Abstract
PARV4 is a small DNA human virus that is strongly associated with hepatitis C virus (HCV) and HIV infections. The immunologic control of acute PARV4 infection has not been previously described. We define the acute onset of PARV4 infection and the characteristics of the acute-phase and memory immune responses to PARV4 in a group of HCV- and HIV-negative, active intravenous drug users. Ninety-eight individuals at risk of blood-borne infections were tested for PARV4 IgG. Gamma interferon enzyme-linked immunosorbent spot assays, intracellular cytokine staining, and a tetrameric HLA-A2–peptide complex were used to define the T cell populations responding to PARV4 peptides in those individuals who acquired infection during the study. Thirty-five individuals were found to be PARV4 seropositive at the end of the study, eight of whose baseline samples were found to be seronegative. Persistent and functional T cell responses were detected in the acute infection phase. These responses had an active, mature, and cytotoxic phenotype and were maintained several years after infection. Thus, PARV4 infection is common in individuals exposed to blood-borne infections, independent of their HCV or HIV status. Since PARV4 elicits strong, broad, and persistent T cell responses, understanding of the processes responsible may prove useful for future vaccine design.
INTRODUCTION
PARV4 is a small, nonenveloped single-stranded DNA virus of the Parvoviridae family that has been commonly associated with parenteral transmission (1–4). The PARV4 genome contains two open reading frames that encode a nonstructural (NS) and a capsid (VP) protein. Though PARV4 is generally absent from healthy individuals in western countries, 8 to 30% of hepatitis C virus (HCV)-infected individuals have been found to be PARV4 DNA or IgG positive (2, 4–8). This level can reach up to 95% among HIV- and HCV-coinfected individuals (9). Despite the growing body of evidence emerging on the prevalence of PARV4 exposure in remotely infected cohorts, relatively little is known about the features that accompany acute acquisition of PARV4 in such at-risk cohorts (3, 10–12).
We previously analyzed the immune responses to PARV4 and described a striking T cell response to the NS protein in HCV+ and HIV+ individuals (13). However, this analysis was cross-sectional and the time point of infection was not known in these cases. Additionally, we were interested in studying PARV4 infection independently of other coinfections. Therefore, we subsequently sought a cohort of individuals who were HCV and HIV-1 negative but had a risk of acquiring PARV4 so that we could study acute acquisition of the virus and the evolution of immune responses in relation to viremia and seroconversion.
We describe here a rare cohort of active intravenous drug users (IDUs), both HIV and HCV negative, who acquired PARV4 during the period they were under study. Because of the detailed nature of the study, with monthly follow-up over several years, it was possible to precisely identify the time of PARV4 seroconversion. We describe here the incidence of PARV4 in this cohort and the duration of viremia and characterize the humoral and cellular immune responses in the acute phase of PARV4 infection through the analysis of longitudinal plasma and peripheral blood mononuclear cell (PBMC) samples. Our findings confirm a transient detectable viremia in the acute phase of disease that is associated with early seroconversion but a late evolution of T cell responses.
MATERIALS AND METHODS
Patient cohort and study design.
This study was approved by the Johns Hopkins School of Medicine Institutional Review Board. Informed patient consent was obtained from 98 HCV- and HIV-negative active IDUs from the Baltimore Before-and-After Acute Study of Hepatitis (BBAASH) to have blood drawn for isolation of plasma and PBMCs in a protocol designed for monthly follow-up. These individuals were between 15 and 30 years of age and acknowledged the use of injection drugs (14). These individuals were selected from the BBAASH cohort according to the following criteria: that they remain HIV and HCV uninfected during the course of the study and that they be followed up for 24 months or more. At enrolment, the time of intravenous drug use was less than 2 years for 90% of the individuals. Figure 1 illustrates the design of this study.
Fig 1.
Study design of acute PARV4 infection in the BBAASH cohort. Shown is a schematic representation of the assays of samples from this cohort carried out to assess the characteristics of the immune response to acute PARV4 infection.
Serological screening.
Plasma samples were screened for anti-PARV4 IgG as previously described (4). Seroconversion in this study refers to PARV4 IgG status. Levels of anti-PARV4 IgG over time were assessed by testing all available plasma samples from subjects BA1 to BA8 (median time span, 33 months; range, 14 to 63 months). To normalize between runs, net optical density readings of samples were converted to arbitrary units by comparison to an anti-PARV4 VP2 reference serum of 100 arbitrary units/μl that was used throughout the study.
DNA extraction, PCR amplification, and viral loads.
DNA was extracted from plasma samples from subjects BA1 to BA8 by using the AllPrep DNA/RNA kit (Qiagen) according to the manufacturer's instructions. Nested PCR assays for PARV4 VP and estimation of viral loads were carried out as described elsewhere (3). The minimum length of viremia was measured from the first day when PARV4 DNA was detected to the last. The maximum duration of viremia was defined from the day after the last DNA negative time point before viremia until 1 day before the next DNA-negative time point.
T cell assays.
Gamma interferon enzyme-linked immunosorbent spot (ELISpot) assays were carried out for individuals BA1 to BA8 by using PARV4 NS peptides as previously described (13). Dimethyl sulfoxide and concanavalin A (20 μg/ml; Sigma) were used as negative and positive controls, respectively. All time points postseroconversion were tested, including the time point prior to seroconversion (median time span, 11 months; range, 4 to 63 months). A cutoff of 55 spot-forming units (SFU)/106 PBMCs per pool of peptides was considered positive, after the subtraction against the average background well, as defined previously (13).
Phenotypic analysis using the corresponding fluorochrome-conjugated tetramer complex HLA-A2 RMT–phycoerythrin (PE) was carried out with samples from individuals BA1 (five samples spanning 22 months) and BA4 (four samples spanning 15 months), as they showed a T cell response to previously defined epitope RMTENIVEV (13). Four PBMC samples from BA1, spanning 22 months, were also tested by intracellular cytokine staining (ICS) as described previously (13). Cells were incubated with epitope RMTENIVEV for 5 to 6 h, and brefeldin A (2 μg/ml, Sigma) was added after 1 h of stimulation.
Cells were stained with live-dead marker (L10119), CD3-Pacific Orange, CD4-QDot 605 (Invitrogen), CD8-Pacific Blue, CCR7-PECy7, CD45RA-fluorescein isothiocyanate (FITC), perforin-FITC, granzyme B-Alexa 700, PD-1–PECy7, gamma interferon (IFN-γ)-Alexa Fluor 700, MIP1-β–PE, tumor necrosis factor alpha (TNF-α)-PECy7, CD107a-PECy5, interleukin-2 (IL-2)–allophycocyanin (APC; BD Pharmingen), CD57-Pacific Blue, CD38-PerCPCy5.5, granzyme A-PerCPCy5.5 (Biolegend), and CD127-APC (MACS Miltenyi Biotech) antibodies. All samples were processed on a BD LSR II and analyzed by using FlowJo software. Cells were gated on live CD3+ lymphocytes unless otherwise indicated. SPICE software was used to analyze single-cell function (15).
RESULTS
High prevalence of PARV4 infection and identification of acute infection.
Ninety-eight active IDUs that were HCV and HIV negative were tested for PARV4 infection. Plasma samples from the latest time point studied were screened for anti-PARV4 IgG. Thirty-five (36%) of the 98 were PARV4 seropositive (data not shown). Thirty-three seropositive individuals (two were unavailable for further study) were also tested for these antibodies by using the first sample available. Eight individuals (BA1 to BA8) were IgG negative at the earliest time point and thus had seroconverted for PARV4 IgG during the study (Fig. 1 and Table 1).
Table 1.
Follow-up and detection of PARV4 IgG, IgM, and viremia in individuals BA1 to BA8
| Individual | Follow-up duration (mo) | IgG detection (mo after first visit) | IgM detection (mo after first visit) | IgM duration (days)b | Viremia detection (mo after first visit) | Viremia duration (days)a | Peak viral load/ml plasma |
|---|---|---|---|---|---|---|---|
| BA1 | 63 | 1 | At first time point | NA | At first time point | NA | 104 |
| BA2 | 37 | 13 | 13 | NA | 13, 14 | 33–109 | 103 |
| BA3 | 30 | 19 | 19, 20 | 32–93 | 18, 19 | 32–98 | 104 |
| BA4 | 14 | 2 | Not detected | Not detected | |||
| BA5 | 32 | 27 | 27 | NA | 27 | Max, 102 | 5 × 102 |
| BA6 | 31 | 22 | 22 | NA | 22 | Max, 63 | 105 |
| BA7 | 33 | 30 | 30 | NA | 30, 31 | 30–124 | 104 |
| BA8 | 40 | 40 | Not detected | 40 | NA | 103 |
The minimum viremia duration was measured from the first day when PARV4 DNA was detected to the last. The maximum viremia duration was defined from the day after the last DNA-negative time point before viremia until 1 day before the next DNA-negative time point.
NA, not available as IgM or viremia was detected at only one time point.
The rate of PARV4 infection was deduced from the rates of IgG seroconversion. Clinic attendance data were available for 60 individuals, 23 of whom were PARV4 seropositive at the end of the study. Subjects attended the clinic for a mean time of 36 months (range, 5 to 81 months), and more than 90% had been using intravenous drugs for less than 2 years at enrolment. This translates to a total time of illicit drug use of 5 years (36 months plus 2 years). If it is assumed that these individuals acquired PARV4 through intravenous drug use, then 23/60 individuals were infected with PARV4 over 5 years. This translates to an incidence rate of (23/60)/5 per year, i.e., 7.6%/year overall. This can be dissected into the rate of incidence at enrolment (25 IgG+ individuals out of 96, over 2 years of IDU), i.e., (25/96)/2 = 13%/year, followed by eight new seroconverters over the 3 years of follow-up, i.e., [8/(96 − 25)]/3 = 3.8%/year.
Serum samples collected before, at, and after seroconversion were tested for PARV4 IgM. IgM was detected in six of these eight individuals (Table 1), providing additional evidence of acute infection. IgM was detected at only one time point in each individual, except for BA3, in whom IgM persisted for over 2 months (Table 1).
Viremia duration was calculated by using the length of time that viral DNA could be detected in the samples around the time of seroconversion (see Materials and Methods). In those individuals for whom such detailed longitudinal analyses were possible (n = 4), viremia was estimated to have lasted between 32 and 104 days (range, 30 to 125 days). Viral loads were between 5 × 102 and 105 (median, 4.7 × 103)/ml of plasma (Table 1).
Acute-phase PARV4 infection elicits a strong humoral and/or cellular immune response.
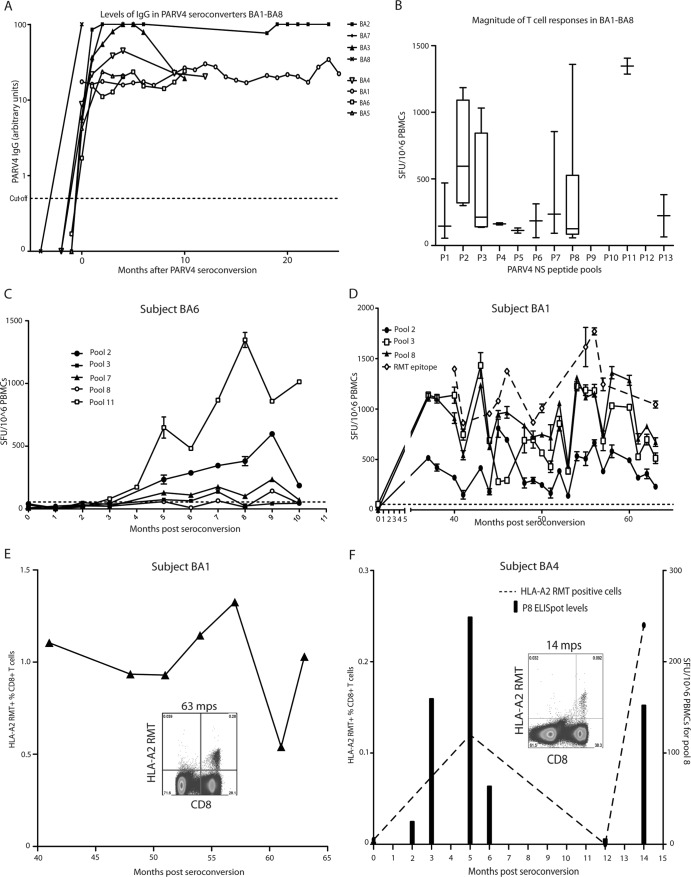
Anti-PARV4 IgG levels were tested at every time point available for subjects BA1 to BA8 and were plotted relative to the date of seroconversion (Fig. 2A). Following seroconversion, IgG antibody titers rose rapidly to high levels in all patients, with subjects BA2, BA3, BA7, and BA8 reaching or exceeding levels detected in the reference serum (Fig. 2A).
Fig 2.
Humoral and cellular immune responses to PARV4 in the acute phase of infection. (A) Levels of anti-PARV4 IgG increase steeply or remain below 50 arbitrary units. The data were normalized to the date of seroconversion of each subject. The cutoff was set at 0.5 arbitrary unit (4) (B) The magnitudes of T cell responses to PARV4 NS across all BA individuals, as measured by IFN-γ ELISpot assay, were plotted by using the time point with the highest value when several were available. (C) Broad T cell responses are elicited in BA6. The dashed line indicates the cutoff at 55 SFU/106 PBMCs. (D) T cell responses are maintained at high levels several years after infection in BA1. (E) RMT-specific T cells detected by tetramer (HLA-A2 RMT) staining in BA1. The inset shows the FACS plot at 63 mps. (F) RMT-specific T cells detected by tetramer (HLA-A2 RMT) staining in BA4 together with the levels of T cell responses to pool 8 (P8; containing the RMT epitope) shown by ELISpot assay. The inset shows the FACS plot at 14 mps. The connecting line is dashed, as samples from only four time points were measured for RMT-specific cells.
Samples from all of these time points were tested for T cell responses by IFN-γ ELISpot assay with PARV4 NS peptide pools. Several individuals showed strong, broad, and long-lasting T cell responses to NS that reached >1,000 SFU/106 PBMCs (Fig. 2B to D and 3). T cell responses were consistently seen several years after infection, as demonstrated in subject BA1, from whom all PBMC samples but one were taken 3 years after seroconversion (Fig. 2D). In this individual, T cell responses to peptides 2.3 (pool 2) and 3.6 (pool 3) and to the previously identified RMT epitope (RMTENIVEV [13], pool 8), which triggered the highest T cell response in this patient, were elicited (Fig. 2D). The detection of epitope-specific cells (here described as RMT-specific cells) with fluorochrome-conjugated tetramer complex HLA-A2 RMT in subjects BA1 and BA4 confirmed that these RMT-specific cells are maintained over years (Fig. 2E and F). RMT-specific cells represented 0.5 to 1.3% of the BA1 CD8+ T cells (Fig. 2E) and 0.1 to 0.2% of the BA4 CD8+ T cells (Fig. 2F). Although T cell responses in subject BA4 tested by IFN-γ ELISpot assay were observed at 5 months postseroconversion (mps), they had disappeared by 12 months (Fig. 2F, columns). However, they did reappear 2 months later. This was confirmed through tetramer staining, as RMT-specific cells were absent at 12 months but recovered to higher levels 2 months later (Fig. 2F, line).
Fig 3.
Prolonged T cell responses in PARV4 seroconverters. Shown is a summary of positive T cell responses (columns) and anti-PARV4 IgG levels (curves) in subjects BA1 to BA8. The number of pools eliciting positive responses is indicated below each time point. The dashed horizontal line corresponds to the cutoff for IgG seropositivity (referring to the left-hand y axis) as established previously by Sharp et al. (4). For individual BA1, samples between the first time point and 37 months of follow-up were missing as the subject did not attend the clinic during that time. This is indicated by the dashed IgG curve of this individual. The presence of viral DNA is indicated by the asterisk below each graph.
Though antibody levels were consistently high in all of our individuals, there was more diversity in T cell responses, which were strong in subjects BA1 and BA6 but weak in subjects BA3 and BA7 (Fig. 3). PARV4 NS triggered a late T cell response in most of the individuals that peaked at around 5 months after seroconversion. No CD4+ T cell responses were detected in response to PARV4 NS, as seen in our previous study of chronic infection (data not shown; 13).
PARV4 RMT-specific T cells have an effector memory, mature, and activated phenotype.
Having confirmed the late evolution of a strong CD8+ T cell response, we next addressed the phenotype and functionality of these memory populations. RMT-specific cells from patient BA4 were 53 to 62% CCR7− CD45RA− (effector memory cells, TEM, Fig. 4A and B). RMT-specific cells from patient BA1 also had an effector memory phenotype, but that was more terminally differentiated and ranged from 65 to 76% CCR7− CD45RA+ (effector memory CD45RA+ cells, TEMRA), throughout the course of study (Fig. 4A and B).
Fig 4.
PARV4 epitope-specific CD8+ T cells have an effector memory phenotype. The memory phenotype was assessed from the expression of CCR7 and CD45RA on tetramer-positive cells. Representative FACS plots are shown for BA4 and BA1 in panel A, which is gated on live CD3+ CD8+ HLA-A2 RMT tetramer-positive cells, and the changes in phenotype over time are shown in panel B. PARV4-specific CD8+ T cells have a memory phenotype distinct from that of other CD8+ T cells, as shown in panel C. The panels on the left are gated on live CD3+ CD8+ HLA-A2 RMT tetramer-positive cells, the panels in the middle are gated on live CD3+ CD8+ HLA-A2 RMT tetramer-negative (neg) cells, and the panels on the right are gated on live CD3+ CD8+ T cells.
CD127 and CD57 were studied to assess antigen exposure and cellular senescence, respectively. From 0.6 to 20% of the BA1 antigen-specific cells were CD127+ (Fig. 5A), and 2% of the BA4 cells were CD127+ (Fig. 5B). Because of limited samples, CD57 staining was carried out only with PBMCs from BA1. Antigen-specific cells were 80 to 85% CD57+ throughout the duration of the study (Fig. 5A).
Fig 5.
PARV4-specific CD8+ T cells are activated and mature and contain cytolytic proteins. RMT-specific T cells detected by tetramer staining were stained for IL-7 receptor CD127, maturation marker CD57, and activation markers PD-1 and CD38 in samples from subjects BA1 (A) and BA4 (B). Their cytotoxic potential was assessed through the expression of the cytolytic proteins perforin and granzymes A and B for individuals BA1 (C) and BA4 (D).
To test for T cell activation and exhaustion, RMT-specific cells were stained for PD-1 and CD38, as well as the cytotoxic markers perforin and granzymes A and B. Although several time points were tested, the levels of the markers did not change significantly; RMT-specific cells in BA1 remained principally CCR7− CD45RA+ CD57+ CD127− CD38+ perforin+ granzyme A+ granzyme B+, while in BA4 they were CCR7− CD45RA− CD127− CD38+ perforin− granzyme A+ granzyme B− (Fig. 5A to D).
PARV4-specific T cells are polyfunctional.
From the data above, the cell populations elicited late after PARV4 infection appeared to be effector memory pools. To further assess function, PBMC samples from subject BA1 were tested by ICS after stimulation with the RMT epitope. Figure 6 illustrates the broad polyfunctionality of T cells in subject BA1 through representative fluorescence-activated cell sorter (FACS) plots over several time points and SPICE pie charts that show polyfunctionality at the single-cell level. The RMT PARV4-specific CD8+ T cells predominantly expressed IFN-γ, MIP1-β (Fig. 6A and B), and TNF-α, as well as IL-2 and CD107a (Fig. 6B). Though these cells consistently produced multiple cytokines, this did decrease over time compared to the frequency of tetramer-positive cells (Fig. 6C).
Fig 6.
CD8+ T cells responding to PARV4 epitope RMT are polyfunctional. (A) Representative FACS plots showing the production of IFN-γ and MIP1-β by CD8+ T cells determined by ICS (plots gated on live lymphocytes, CD3+ CD8+ cells). (B) SPICE charts illustrate the polyfunctionality of these CD8+ T cells in response to epitope RMT, through the production of IFN-γ, MIP1-β, CD107a, TNF-α, and IL-2. (C) Comparison of the frequency of HLA-A2 tetramer-positive cells (red, left axis) and the percentage of CD8+ T cells producing cytokines (green, right axis). PMA iono, phorbol myristate acetate and ionomycin (positive control).
DISCUSSION
This is the first prospective study of cellular immune responses to PARV4 in a cohort of acutely infected individuals. PARV4 has been strongly associated with intravenous drug use in many studies (1–4, 9), and rare are the subjects in whom PARV4 can be studied independently of major coinfections such as HIV and HCV. Only one study of PARV4 has previously looked at a group of 10 IDUs who were HCV negative, and only 1 of these was PARV4 viremic (2). Our cohort provided an ideal setting in which to study PARV4 infection alone.
Although this infection is commonly associated with HCV and HIV infections, 36% of the 98 individuals in this cohort were PARV4 seropositive despite remaining HCV and HIV negative. This seropositivity rate is similar to that of other HCV-infected cohorts, strengthening the correlation between PARV4 and intravenous drug use, rather than HCV per se (4, 5, 13). The most conservative estimate of incidence was calculated to be a PARV4 seroconversion rate of 7.6%/year overall. This estimate is much lower than the HCV incidence in this cohort of 27.2 HCV seroconversions per 100 person-years (14), suggesting that these two infections do not always occur together, as is seen in many other cohorts. This still suggests that PARV4 is a common percutaneously acquired virus, highlighting its importance in the safety of blood products. We did note a decrease in the rate of incidence from enrolment to the end of the study (13 to 3.8%). This could be attributed to the counseling the individuals receive when they enroll in the program (16). As these individuals were selected for never seroconverting for HCV, it may be that they have lower-risk behavior. Additionally, these rates of incidence were calculated on the basis of the approximate time of intravenous drug use as a means of transmission; however, other parenteral routes of PARV4 transmission, such as intranasal and skin injury, may play a role and therefore may affect the rates of incidence in different subsets of patients. Further information on possible PARV4 transmission and detailed behavioral information is required to answer these questions.
Viremia and PARV4-specific IgM are short-lived in the acute phase of PARV4 infection.
Two previous studies found that PARV4 viremia lasted 1 and 9 months, respectively; however, these estimates were based on only one individual each time, in cohorts where most of the subjects were HIV infected (3, 11). The present study suggests that viral DNA remains in peripheral blood for 1 to 3 months, on the basis of monthly data from four individuals. PARV4 viral loads were determined for 6/8 patients and averaged at 5 × 103 copies/ml. These low levels are characteristic of PARV4 infection (5, 7, 17). Anti-PARV4 IgG was detected either simultaneously with viral DNA or 1 month later. IgM was also detected at a similar time, but with monthly samples, it was difficult to assess the exact timing of IgM with respect to IgG and viremia. Nonetheless, acute PARV4 infection can be defined by short-lived viremia and the concomitant appearance of IgM. IgG levels increased progressively and peaked between 4 and 7 months after seroconversion. The time of appearance of IgG was similar to that in HCV and HIV-1 infections, which is estimated to be around 2 to 6 weeks (18, 19).
Figure 3 shows that although antibody levels were consistently high, T cell responses varied between individuals. We previously observed a discrepancy between PARV4 IgG and T cell responses in a cohort of remotely infected individuals (13). It is possible, as seen in HIV-1 and HCV infections, that host factors such as HLA types may affect their ability to elicit different types of immune responses to PARV4. It can also be speculated that if antibody levels are high enough to eliminate viremia, no or little antigen would remain to trigger the strong late T cell responses (e.g., in subjects BA3, BA7, and BA8, Fig. 3).
Rather than leading to an acute T cell flare, PARV4 NS triggered a late cellular response that increased over time, peaking at around 5 mps. After the expansion phase, T cell responses normally contract within a few weeks. As shown in Fig. 2 and 3, PARV4 NS T cell responses were maintained over time, up to 63 mps, and at levels of >1,000 SFU/106 PBMCs per peptide (Fig. 2D). The late T cell responses observed were not due to subsequent reinfection, as plasma samples at later time points proved DNA negative (data not shown). The kinetics of these memory cells are reminiscent of those observed in cytomegalovirus (CMV) infection, sometimes described as memory inflation, which is also seen in parvovirus B19 infection (20–23). T cell responses to both PARV4 and B19 are persistent over the years, and this finding is consistent with our previous data on a cohort of chronically HCV-infected subjects in whom PARV4 T cell responses were detected decades after the primary infection (13, 24). It was previously reported that up to 4.5% of CD8+ T cells responded to a specific parvovirus B19 epitope (20), while PARV4 RMTENIVEV epitope-specific T cells represented 0.1 to 1.5% of the CD8+ T cells in two individuals, at levels similar to those seen for CMV or other persistent infections (25–30).
This study found that the PARV4 T cell responses of individuals BA4 and BA1 had an effector memory phenotype (TEM or TEMRA). This effector memory phenotype is consistent with what we had previously reported for these RMT-specific T cells in an individual from an HCV-infected cohort (13). It is hypothesized that intermittent and/or low antigen levels, such as those observed in latent CMV infection, may allow for the differentiation to a “late memory” TEMRA phenotype, whereas a high, continuous antigen load, as in HIV-1 infection, may lead to an early abrogation of differentiation, explaining why most HIV-1-specific T cells are TEM (31–33). Because of the limited number of samples available for study and with a response to the RMT epitope, we were unable to characterize the memory phenotype more precisely. However, judging from these subjects and our previous study, PARV4-specific T cells appear to consistently have an effector memory phenotype, whether further differentiated to CD45RA+ or not.
Other phenotypic features of the cells are consistent with repetitive antigenic stimulation. Eighty percent of the PARV4-specific T cells were CD57+, consistent with the phenotype observed in a previous cohort (data not shown; 13). The CD8+ CD57+ T cell subset has been shown to expand during chronic activation in several viral infections—including parvovirus B19—and is thought to be a result of persistent antigenic stimulation (20, 34). CD127 (IL-7Rα) is required for the maintenance of memory T cells in the absence of antigen (35). Several studies have shown that CD8+ T cells specific for viruses that are successfully cleared, such as influenza virus and respiratory syncytial virus, expressed CD127, whereas CD8+ T cells specific for persistent viruses such as CMV and HIV were CD127− (36, 37). RMT-specific cells from BA1 and BA4 were CD127lo for the duration of the study, spanning 3 to 5 years postinfection for BA1, which suggests that antigen may still persist at 5 years postinfection. As patient BA1 was an active IDU, it was possible that continuous exposure to PARV4 would result in a constant renewal of antigen. However, a sample tested 3 years after primary infection was DNA negative. This suggests that PARV4 antigen may persist at levels below detection.
Fifty percent of the PARV4-specific T cells expressed CD38+, as also seen in a cohort previously studied (data not shown; 13), which may reflect an intermediate state of activation due to low levels of persisting PARV4. Eighty percent of the RMT-specific T cells expressed PD-1. PD-1 on virus-specific T cells has been characterized as a marker of T cell exhaustion in HIV and HCV infections, and this is thought to be a possible mechanism of viral evasion (38, 39). However, PD-1 is also expressed on 60% of the memory CD8+ T cells in healthy individuals and on efficient yellow fever virus- and vaccinia virus-specific CD8+ T cells (40, 41). Importantly, we show here that after several years postseroconversion, RMT-specific CD8+ T cells still produce a variety of cytokines, a population relevant to long-term viral control, though function does appear to decrease over time (42–47). Further study samples are required to test the consistency and significance of this result. It is likely that a combination of high-magnitude, broad, and polyfunctional T cell responses is important for optimal protection (45, 48). In this respect, PARV4 or mechanisms related to its persistence and triggering could be considered interesting properties for a vaccine vector.
In conclusion, the nature of this cohort and the monthly follow-up of the subjects have allowed the precise characterization of acute PARV4 infection. The high incidence of PARV4 in this cohort emphasizes the risk of transmission of novel viruses through blood products. The immunologic data clearly show that PARV4-specific cells expand late and are retained in a mature and activated state, which is very similar to that of B19- and CMV-specific CD8+ T cells (20, 49), and suggest that, similar to these viruses, PARV4 antigen persists, although its tropism is still unknown. Definition of the features underlying this process is of relevance not only to parvovirus infection but also potentially more broadly to vaccine design.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust (including WT091663MA), the National Institutes of Health (NIAID 5U19A082630-04 and 1U19AI08879), the Medical Research Council UK, and the National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford, United Kingdom. The development and use of the serological assay for anti-PARV4 antibodies were supported by an unrestricted investigator-initiated grant from Baxter Healthcare and by the National Institute of Child Health and Human Development, National Institutes of Health (R01 HD41224).
Footnotes
Published ahead of print 2 January 2013
REFERENCES
- 1. Jones MS, Kapoor A, Lukashov VV, Simmonds P, Hecht F, Delwart E. 2005. New DNA viruses identified in patients with acute viral infection syndrome. J. Virol. 79:8230–8236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lurcharchaiwong W, Chieochansin T, Payungporn S, Theamboonlers A, Poovorawan Y. 2008. Parvovirus 4 (PARV4) in serum of intravenous drug users and blood donors. Infection 36:488–491 [DOI] [PubMed] [Google Scholar]
- 3. Sharp CP, Lail A, Donfield S, Gomperts ED, Simmonds P. 2012. Virologic and clinical features of primary infection with human parvovirus 4 in subjects with hemophilia: frequent transmission by virally inactivated clotting factor concentrates. Transfusion 52:1482–1489 [DOI] [PubMed] [Google Scholar]
- 4. Sharp CP, Lail A, Donfield S, Simmons R, Leen C, Klenerman P, Delwart E, Gomperts ED, Simmonds P. 2009. High frequencies of exposure to the novel human parvovirus PARV4 in hemophiliacs and injection drug users, as detected by a serological assay for PARV4 antibodies. J. Infect. Dis. 200:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fryer JF, Lucas SB, Padley D, Baylis SA. 2007. Parvoviruses PARV4/5 in hepatitis C virus-infected patient. Emerg. Infect. Dis. 13:175–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmonds P, Manning A, Kenneil R, Carnie FW, Bell JE. 2007. Parenteral transmission of the novel human parvovirus PARV4. Emerg. Infect. Dis. 13:1386–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuke PW, Parry RP, Appleton H. 2010. Parvovirus PARV4 visualization and detection. J. Gen. Virol. 91:541–544 [DOI] [PubMed] [Google Scholar]
- 8. Vallerini D, Barozzi P, Quadrelli C, Bosco R, Potenza L, Riva G, Gregorini G, Sandrini S, Tironi A, Montagnani G, De Palma M, Torelli G, Delwart E, Luppi M. 2008. Parvoviruses in blood donors and transplant patients, Italy. Emerg. Infect. Dis. 14:185–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simmons R, Sharp C, McClure CP, Rohrbach J, Kovari H, Frangou E, Simmonds P, Irving W, Rauch A, Bowness P, Klenerman P, Swiss HIV Cohort Study 2012. Parvovirus 4 infection and clinical outcome in high-risk populations. J. Infect. Dis. 205:1816–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen MY, Yang SJ, Hung CC. 2011. Placental transmission of human parvovirus 4 in newborns with hydrops, Taiwan. Emerg. Infect. Dis. 17:1954–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lahtinen A, Kivela P, Hedman L, Kumar A, Kantele A, Lappalainen M, Liitsola K, Ristola M, Delwart E, Sharp C, Simmonds P, Soderlund-Venermo M, Hedman K. 2011. Serodiagnosis of primary infections with human parvovirus 4, Finland. Emerg. Infect. Dis. 17:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang SJ, Hung CC, Chang SY, Lee KL, Chen MY. 2011. Immunoglobulin G and M antibodies to human parvovirus 4 (PARV4) are frequently detected in patients with HIV-1 infection. J. Clin. Virol. 51:64–67 [DOI] [PubMed] [Google Scholar]
- 13. Simmons R, Sharp C, Sims S, Kloverpris H, Goulder P, Simmonds P, Bowness P, Klenerman P. 2011. High frequency, sustained T cell responses to PARV4 suggest viral persistence in vivo. J. Infect. Dis. 203:1378–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, Chien D, Shyamala V, Ray SC, Thomas DL. 2005. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin. Infect. Dis. 40:951–958 [DOI] [PubMed] [Google Scholar]
- 15. Roederer M, Nozzi JL, Nason MX. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79(2):167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox AL, Page K, Bruneau J, Shoukry NH, Lauer GM, Kim AY, Rosen HR, Radziewicz H, Grakoui A, Fierer DS, Branch AD, Kaplan DE, Chang KM. 2009. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology 136:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider B, Fryer JF, Oldenburg J, Brackmann HH, Baylis SA, Eis-Hubinger AM. 2008. Frequency of contamination of coagulation factor concentrates with novel human parvovirus PARV4. Haemophilia 14:978–986 [DOI] [PubMed] [Google Scholar]
- 18. Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. 2005. Humoral immune response in acute hepatitis C virus infection. Clin. Infect. Dis. 41:667–675 [DOI] [PubMed] [Google Scholar]
- 19. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isa A, Kasprowicz V, Norbeck O, Loughry A, Jeffery K, Broliden K, Klenerman P, Tolfvenstam T, Bowness P. 2005. Prolonged activation of virus-specific CD8+ T cells after acute B19 infection. PLoS Med. 2:e343 doi:10.1371/journal.pmed.0020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022–2029 [DOI] [PubMed] [Google Scholar]
- 22. Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450–458 [DOI] [PubMed] [Google Scholar]
- 23. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norbeck O, Isa A, Pohlmann C, Broliden K, Kasprowicz V, Bowness P, Klenerman P, Tolfvenstam T. 2005. Sustained CD8+ T-cell responses induced after acute parvovirus B19 infection in humans. J. Virol. 79:12117–12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillespie GM, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PA. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74:8140–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin X, Demoitie MA, Donahoe SM, Ogg GS, Bonhoeffer S, Kakimoto WM, Gillespie G, Moss PA, Dyer W, Kurilla MG, Riddell SR, Downie J, Sullivan JS, McMichael AJ, Workman C, Nixon DF. 2000. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J. Infect. Dis. 181:165–175 [DOI] [PubMed] [Google Scholar]
- 27. Klenerman P, Cerundolo V, Dunbar PR. 2002. Tracking T cells with tetramers: new tales from new tools. Nat. Rev. Immunol. 2:263–272 [DOI] [PubMed] [Google Scholar]
- 28. Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C, Williams R, Vergani D, Bertoletti A. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 117:1386–1396 [DOI] [PubMed] [Google Scholar]
- 30. Wilson JD, Ogg GS, Allen RL, Davis C, Shaunak S, Downie J, Dyer W, Workman C, Sullivan S, McMichael AJ, Rowland-Jones SL. 2000. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS 14:225–233 [DOI] [PubMed] [Google Scholar]
- 31. Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111 [DOI] [PubMed] [Google Scholar]
- 32. van Lier RA, ten Berge IJ, Gamadia LE. 2003. Human CD8(+) T-cell differentiation in response to viruses. Nat. Rev. Immunol. 3:931–939 [DOI] [PubMed] [Google Scholar]
- 33. Waller EC, Day E, Sissons JG, Wills MR. 2008. Dynamics of T cell memory in human cytomegalovirus infection. Med. Microbiol. Immunol. 197:83–96 [DOI] [PubMed] [Google Scholar]
- 34. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711–2720 [DOI] [PubMed] [Google Scholar]
- 35. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198 [DOI] [PubMed] [Google Scholar]
- 36. Mojumdar K, Vajpayee M, Chauhan NK, Singh A, Singh R, Kurapati S. 2011. Loss of CD127 & increased immunosenescence of T cell subsets in HIV infected individuals. Indian J. Med. Res. 134:972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 106:2091–2098 [DOI] [PubMed] [Google Scholar]
- 38. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 39. Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed R, Akondy RS. 2011. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol. Cell Biol. 89:340–345 [DOI] [PubMed] [Google Scholar]
- 41. Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R. 2011. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J. Immunol. 186:4200–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akinsiku OT, Bansal A, Sabbaj S, Heath SL, Goepfert PA. 2011. Interleukin-2 production by polyfunctional HIV-1-specific CD8 T-cells is associated with enhanced viral suppression. J. Acquir. Immune Defic. Syndr. 58(2):132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. 2008. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 82:10017–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Julg B, Williams KL, Reddy S, Bishop K, Qi Y, Carrington M, Goulder PJ, Ndung'u T, Walker BD. 2010. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84:5540–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81:12071–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, Hallahan CW, Davey RT, Jr, Dybul M, Vogel S, Metcalf J, Connors M. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900–10909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moss P, Khan N. 2004. CD8(+) T-cell immunity to cytomegalovirus. Hum. Immunol. 65:456–464 [DOI] [PubMed] [Google Scholar]