag (M1) and 0.54 ± 0.04 of the Gag signal overlapping with PH-mCherry (M2) (n = 10). Consistent with previous reports, epifluorescence of HIV-1 Gag-GFP (34) (a gift from Marilyn Resh, Memorial Sloan-Kettering Cancer Center) also overlapped with PH-mCherry (M1 = 0.29 ± 0.04, M2 = 0.49 ± 0.03; n = 10) (30) (Fig. 1A and B). Therefore, in QT6 cells, both RSV and HIV-1 Gag proteins were recruited to similar regions of the PM as the PI(4,5)P2-binding protein PH-mCherry.
Fig 1.
RSV Gag-GFP and HIV Gag-GFP colocalize with P(4,5)P2-bound pPH-mCherry. (A) Schematic representation of Gag expression constructs. The wild-type RSV Gag protein is shown at the top, with MA, p2, p10, CA, NC, and PR domains denoted. In RSV Gag-GFP derivatives, GFP replaces 7 amino acids of NC and the entire PR domain. HIV.Gag-GFP is myristoylated (zigzag line), and its expression is Rev independent. (B) QT6 cells were transfected with 1 μg each of plasmid DNAs encoding PH-mCherry (PH-mCh) and RSV Gag-GFP (top panel) or HIV Gag-GFP (bottom panel). DAPI (4′,6-diamidino-2-phenylindole) (blue) was used to visualize the nucleus. The boxed area in each image was enlarged and shown in the fourth column. Mander's colocalization coefficients for PH-mCherry with Gag (M1) or Gag with PH-mCherry (M2) were calculated using Just Another Colocalization Plugin (JACoP) (59) for ImageJ (60) and are illustrated as the mean ± the standard error of the mean for 10 cells. Two-tailed Student's t tests were used to determine the statistical significance of variations in M1 and M2 values between Gag mutants.
To examine whether RSV Gag localization was altered by enzymatic depletion of PI(4,5)P2 and PI(3,4,5)P3, we expressed myc-tagged 5ptaseIV (pcDNA4TO/Myc5ptaseIV) (28) or 5ptaseIVΔ1 (30), a catalytically inactive mutant of 5ptaseIV (gifts of E. Freed, NCI-Frederick), in QT6 cells and visualized the proteins with rabbit anti-Myc antibody (AbCam) and goat anti-rabbit secondary antibody conjugated to Cy3 (Sigma) or Cy5 (Invitrogen). The subcellular localization of Gag was visually scored as predominantly localized to the PM (M) or cytoplasm (C) in >20 cells transfected on separate days. To avoid bias, researchers were blinded to the identity of the sample at every stage of the experiment (during transfection, image acquisition, and scoring of subcellular localization). Scoring of Gag subcellular localization was performed independently by two investigators, with excellent reproducibility of the results. The statistical significance of changes in Gag localization was determined by Fisher's exact test (GraphPad Prism).
RSV Gag-GFP was localized to the PM under steady-state conditions in the majority of cells (M = 90%, C = 10%, n = 32) (Fig. 2A). In contrast, 5ptaseIV coexpression resulted in a significant reduction in the PM localization of Gag (M = 31%, C = 69%, n = 20; p < 0.0001), whereas 5ptaseIVΔ1 did not markedly alter Gag localization (M = 70%, C = 30%, n = 32; p > 0.10). To determine whether VLP production was also affected by the reduction in intracellular PI(4,5)P2 and PI(3,4,5)P3 levels, cells expressing Gag-GFP with or without 5ptaseIV or 5ptaseIVΔ1 were metabolically labeled with [35S]methionine-cysteine. Cell lysates and clarified supernatants were immunoprecipitated using anti-RSV antibody, and radioactive bands were quantitated by PhosphorImager analysis as previously described (35–37). Budding efficiency was calculated as the ratio of Gag-GFP in the supernatant to the total Gag-GFP (cell lysate plus supernatant). Expression of 5ptaseIV reduced Gag-mediated VLP production by 39% compared to untreated cells (p < 0.01), whereas 5ptaseIVΔ1 had a minimal influence on VLP production (9% reduction; p > 0.10) (Fig. 2B). Together, these data indicate that PM localization of RSV Gag and efficient VLP assembly require normal intracellular levels of PI(4,5)P2 and PI(3,4,5)P3. However, the effect of 5ptaseIV expression on RSV budding was less than that observed for HIV-1 Gag, suggesting that additional electrostatic interactions other than PI(4,5)P2 and PI(3,4,5)P3 may contribute to RSV particle assembly.
Fig 2.

Overexpression of 5ptaseIV prevents RSV Gag from localizing to the PM. (A) QT6 cells were transfected with RSV pGag-GFP alone (top row) or cotransfected with Myc-tagged 5ptaseIV (middle row) or Myc-tagged 5ptaseIVΔ1 (bottom row). Graphs showing the percentage of cells with cytoplasmic (C) or PM-associated (M) Gag are shown at the right of the images. Fisher's exact test (GraphPad Prism) was used to determine whether there were statistically significant differences in Gag localization for untreated, 5ptaseIV-, and 5ptaseIVΔ1-treated cells and for the Gag variants compared to the wild type. (B) QT6 cells expressing Gag-GFP alone (mock) or Gag-GFP plus 5ptaseIV or 5ptaseIVΔ1 were metabolically labeled with [35S]methionine-cysteine, the medium was collected after a 2.5-h labeling period, and the cells were lysed. After immunoprecipitation of cell lysates and supernatants with anti-RSV antiserum (a gift from John Wills and Rebecca Craven, Penn State College of Medicine) (32), the amount of Gag-GFP in the lysates and media was quantified by PhosphorImager analysis (35–37). Budding efficiency, the amount of Gag-GFP in the medium divided by the sum of the amounts in the medium and lysate [M/(M+L)], was calculated. Budding efficiency of Gag-GFP under steady-state conditions was set at 100%. Data from three independent experiments were averaged, with the bars representing the mean ± the standard error of the mean. Statistical analysis was performed using two-tailed Student's t test, with a single asterisk signifying a p value of <0.005 and two asterisks signifying a p value of <0.0005. Autoradiography images from a representative experiment are shown to the right of the graph.
The NC domain of Gag mediates Gag-Gag and Gag-RNA interactions, and RSV Gag NC domain truncation mutants do not localize to the PM or produce VLPs efficiently (35, 38). However, both PM localization and VLP assembly can be rescued by replacement of NC with a leucine zipper protein-protein dimerization motif from CREB1 (39) (pMA-CA-Zip, also called Gag.Zip; a gift of V. Vogt, Cornell University). In QT6 cells, GagΔNC-YFP (Fig. 3A) (40), which lacks the ability to oligomerize or bind RNA, was diffusely cytoplasmic (M = 0%, C = 100%, n = 22) and was completely unaffected by expression of 5ptaseIV or 5ptaseIVΔ1 (Fig. 3B).
Fig 3.
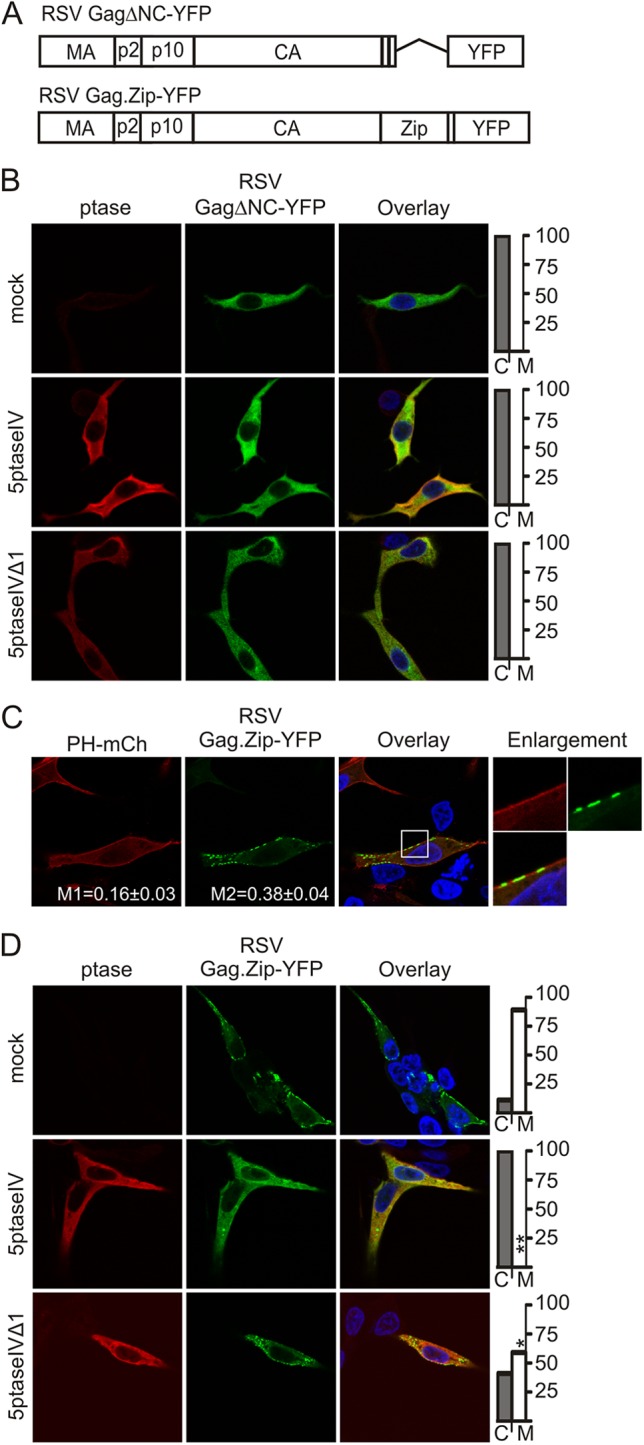
NC-mediated Gag multimerization is required for RSV Gag PI(4,5)P2-dependent membrane localization. (A) Schematic diagrams of RSV GagΔNC-YFP, which has deletion of amino acids 495 to 577 in the NC domain, and RSV Gag.Zip-GFP, which has the leucine zipper domain of the human CREB1-binding protein in place of the NC domain. (B) QT6 cells were transfected with pGagΔNC-YFP alone (top row) or cotransfected with pGagΔNC-YFP and either pMyc-5ptaseIV (2nd row) or pMyc-5ptaseIVΔ1 (bottom row). (C) QT6 cells were transfected with pGag.Zip-GFP and pPH-mCherry. Mander's colocalization analysis was conducted as described in Fig. 1B. (D) QT6 cells were transfected with pGag.Zip-GFP (top row) or cotransfected with either pMyc-5ptaseIV (middle row) or pMyc-5ptaseIVΔ1 (bottom row). Graphs showing the percentage of cells with cytoplasmic (C) or PM-associated (M) Gag are shown at the right of the images. Statistically significant differences in Gag localization were identified as described in Fig. 2A.
In contrast, Gag.Zip-YFP (Fig. 3A) was localized in the PM in a majority of cells (M = 89%, C = 11%, n = 33) and formed long patches along the PM rather than distinct foci (Fig. 3C). Interestingly, the signal from Gag.Zip-YFP overlapped less with PH-mCherry than wild-type Gag (M1 = 0.16 ± 0.03, p = 0.01; M2 = 0.38 ± 0.04, p = 0.02; n = 10), suggesting that either Gag.Zip competes with PH-mCherry for binding to PI(4,5)P2 or Gag.Zip PM binding is independent of PI(4,5)P2. To discriminate between these possibilities, we tested the sensitivity of Gag.Zip to 5ptaseIV expression. Strikingly, Gag.Zip-YFP was cytosolic with 5ptaseIV expression in every cell analyzed (M = 0%, C = 100%, n = 34; p < 0.0001) (Fig. 3D), a significant increase compared to the effect of PI(4,5)P2/PI(3,4,5)P3 depletion on wild-type Gag-GFP PM localization (p < 0.002). Expression of 5ptaseIVΔ1 also reduced the proportion of cells in which Gag.Zip-YFP was PM localized (M = 59%, C = 41%, n = 30; p < 0.05), although the magnitude of the change was similar to that of wild-type Gag-GFP (p > 0.50). Thus, replacing the NC domain with a leucine zipper may make Gag more dependent on PI(4,5)P2 and/or PI(3,4,5)P3 for PM localization despite the presence of a wild-type MA domain.
We next tested whether alterations of the RSV Gag MBD would change the sensitivity of Gag to depletion of intracellular levels of PI(4,5)P2 and/or PI(3,4,5)P3. To this end, we examined the localization of Gag.MyrIE, in which the membrane-binding domain of the v-Src oncoprotein was added as an extension at the N terminus of RSV Gag (Fig. 4A) (41). The v-Src MBD is myristoylated and consists of 10 amino acids enriched in basic residues (42, 43). Addition of this sequence results in stronger PM binding and enhancement of budding compared to those of the wild-type Gag protein (35, 41). In QT6 cells, Myr1E.Gag-GFP localized near the same sites at the PM as PH-mCherry, and this Gag variant was PM associated in 100% of cells examined (n = 24) (Fig. 4B). However, Myr1E.Gag-GFP localization was largely unaffected by 5ptaseIV (M = 88%, C = 13%, n = 24; p > 0.20) (Fig. 4C) and was significantly less sensitive to 5ptaseIV than wild-type Gag (p < 0.0001). Myr1E.Gag-GFP localization was also unaffected by 5ptaseIVΔ1 expression (M = 100%, C = 0%, n = 25) (Fig. 4C). These results suggest that Myr1E.Gag-GFP either does not interact with PI(4,5)P2 or PI(3,4,5)P3 to the same degree as wild-type Gag or additional mechanisms (e.g., hydrophobic interaction of myristate and/or electrostatic interactions with other negatively charged lipids) stabilize membrane binding, making the mutant protein independent of phosphoinositide levels once it reaches the PM.
Fig 4.
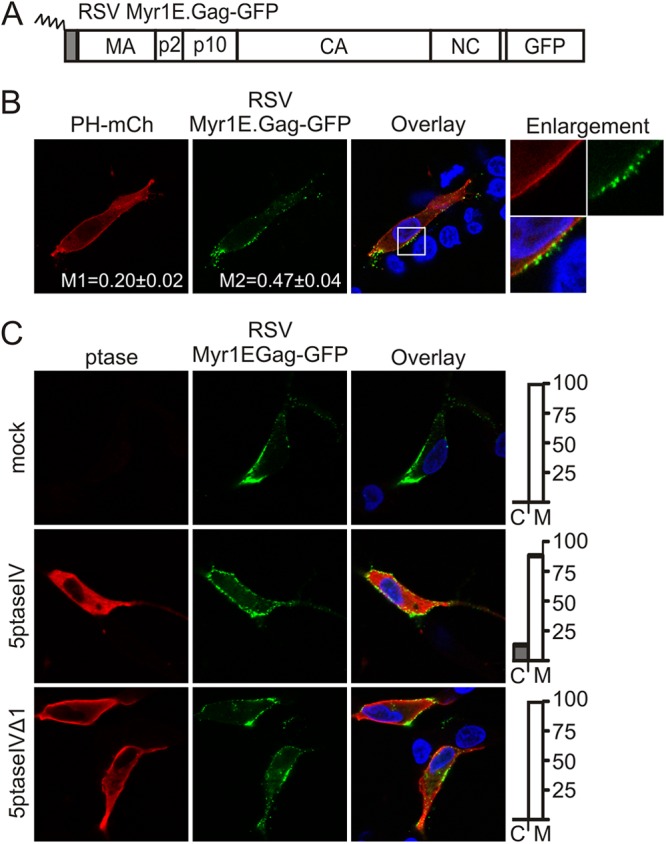
Effect of 5ptaseIV expression on RSV Gag.Myr1E-GFP localization. (A) Schematic representation of RSV Gag.Myr1E-GFP showing the 10-amino-acid membrane-binding domain of the v-Src protein as a gray box and myristylation as a zigzag line. (B) QT6 cells were transfected with pGag.Myr1E-GFP and pPH-mCherry. Mander's colocalization analysis was conducted as described in Fig. 1B. (C) QT6 cells were transfected with pGag.Myr1E-GFP alone (top row) or cotransfected with pMyc-5ptaseIV (middle row) or pMyc-5ptaseIVΔ (bottom row). Graphs on the right show the percentage of cells with Gag in the cytoplasm (C) or at the plasma membrane (M). Statistically significant differences in Gag localization were determined as described in Fig. 2A.
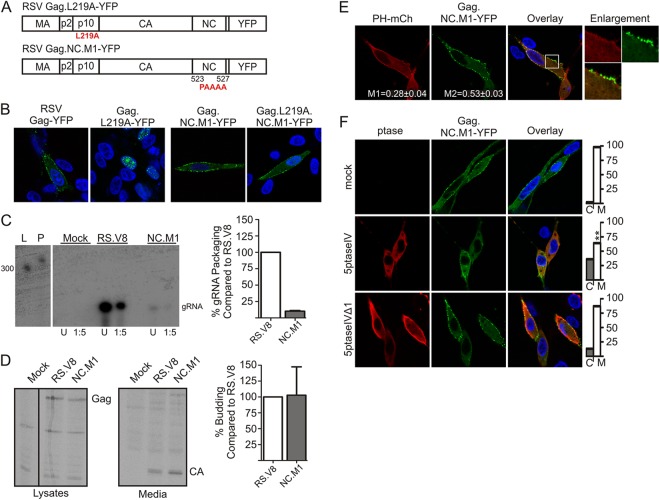
Myr1E.Gag exhibits reduced nuclear trafficking, and when expressed in a proviral context, the Myr1E viral mutant (RC.Myr1E) is impaired in genomic RNA incorporation (22, 41). We recently identified another Gag mutant with similar properties, although the MA domain was not altered in this case. Instead, the basic residues KKRK in the NC domain, implicated in binding to RSV genomic RNA (44, 45), were replaced by alanine residues (Gag.NC.M1) (Fig. 5A). We previously reported that these basic residues constitute an NLS in NC (21, 46, 47), so we examined whether the loss of the KKRK residues would reduce the nuclear localization of Gag.NC.M1 in the presence of a point mutation in p10 that disrupts the NES. Gag.L219A-YFP (Fig. 5A) accumulated in the nucleus as previously reported (Fig. 5B) (23, 40), whereas Gag.L219A.NC.M1-YFP remained at the PM, indicating that the KKRK residues in NC are required for Gag nuclear import (Fig. 5B). Furthermore, when the NC.M1 mutation was introduced into the proviral vector pRS.V8 (35, 48) and expressed in QT6 cells, genomic RNA packaging was reduced to ∼10% of the wild-type level with no effect on budding (Fig. 5C and D).
Fig 5.
Nuclear trafficking of RSV Gag is linked to 5ptaseIV sensitivity. (A) Schematic representation of RSV Gag.L219A-YFP and RSV Gag.NC.M1-YFP Gag constructs. (B) Subcellular localization of RSV Gag-YFP, RSV Gag.L219A-YFP, RSV Gag.NC.M1-YFP, and RSV Gag.L219A.NC.M1-YFP. Nuclei were stained with DAPI. (C) The Gag.NC.M1 mutation was introduced into the proviral RS.V8, which contains the gfp sequence in place of v-src (48, 61), and transfected into QT6 cells. Mutant or wild-type virus particles were harvested, normalized by exogenous reverse transcription activity (32), and assayed for genomic RNA packaging by an RNase protection assay (32, 36, 62). An autoradiographic image from a representative experiment is shown on the left, where L corresponds to the ladder and P corresponds to the undigested probe. To ensure that samples were in the linear range of detection, samples were run undiluted (U) or at a 1:5 dilution (1:5). The graph shows the mean of three separate experiments ± the standard error of the mean. (D) The effect of the NC.M1 mutation on viral budding was determined by radioimmunoprecipitation of [35S]methionine-cysteine-labeled Gag from lysates and media of QT6 cells expressing wild-type RS.V8 or RS.NC.M1 mutant viruses and quantitated by PhosphorImager analysis (35–37). A representative autoradiogram is shown on the left. Budding efficiency was calculated as in Fig. 2B, and the graph shows the mean budding efficiency from three separate experiments ± the standard error of the mean. For panels C and D, statistical significance was determined by two-tailed Student's t test. (E) QT6 cells transfected with RSV pGag.NC.M1-YFP and pPH-mCherry. Mander's analysis and statistical testing were conducted as described in Fig. 1B. (F) QT6 cells were transfected with RSV pGag.NC.M1-YFP alone (top row) or cotransfected with pMyc-5ptaseIV (middle row) or pMyc-5ptaseIVΔ (bottom row). Graphs on the right show the percentage of cells with Gag located in the cytoplasm (C) or at the PM (M). Fisher's exact test was used to determine statistical significance of the change in Gag localization, as described in Fig. 2A.
Analysis of Gag.NC.M1-YFP localization indicated partial overlap with PH-mCherry at the PM (M1 = 0.28 ± 0.04; M2 = 0.53 ± 0.03) (Fig. 5E), and the mutant protein was PM localized in nearly all cells (M = 98%, C = 2%, n = 43) (Fig. 5F). Although 5ptaseIV coexpression relocalized Gag.NC.M1-YFP away from the PM (M = 64%, C = 36%, n = 49; p < 0.0001) (Fig. 5F), the mutant was less sensitive to 5ptaseIV activity than wild-type Gag-GFP (p < 0.01). Interestingly, Gag.NC.M1 was slightly more sensitive to 5ptaseIV than was Myr1E.Gag-GFP (p < 0.05), possibly due to the increased hydrophobic or electrostatic interaction of the myristoylated Myr1E.Gag N terminus with the membrane. However, Gag.NC.M1-YFP was largely unaffected by 5ptaseIVΔ1 expression (M = 87%, C = 13%, n = 60; p > 0.05) (Fig. 5F). The observation that Myr1E.Gag and Gag.NC.M1 are both deficient in nuclear trafficking and relatively insensitive to 5ptaseIV-mediated depletion of PI(4,5)P2 and PI(3,4,5)P3 suggests an intriguing link between Gag nuclear localization and phosphoinositide-mediated PM localization. In support of this idea, those Gag proteins that do undergo nuclear trafficking (wild type and Gag.Zip) (22, 40) were more sensitive to 5ptaseIV treatment.
The aim of this study was to determine whether phosphoinositides at the PM are involved in directing RSV Gag to the PM for particle assembly. All of the wild-type and mutant Gag proteins tested were targeted to a similar location as PH-mCherry, which binds to PI(4,5)P2 at the inner leaflet of the PM (Fig. 1B, 3C, 4B, and 5E). However, our finding that depletion of PI(4,5)P2 and PI(3,4,5)P3 results in cytoplasmic Gag accumulation and a moderate reduction in VLP assembly is in contrast to those published by Chan et al. (27). Although the reasons for this discrepancy are not entirely clear, there were some differences in how the experiments were conducted that may provide an explanation. For example, we were careful to limit cellular toxicity of 5ptaseIV expression in our experiments to ensure that the cells remained healthy enough to observe the effect of 5ptaseIV expression on Gag trafficking and VLP production. We transfected 0.5 μg of 5ptaseIV plasmid DNA and we imaged the transfected cells within 15 h of transfection, whereas Chan et al. used 2 μg of DNA for transfection and analyzed cells at 20 to 24 h posttransfection. Moreover, we performed our studies in an investigator-blinded fashion to avoid bias, and experiments were statistically analyzed to ensure reproducibility. Finally, we performed budding assays after a 2.5-hour collection of radiolabeled, immunoprecipitated Gag-GFP proteins with quantitative analysis. In contrast, performing immunoblots of VLPs collected for 24 h (27) may limit the sensitivity of the assay given that the half-time of budding for RSV Gag is approximately 30 min (23).
Additional evidence that our experimental conditions did not appear to cause nonspecific mislocalization of PM-bound proteins or excessive cellular toxicity was provided by the finding of variable degrees of 5ptaseIV sensitivity among different Gag mutants. Interestingly, RSV Gag.Zip-YFP was more sensitive to 5ptaseIV expression than wild-type Gag, suggesting that that mutant is more dependent on PI(4,5)P2 and/or PI(3,4,5)P2 for stable membrane binding or that a conformational change in the mutant protein modifies the way it binds to the PM compared to the wild-type protein. In either case, the behavior of Gag.Zip-YFP suggests that RNA-driven multimerization of Gag may facilitate attachment to the PM by a different mechanism than those interactions driven by protein-protein oligomerization. Thus, it is possible that RNA interactions influence RSV Gag PM targeting and binding, as suggested for HIV-1 Gag (11).
Interestingly, our data indicate that adding the v-Src membrane-binding domain to Gag results in membrane binding that is less sensitive to depletion of PI(4,5)P2 and/or PI(3,4,5)P2 (Fig. 4C), potentially due to additional hydrophobic or electrostatic interactions of the Gag mutant with the PM. Although v-Src and HIV-1 Gag have similar MBDs composed of N-terminal myristic acid plus nearby basic residues, they differ markedly in their mechanism of membrane interaction, with HIV-1 Gag being much more sensitive to PI(4,5)P2 levels for stable membrane binding than v-Src. These findings are consistent with those reported by Ono et al., who found that a chimeric Fyn HIV-1 Gag construct was markedly less sensitive to 5ptaseIV expression (30), likely due to strong hydrophobic interactions of its myristic acid and palmitic acid moieties with the PM.
Unexpectedly, we found that altering the NLS in NC caused the mutant Gag.NC.M1 protein to be strongly localized to the PM and reduced in its sensitivity to 5ptaseIV treatment. This finding was surprising because we had not previously identified a mutant in the NC domain that altered trafficking to the PM. Furthermore, we expected the remaining NLS in MA to retain nuclear trafficking activity even in the absence of the KKRK NLS in NC, especially given that a mutant having a deletion of the entire NC sequence still traffics through the nucleus (21). Thus, it is possible that mutating the basic residues in NC alters the global conformation of Gag, inducing a structural rearrangement of the MBD that eliminates MA NLS activity while simultaneously strengthening PM binding. It is plausible that Gag.NC.M1 may adopt an “extended” conformation (49) that favors PM targeting and interferes with binding of nuclear import factors to the NLS in MA.
Gag.NC.M1 and Myr1E.Gag are both impaired in nuclear trafficking, and their membrane-binding mechanisms are less dependent on 5ptaseIV expression, suggesting a potential link between nuclear localization of Gag and PI(4,5)P2/PI(3,4,5)P3-mediated PM binding. We find this possibility to be quite intriguing because PI(4,5)P2 is present in the nucleus and has been implicated in mRNA processing, mRNA export, chromatin remodeling, and transcriptional regulation (50–57), whereas nuclear PI(3,4,5)P3 has been linked to the inhibition of apoptosis through its interaction with the nucleolar protein B23 (58). Thus, we propose the hypothesis that Gag may recruit nuclear PI(4,5)P2 or PI(3,4,5)P3 into the Gag-genomic RNA complex in the nucleus or nucleolus, as we have recently demonstrated nucleolar trafficking of RSV Gag (47). After exiting the nucleus through the nuclear pore, PI(4,5)P2 or PI(3,4,5)P3 may play a role in directing the viral ribonucleoprotein complex to the PM for final assembly of the virus particle. Because they bypass the nucleus, Gag.NC.M1 and Myr1E may not interact with nuclear phosphoinositides, altering the mechanisms by which these mutant proteins traffic to and bind to the PM. We also consider the possibility that other nuclear factors may be involved, and future studies will aim to identify host factors involved in the intracellular transport of the RSV Gag ribonucleoprotein complex from the nucleus to the PM for virion assembly.
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from the NIH to L.J.P. (R01 CA076534), D.V.B. (F30 CA165774), T.L.L. (T32 CA60395, R. Courtney, PI), and S.N.-H. (T32 CA60395, H. Isom, PI) and CURE Funds from the Pennsylvania Department of Health (L.J.P. and N.G.-O.).
We appreciate technical assistance from Malgorzata Sudol, and we acknowledge the Penn State College of Medicine Core Facilities (Imaging Core and Macromolecular Synthesis Core). We are grateful to those scientists who provided us with reagents (C. Carter, R. Craven, E. Freed, M. Resh, R. Tsien, V. Vogt, and J. Wills).
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Published ahead of print 16 January 2013
REFERENCES
- 1. Hamard-Peron E, Juillard F, Saad JS, Roy C, Roingeard P, Summers MF, Darlix JL, Picart C, Muriaux D. 2010. Targeting of murine leukemia virus gag to the plasma membrane is mediated by PI(4,5)P2/PS and a polybasic region in the matrix. J. Virol. 84:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen M, Jelinek L, Whiting S, Barklis E. 1990. Transport and assembly of gag proteins into Moloney murine leukemia virus. J. Virol. 64:5306–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prchal J, Srb P, Hunter E, Ruml T, Hrabal R. 2012. The structure of myristoylated Mason-Pfizer monkey virus matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in its membrane binding. J. Mol. Biol. 423:427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Provitera P, Bouamr F, Murray D, Carter C, Scarlata S. 2000. Binding of equine infectious anemia virus matrix protein to membrane bilayers involves multiple interactions. J. Mol. Biol. 296:887–898 [DOI] [PubMed] [Google Scholar]
- 5. Rein A, McClure MR, Rice NR, Luftig RB, Schultz AM. 1986. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc. Natl. Acad. Sci. U. S. A. 83:7246–7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee SS, Hunter E. 1991. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 10:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stansell E, Apkarian R, Haubova S, Diehl WE, Tytler EM, Hunter E. 2007. Basic residues in the Mason-Pfizer monkey virus Gag matrix domain regulate intracellular trafficking and capsid-membrane interactions. J. Virol. 81:8977–8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verderame MF, Nelle TD, Wills JW. 1996. The membrane-binding domain of the Rous sarcoma virus Gag protein. J. Virol. 70:2664–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou W, Parent LJ, Wills JW, Resh MD. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. 2008. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient Gag membrane binding. J. Virol. 82:2405–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chukkapalli V, Oh SJ, Ono A. 2010. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc. Natl. Acad. Sci. U. S. A. 107:1600–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saad JS, Ablan SD, Ghanam RH, Kim A, Andrews K, Nagashima K, Soheilian F, Freed EO, Summers MF. 2008. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J. Mol. Biol. 382:434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shkriabai N, Datta SA, Zhao Z, Hess S, Rein A, Kvaratskhelia M. 2006. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry 45:4077–4083 [DOI] [PubMed] [Google Scholar]
- 14. Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. 2008. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J. Virol. 82:11228–11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. 2006. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. U. S. A. 103:11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen K, Bachtiar I, Piszczek G, Bouamr F, Carter C, Tjandra N. 2008. Solution NMR characterizations of oligomerization and dynamics of equine infectious anemia virus matrix protein and its interaction with PIP2. Biochemistry 47:1928–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandes F, Chen K, Ehrlich LS, Jin J, Chen MH, Medina GN, Symons M, Montelaro R, Donaldson J, Tjandra N, Carter CA. 2011. Phosphoinositides direct equine infectious anemia virus gag trafficking and release. Traffic 12:438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inlora J, Chukkapalli V, Derse D, Ono A. 2011. Gag localization and virus-like particle release mediated by the matrix domain of human T-lymphotropic virus type 1 Gag are less dependent on phosphatidylinositol-(4,5)-bisphosphate than those mediated by the matrix domain of HIV-1 Gag. J. Virol. 85:3802–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmiter RD, Gagnon J, Vogt VM, Ripley S, Eisenman RN. 1978. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology 91:423–433 [DOI] [PubMed] [Google Scholar]
- 20. Schultz AM, Henderson LE, Oroszlan S. 1988. Fatty acylation of proteins. Annu. Rev. Cell Biol. 4:611–647 [DOI] [PubMed] [Google Scholar]
- 21. Butterfield-Gerson KL, Scheifele LZ, Ryan EP, Hopper AK, Parent LJ. 2006. Importin-β family members mediate alpharetrovirus Gag nuclear entry via interactions with matrix and nucleocapsid. J. Virol. 80:1798–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheifele LZ, Garbitt RA, Rhoads JD, Parent LJ. 2002. Nuclear entry and CRM1-dependent nuclear export of the Rous sarcoma virus Gag polyprotein. Proc. Natl. Acad. Sci. U. S. A. 99:3944–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheifele LZ, Ryan EP, Parent LJ. 2005. Detailed mapping of the nuclear export signal in the Rous sarcoma virus Gag protein. J. Virol. 79:8732–8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonnell JM, Fushman D, Cahill SM, Zhou W, Wolven A, Wilson CB, Nelle TD, Resh MD, Wills J, Cowburn D. 1998. Solution structure and dynamics of the bioactive retroviral M domain from Rous sarcoma virus. J. Mol. Biol. 279:921–928 [DOI] [PubMed] [Google Scholar]
- 25. Nelle TD, Wills JW. 1996. A large region within the Rous sarcoma virus matrix protein is dispensable for budding and infectivity. J. Virol. 70:2269–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray PS, Li Z, Wang J, Tang CL, Honig B, Murray D. 2005. Retroviral matrix domains share electrostatic homology: models for membrane binding function throughout the viral life cycle. Structure 13:1521–1531 [DOI] [PubMed] [Google Scholar]
- 27. Chan J, Dick RA, Vogt VM. 2011. Rous sarcoma virus Gag has no specific requirement for phosphatidylinositol-(4,5)-bisphosphate for plasma membrane association in vivo or for liposome interaction in vitro. J. Virol. 85:10851–10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kisseleva MV, Cao L, Majerus PW. 2002. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 277:6266–6272 [DOI] [PubMed] [Google Scholar]
- 29. Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. 2001. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154:1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. 2004. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 101:14889–14894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22:1567–1572 [DOI] [PubMed] [Google Scholar]
- 32. Craven RC, Leure-duPree AE, Weldon RA, Jr, Wills JW. 1995. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J. Virol. 69:4213–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Craven RC, Leure-duPree AE, Erdie CR, Wilson CB, Wills JW. 1993. Necessity of the spacer peptide between CA and NC in the Rous sarcoma virus Gag protein. J. Virol. 67:6246–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hermida-Matsumoto L, Resh MD. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Callahan EM, Wills JW. 2003. Link between genome packaging and rate of budding for Rous sarcoma virus. J. Virol. 77:9388–9398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garbitt RA, Bone KR, Parent LJ. 2004. Insertion of a classical nuclear import signal into the matrix domain of the Rous sarcoma virus Gag protein interferes with virus replication. J. Virol. 78:13534–13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weldon RA, Jr, Erdie CR, Oliver MG, Wills JW. 1990. Incorporation of chimeric Gag protein into retroviral particles. J. Virol. 64:4169–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weldon RA, Jr, Wills JW. 1993. Characterization of a small (25-kilodalton) derivative of the Rous sarcoma virus Gag protein competent for particle release. J. Virol. 67:5550–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson MC, Scobie HM, Ma YM, Vogt VM. 2002. Nucleic acid-independent retrovirus assembly can be driven by dimerization. J. Virol. 76:11177–11185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kenney SP, Lochmann TL, Schmid CL, Parent LJ. 2008. Intermolecular interactions between retroviral Gag proteins in the nucleus. J. Virol. 82:683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parent LJ, Cairns TM, Albert JA, Wilson CB, Wills JW, Craven RC. 2000. RNA dimerization defect in a Rous sarcoma virus matrix mutant. J. Virol. 74:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sigal CT, Zhou W, Buser CA, McLaughlin S, Resh MD. 1994. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc. Natl. Acad. Sci. U. S. A. 91:12253–12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silverman L, Resh MD. 1992. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J. Cell Biol. 119:415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou J, Bean RL, Vogt VM, Summers M. 2007. Solution structure of the Rous sarcoma virus nucleocapsid protein: muPsi RNA packaging signal complex. J. Mol. Biol. 365:453–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou J, McAllen JK, Tailor Y, Summers MF. 2005. High affinity nucleocapsid protein binding to the muPsi RNA packaging signal of Rous sarcoma virus. J. Mol. Biol. 349:976–988 [DOI] [PubMed] [Google Scholar]
- 46. Gudleski N, Flanagan JM, Ryan EP, Bewley MC, Parent LJ. 2010. Directionality of nucleocytoplasmic transport of the retroviral gag protein depends on sequential binding of karyopherins and viral RNA. Proc. Natl. Acad. Sci. U. S. A. 107:9358–9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lochmann TL, Bann DV, Ryan EP, Beyer AR, Mao A, Cochrane A, Parent LJ. 2 October 2012. NC-mediated nucleolar localization of retroviral Gag proteins. Virus Res. [Epub ahead of print.] doi:10.1016/j.virusres.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Federspiel MJ, Hughes SH. 1994. Effects of the gag region on genome stability: avian retroviral vectors that contain sequences from the Bryan strain of Rous sarcoma virus. Virology 203:211–220 [DOI] [PubMed] [Google Scholar]
- 49. Datta SA, Heinrich F, Raghunandan S, Krueger S, Curtis JE, Rein A, Nanda H. 2011. HIV-1 Gag extension: conformational changes require simultaneous interaction with membrane and nucleic acid. J. Mol. Biol. 406:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Albi E, Viola Magni MP. 2004. The role of intranuclear lipids. Biol. Cell 96:657–667 [DOI] [PubMed] [Google Scholar]
- 51. Barlow CA, Laishram RS, Anderson RA. 2010. Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol. 20:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boronenkov IV, Loijens JC, Umeda M, Anderson RA. 1998. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell 9:3547–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bunce MW, Bergendahl K, Anderson RA. 2006. Nuclear PI(4,5)P(2): a new place for an old signal. Biochim. Biophys. Acta 1761:560–569 [DOI] [PubMed] [Google Scholar]
- 54. Keune W, Bultsma Y, Sommer L, Jones D, Divecha N. 2011. Phosphoinositide signalling in the nucleus. Adv. Enzyme Regul. 51:91–99 [DOI] [PubMed] [Google Scholar]
- 55. Mellman DL, Anderson RA. 2009. A novel gene expression pathway regulated by nuclear phosphoinositides. Adv. Enzyme Regul. 49:11–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. 2001. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 114:2501–2511 [DOI] [PubMed] [Google Scholar]
- 57. Toska E, Campbell HA, Shandilya J, Goodfellow SJ, Shore P, Medler KF, Roberts SG. 2012. Repression of transcription by WT1-BASP1 requires the myristoylation of BASP1 and the PIP2-dependent recruitment of histone deacetylase. Cell Rep. 2:462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahn JY, Liu X, Cheng D, Peng J, Chan PK, Wade PA, Ye K. 2005. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol. Cell 18:435–445 [DOI] [PubMed] [Google Scholar]
- 59. Bolte S, Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224:213–232 [DOI] [PubMed] [Google Scholar]
- 60. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Callahan EM, Wills JW. 2000. Repositioning basic residues in the M domain of the Rous sarcoma virus Gag protein. J. Virol. 74:11222–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garbitt RA, Albert JA, Kessler MD, Parent LJ. 2001. trans-Acting inhibition of genomic RNA dimerization by Rous sarcoma virus matrix mutants. J. Virol. 75:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]