Abstract
Apoptosis induction is an important host defense mechanism to control viral infection, which is antagonized by viral proteins. Murine cytomegalovirus m41.1 encodes a viral inhibitor of BAK oligomerization (vIBO) that blocks the mitochondrial apoptosis mediator BAK. However, its importance for viral fitness in vivo has not been investigated. Here, we show that an m41.1-deficient virus attains reduced titers in salivary glands of wild-type but not Bak1−/− mice, indicating a requirement of BAK inhibition for optimal dissemination in vivo.
TEXT
Programmed cell death is thought to function as an innate antiviral defense mechanism. This hypothesis is supported by the fact that many viruses have evolved cell death-inhibiting proteins (1). However, in only a few instances has the importance of these proteins for viral fitness been determined in vivo (2–6). Murine cytomegalovirus (MCMV), a betaherpesvirus, has a protracted replication cycle and causes persistent infections in its host. MCMV expresses several cell death inhibitors, which are presumably needed to keep the host cell alive and promote viral replication and dissemination (7, 8). Since MCMV's natural host, the mouse, is an easily available and genetically manipulable organism, the MCMV-infected mouse model can serve as an excellent system to study the in vivo relevance of different cell death pathways.
In MCMV-infected cells, the extrinsic, death receptor-induced apoptosis pathway is blocked by the MCMV protein M36, which inhibits caspase-8 activation (9). An MCMV ΔM36 mutant was strongly attenuated in vitro and in vivo (2). MCMVs carrying mutations in M45 showed an even stronger attenuation in vivo with barely detectable organ titers (5, 10). The viral M45 protein inhibits both death receptor-induced and virus-induced programmed necrosis by interacting with receptor-interacting protein 1 (RIP1) and RIP3 (11, 12). The in vivo attenuation of an M45 mutant MCMV was abrogated in RIP3-deficient mice, which elegantly confirmed the importance of RIP3-dependent necrosis as a host defense mechanism (10).
Apoptosis can also be induced by different intracellular stress signals via the intrinsic mitochondrial pathway (13). These stress signals activate the proapoptotic Bcl-2 family members BAX and BAK. Activated BAX and BAK oligomerize within the outer mitochondrial membrane and increase membrane permeability, resulting in the release of cytochrome c and other proapoptotic factors into the cytosol. Cytochrome c then induces the formation of a so-called apoptosome complex and subsequent activation of caspases (13). MCMV inhibits the intrinsic apoptosis pathway at mitochondria with two separate viral proteins: a BAX-specific inhibitor, encoded by open reading frame (ORF) m38.5 (14–17), and a BAK-specific inhibitor, encoded by ORF m41.1 (18). The mitochondrion-localized m41.1 protein is expressed from the m41 locus and functions as viral inhibitor of BAK oligomerization (18). In this study, we investigated the role of m41.1/viral inhibitor of BAK oligomerization (vIBO) for MCMV replication and dissemination in mice.
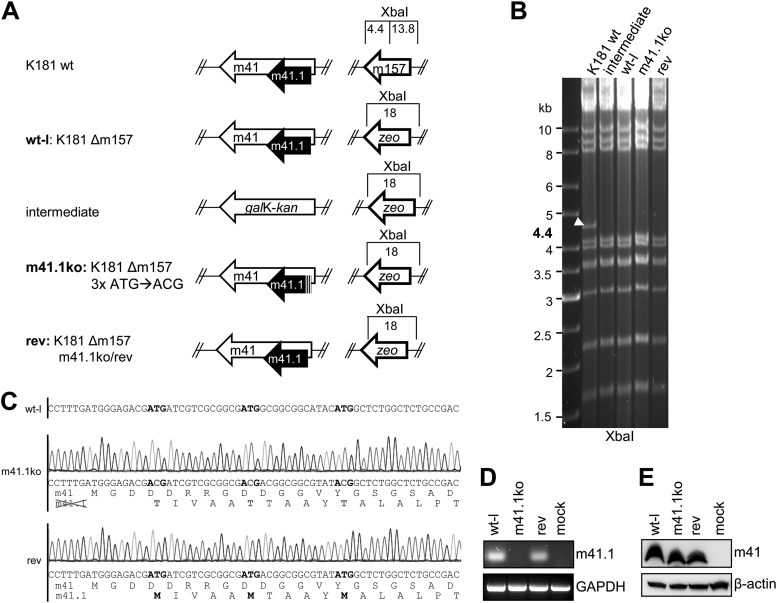
Since the previously constructed m41.1 mutants (18) were not suitable for in vivo infection experiments, we constructed a new m41.1 knockout (m41.1ko) virus and a corresponding revertant. Mutagenesis was done by homologous recombination using the bacterial artificial chromosome (BAC) clone of MCMV strain K181 (19). First, the viral m157 gene was deleted by replacing it with a zeocin resistance (zeo) marker, as described previously (20) (Fig. 1A). This deletion was necessary in order to infect C57BL/6 mice, which are resistant to MCMV infection due to expression of the NK cell receptor Ly49H (21–24). Resistance can be overcome by removal of m157, which encodes a Ly49H ligand (20). In this Δm157 wild-type-like (wt-l) virus genome, the m41 locus was first replaced by a galK-kan cassette using kanamycin for positive selection. Using 2-deoxy-galactose for negative selection, galK-kan was then replaced by an m41 sequence in which all three m41.1 ATG codons were mutated to ACG, leaving the protein sequence of the overlapping m41 ORF unchanged (18). Using the same two-step replacement strategy (which is described in detail elsewhere [25]), the revertant (rev) was constructed (Fig. 1A). All BACs were analyzed by restriction digestion with XbaI (Fig. 1B), as well as SnaBI and ScaI (not shown), and the entire m41 locus was sequenced to ensure that only the intended changes were introduced (Fig. 1C). Recombinant viruses were reconstituted by transfecting BAC DNA into 10.1 fibroblasts (26) using Polyfect transfection reagent (Qiagen). Viral DNA was purified from virions as described previously (27) and analyzed by restriction digestion. The restriction patterns of the wt-l, m41.1ko, and rev viruses were as predicted (data not shown). To verify that m41.1 was knocked out and expression of the unrelated m41 protein had remained unchanged, lysates of infected cells were analyzed for m41 expression by immunoblotting using the m41-specific antibody 2A6 and for m41.1 expression by reverse transcription (RT)-PCR (Fig. 1D and E).
Fig 1.
Construction and genetic analysis of mutant MCMVs. (A) The m157 gene was replaced by a zeo marker within the MCMV K181 BAC to facilitate infection of C57BL/6 mice. This K181Δm157 virus was used as a wild-type-like (wt-l) virus in all experiments. To generate an m41.1ko virus, the m41 locus was first replaced by galK-kan followed by reinsertion of a mutated m41 ORF, in which the three ATGs of m41.1 had been changed to ACG. The revertant virus was constructed using the same two-step strategy. (B) BAC DNA of wt and mutant MCMV genomes was digested to check for m157 deletion with XbaI and separated on 0.6% agarose gels. A 4.4-kb fragment in the parental K181 BAC is indicated by an arrowhead. (C) Sequencing of wt-l, m41.1ko, and rev BAC DNA. In the wt-l sequence, ATG is highlighted in boldface. Point mutations within the m41.1 ATG codons are indicated in gray. Translated amino acid sequences are shown beneath the nucleotide sequences. (D) 10.1 fibroblasts were infected with wt-l, m41.1ko, and rev viruses. At 24 hpi, m41.1 expression was analyzed by RT-PCR using a forward primer that ends on the T of the second m41.1 ATG codon (5′-ATGATCGTCGCGGCGAT-3′ and 5′-GGAGGGCGCGACGAAAG-3′). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (E) Expression of m41 was analyzed by immunoblotting in virus-infected RAW264.7 macrophages using the m41-specific antibody 2A6 (18).
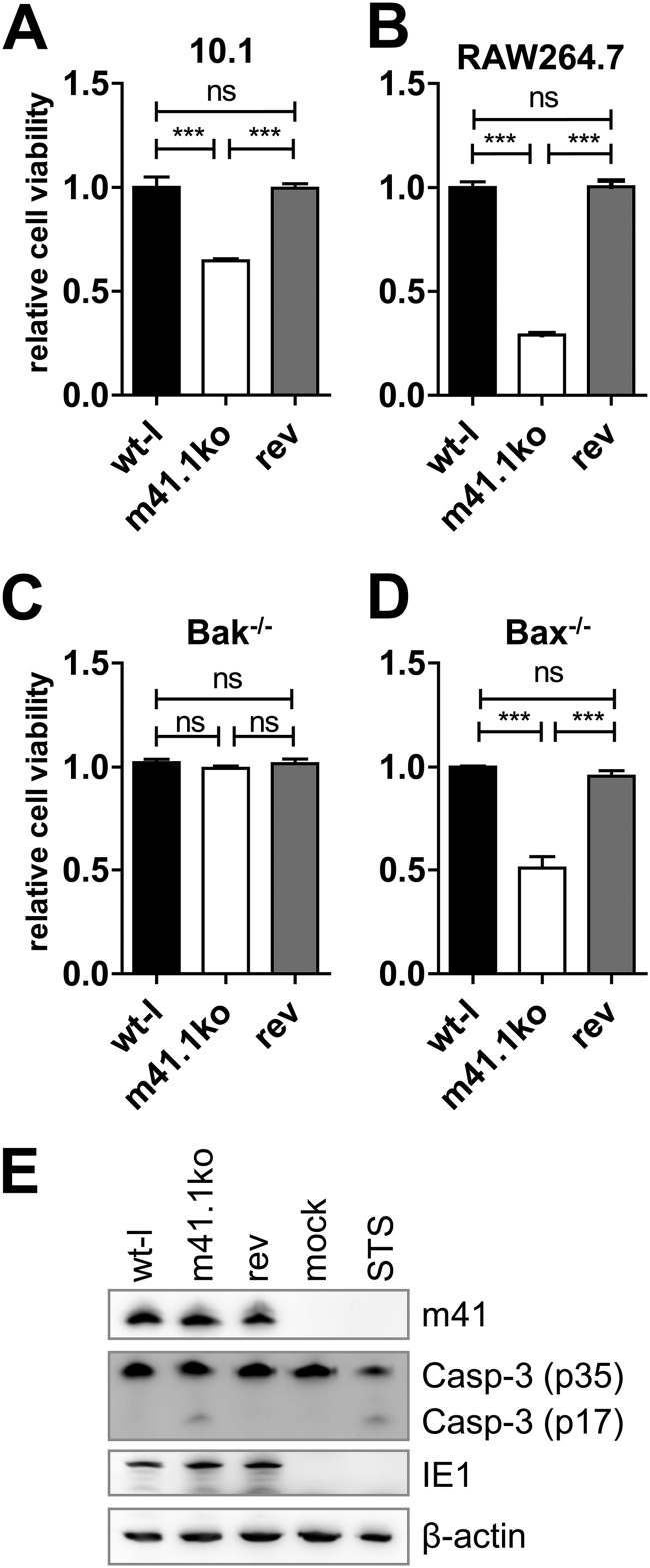
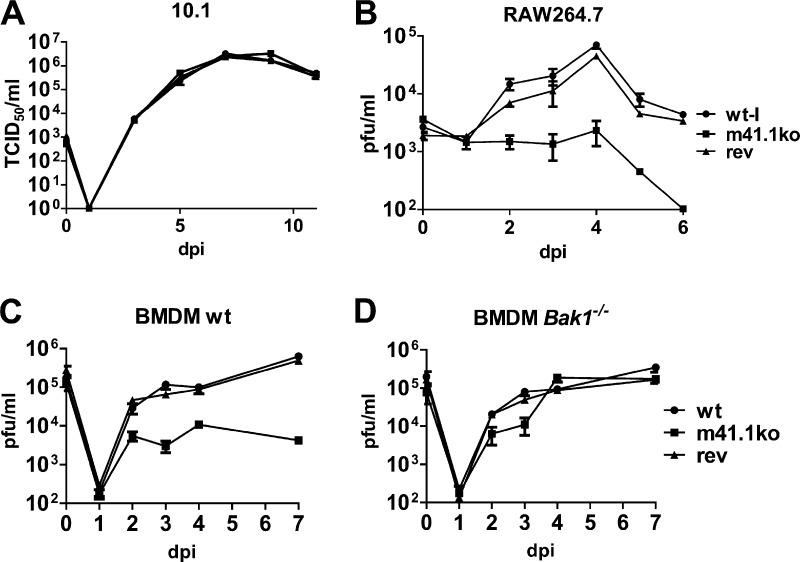
Before using the newly constructed K181-derived viruses for in vivo experiments, we functionally characterized them in cell culture-based assays. First we infected 10.1 fibroblasts at a multiplicity of infection (MOI) of 5. Six hours postinfection (hpi), cells were stimulated with 200 nM staurosporine (STS) (Sigma) to induce apoptosis. Cell viability was measured 24 hpi by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium] assay (CellTiter 96 AQueous cell proliferation assay; Promega). As shown in Fig. 2A, cells infected with the m41.1ko virus were more sensitive to STS-induced apoptosis than the parental wt-l or rev viruses. Next we infected RAW264.7 macrophages (ATCC TIB-71), which are more sensitive than fibroblasts to infection-induced proapoptotic stress. Two days postinfection (dpi), macrophages infected with m41.1ko virus showed clearly reduced viability compared to cells infected with the control viruses (Fig. 2B). To confirm that the observed phenotype was BAK dependent, BAK and BAX knockout cells (28) were infected for 72 h. Cell viability was then analyzed by MTS assay. As shown in Fig. 2C and D, BAK deficiency but not BAX deficiency compensated for the loss of m41.1. Infection of 10.1 cells with m41.1ko resulted in caspase-3 activation (Fig. 2E), confirming that m41.1 is required for apoptosis inhibition. In addition, the replication kinetics of the three viruses were determined on 10.1 fibroblasts and RAW264.7 macrophages. Replication of the m41.1ko virus was indistinguishable from that of the control viruses in 10.1 fibroblasts but significantly reduced in macrophages (Fig. 3A and B), consistent with previously published results (18). The m41.1ko virus also replicated to lower titers in primary bone marrow-derived macrophages (BMDMs) of C57BL/6J but not BAK-deficient mice (B6.129-Bak1tm1Thsn/J) (29) mice, indicating that the replication defect is BAK dependent (Fig. 3C and D).
Fig 2.
Characterization of mutant MCMVs in cell culture. (A) 10.1 fibroblasts were infected with wt-l, m41.1ko, and rev viruses at an MOI of 5. Six hours postinfection, cells were stimulated with staurosporine (STS). Cell viability was analyzed by MTS assay at 24 hpi. (B) RAW264.7 macrophages were infected with the indicated viruses (MOI of 5). Cell viability was measured 48 hpi by MTS assay. (C and D) Bak- or Bax-deficient mouse embryonic fibroblasts (MEFs) were infected with wt-l, m41.1ko, or rev. Cell viability was determined 72 hpi. Experiments were done with 5 or more replicates; means and standard errors of the means (SEM) are shown. Statistical significance was assessed by Student's t test (***, P < 0.001; ns, not significant). (E) 10.1 fibroblasts were infected with wt-l, m41.1ko, and rev virus or treated with STS to induce apoptosis. At 21 hpi, caspase-3 cleavage was analyzed by immunoblotting using a capase-3 (Casp-3)-specific antibody (no. 9662; Cell Signaling). IE1 was detected as an infection control and β-actin as a loading control using monoclonal antibodies (MAbs) CROMA101 and Ac-74 (Sigma), respectively.
Fig 3.
Replication of mutant MCMVs in cell culture. (A and B) 10.1 fibroblasts or RAW264.7 macrophages were infected with wt-l, m41.1ko, or rev virus at an MOI of 0.02. Supernatants were collected, and viral titers were determined by plaque assay or the TCID50 method. (C and D) BMDMs were isolated as described previously (31). On day 7 after isolation, cells were infected with wt-l, m41.1ko, or rev virus at an MOI of 0.02. Supernatants were collected and viral titers were determined by plaque assay. All experiments were done in duplicate or triplicate; means and SEM are shown.
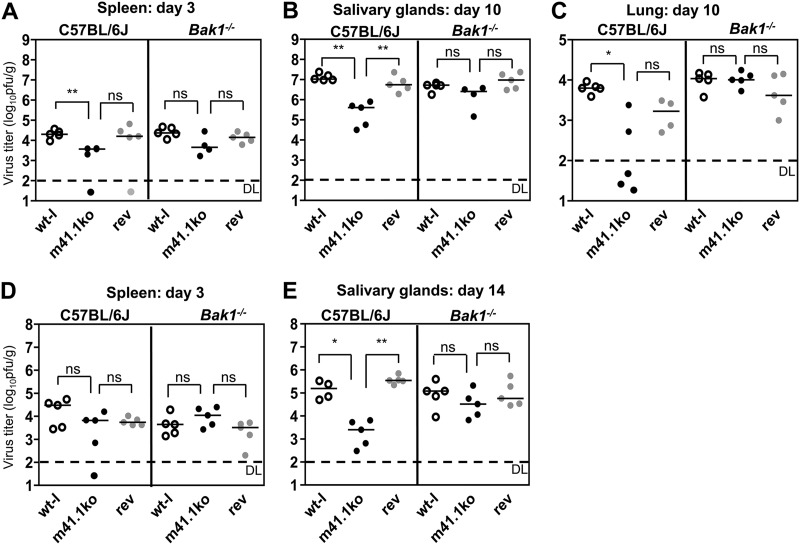
Manzur and colleagues showed that the viral BAX inhibitor m38.5 is important for MCMV replication and dissemination in mice (16). Therefore, we wanted to analyze if BAK inhibition by m41.1 is also of importance for viral replication and spread. To do this, we infected 8- to 10-week-old C57BL/6J and Bak1−/− mice with 2 × 105 PFU of wt-l, m41.1ko, or rev virus intravenously or intraperitoneally (Fig. 4A to E). Mice were sacrificed on days 3 and 14 after intraperitoneal infection and, due to a faster viral dissemination, on days 3 and 10 after intravenous infection. Spleen, salivary gland (SG), and lung titers were determined by plaque assay. On day 3 postinfection, viral titers in the spleen did not differ significantly independent of the mouse strain used (Fig. 4A and D). However, the m41.1ko strain reached moderately (10- to 50-fold) but significantly reduced SG titers compared to wt-l and rev viruses in C57BL/6J mice (Fig. 4B and E). In Bak1−/− mice, the SG titers were similar for all three viruses, providing strong support for the notion that BAK inhibition is necessary for optimal replication and dissemination to SGs. The m41.1ko virus also reached lower titers in lungs of C57BL/6J mice (Fig. 4C), although lung titers generally showed more variability. To rule out that a spontaneous reversion was responsible for the modest attenuation of m41.1ko viruses in vivo, we sequenced the m41 locus of m41.1ko viruses isolated from SGs and lungs of C57BL/6J mice. All sequenced isolates carried the mutant m41.1 sequence (data not shown).
Fig 4.
Replication of the m41.1ko virus in C57BL/6 and Bak1−/− mice. C57BL/6 or Bak1−/− mice were infected either intravenously (A to C) or intraperitoneally (D and E) with 2 × 105 PFU/mouse. On days 3 (A and D) and 10 (B and C) or 14 (E) postinfection, mice were sacrificed. Virus titers in the spleen, lungs, and SGs were determined by plaque assay. Statistical significance was assessed using the Mann-Whitney test (*, P < 0.05; **; P < 0.01; ns, not significant). DL, detection limit.
Previous studies with MCMV ΔM36 and ΔM45 mutants have demonstrated a severely attenuated phenotype of these viruses in vivo (2, 5). In contrast, MCMV mutants with an inactivated m38.5 (16) or m41.1 (this study) gene were only modestly attenuated in mice, reaching about 10- to 100-fold-lower SG titers. As these organs are important for MCMV persistence and transmission, even a modest attenuation is likely to be of biological importance. One should also keep in mind that the m38.5 and m41.1 gene products target two related proapoptotic mitochondrial proteins, BAX and BAK, that have been proposed to act in a largely redundant fashion (30). In fact, an m38.5 m41.1 double-knockout (dko) virus has shown a more prominent phenotype in cultured cells than the single-gene knockouts (18). Hence it might be necessary to test a dko virus in the mouse to fully appreciate the role of the intrinsic, mitochondrially mediated apoptosis pathway as an antiviral defense mechanism. However, MCMV with its highly specific inhibitors offers the unique opportunity to study the contributions of BAX and BAK to infection control individually.
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft grant BR1730/3 to W.B.
We thank Mijo Golemac for help with animal experiments, which were performed at the University of Rijeka according to national and institutional animal care and ethical guidelines and were approved by local ethical committees. All mice were raised under specific-pathogen-free conditions at the Central Animal Facility of the Medical Faculty, University of Rijeka, Croatia.
Footnotes
Published ahead of print 9 January 2013
REFERENCES
- 1. Benedict CA, Norris PS, Ware CF. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013–1018 [DOI] [PubMed] [Google Scholar]
- 2. Cicin-Sain L, Ruzsics Z, Podlech J, Bubic I, Menard C, Jonjic S, Reddehase MJ, Koszinowski UH. 2008. Dominant-negative FADD rescues the in vivo fitness of a cytomegalovirus lacking an antiapoptotic viral gene. J. Virol. 82:2056–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lima BD, May JS, Marques S, Simas JP, Stevenson PG. 2005. Murine gammaherpesvirus 68 bcl-2 homologue contributes to latency establishment in vivo. J. Gen. Virol. 86:31–40 [DOI] [PubMed] [Google Scholar]
- 4. Feng P, Liang C, Shin YC, Xiaofei E, Zhang W, Gravel R, Wu TT, Sun R, Usherwood E, Jung JU. 2007. A novel inhibitory mechanism of mitochondrion-dependent apoptosis by a herpesviral protein. PLoS Pathog. 3:e174 doi:10.1371/journal.ppat.0030174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lembo D, Donalisio M, Hofer A, Cornaglia M, Brune W, Koszinowski U, Thelander L, Landolfo S. 2004. The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J. Virol. 78:4278–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor JM, Barry M. 2006. Near death experiences: poxvirus regulation of apoptotic death. Virology 344:139–150 [DOI] [PubMed] [Google Scholar]
- 7. Andoniou CE. 2010. Suicide watch: how cytomegalovirus interferes with the cell-death pathways of infected cells. Tissue Antigens 76:1–8 [DOI] [PubMed] [Google Scholar]
- 8. Handke W, Krause E, Brune W. 2012. Live or let die: manipulation of cellular suicide programs by murine cytomegalovirus. Med. Microbiol. Immunol. 201:475–486 [DOI] [PubMed] [Google Scholar]
- 9. Ménard C, Wagner M, Ruzsics Z, Holak K, Brune W, Campbell AE, Koszinowski UH. 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77:5557–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mack C, Sickmann A, Lembo D, Brune W. 2008. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. U. S. A. 105:3094–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Upton JW, Kaiser WJ, Mocarski ES. 2008. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 283:16966–16970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green DR, Kroemer G. 2004. The pathophysiology of mitochondrial cell death. Science 305:626–629 [DOI] [PubMed] [Google Scholar]
- 14. Arnoult D, Skaletskaya A, Estaquier J, Dufour C, Goldmacher VS. 2008. The murine cytomegalovirus cell death suppressor m38.5 binds Bax and blocks Bax-mediated mitochondrial outer membrane permeabilization. Apoptosis 13:1100–1110 [DOI] [PubMed] [Google Scholar]
- 15. Jurak I, Schumacher U, Simic H, Voigt S, Brune W. 2008. Murine cytomegalovirus m38.5 protein inhibits Bax-mediated cell death. J. Virol. 82:4812–4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manzur M, Fleming P, Huang DC, Degli-Esposti MA, Andoniou CE. 2009. Virally mediated inhibition of Bax in leukocytes promotes dissemination of murine cytomegalovirus. Cell Death Differ. 16:312–320 [DOI] [PubMed] [Google Scholar]
- 17. Norris KL, Youle RJ. 2008. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J. Virol. 82:6232–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Çam M, Handke W, Picard-Maureau M, Brune W. 2010. Cytomegaloviruses inhibit Bak- and Bax-mediated apoptosis with two separate viral proteins. Cell Death Differ. 17:655–665 [DOI] [PubMed] [Google Scholar]
- 19. Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, Shellam GR. 2005. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. J. Virol. 79:2998–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, Jonjic S, Koszinowski UH. 2004. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J. Virol. 78:7536–7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934–937 [DOI] [PubMed] [Google Scholar]
- 22. Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28:42–45 [DOI] [PubMed] [Google Scholar]
- 24. Scalzo AA, Fitzgerald NA, Simmons A, La Vista AB, Shellam GR. 1990. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J. Exp. Med. 171:1469–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36 doi:10.1093/nar/gni035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvey DM, Levine AJ. 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 5:2375–2385 [DOI] [PubMed] [Google Scholar]
- 27. Schumacher U, Handke W, Jurak I, Brune W. 2010. Mutations in the M112/M113-coding region facilitate murine cytomegalovirus replication in human cells. J. Virol. 84:7994–8006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19:1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6:1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blanc M, Hsieh WY, Robertson KA, Watterson S, Shui G, Lacaze P, Khondoker M, Dickinson P, Sing G, Rodriguez-Martin S, Phelan P, Forster T, Strobl B, Muller M, Riemersma R, Osborne T, Wenk MR, Angulo A, Ghazal P. 2011. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 9:e1000598 doi:10.1371/journal.pbio.1000598 [DOI] [PMC free article] [PubMed] [Google Scholar]