Abstract
Studies on large double-stranded DNA (dsDNA) viruses such as poxviruses have been helpful in identifying a number of viral and cellular growth factors that contribute to our broad understanding of virus-host interaction. Orthopoxviruses and leporipoxviruses are among the most studied viruses in this aspect. However, tanapoxvirus (TPV), a member of the genus Yatapoxvirus, still remains largely unexplored, as the only known hosts for this virus are humans and monkeys. Here, we describe the initial characterization of an epidermal growth factor (EGF)-like growth factor mimicking human neuregulin from TPV, expressed by the TPV-15L gene. Assays using a baculovirus-expressed and tagged TPV-15L protein demonstrated the ability to phosphorylate neuregulin receptors. Neuregulins represent a large family of EGF-like growth factors that play important roles in embryonic endocardium development, Schwann and oligodendrocyte survival and differentiation, localized acetylcholine receptor expression at the neuromuscular junction, and epithelial morphogenesis. Interestingly, certain neuregulin molecules are able to target specific tissues through interactions with heparin sulfate proteoglycans via an immunoglobulin (Ig)-like domain. Analyses of TPV-15L revealed no Ig-like domain, but it retains the ability to bind heparin and phosphorylate neuregulin receptors, providing compelling evidence that TPV-15L is a functional mimetic of neuregulin. TPV-15L knockout virus experiments demonstrate that the virus replicates in human umbilical vein endothelial cells less efficiently than wild-type TPV-Kenya, indicating that this is a nonessential protein for virus viability but can serve a stimulatory role for replication in some cultured cells. However, the precise role of this protein in host-virus interaction still remains to be deduced.
INTRODUCTION
Members of the epidermal growth factor (EGF) family ligands selectively activate various members of the epidermal growth factor receptor (EGFR) family, which are distinctly classified as ErbB1, ErbB2, ErbB3, and ErbB4. When a cognate ligand is bound to EGFRs, various homodimers and/or heterodimers are generated, causing tyrosine phosphorylation of the intracellular portion of the receptor. This activates numerous intracellular pathways causing pleiotropic proliferation and developmental effects (1). The ErbB1 receptor is the classical receptor for EGF, whereas ErbB2 does not bind to any ligand with high affinity (2). The ErbB3 and ErbB4 receptors are activated by a group of EGF-like ligands called neuregulins (NRGs) (3). Of functional significance, ErbB3 is catalytically deficient compared to other EGFRs, thus requiring an initial binding of NRG to ErbB3 before heterodimerization with ErbB1, ErbB2, and ErbB4 for signaling activity (4). In certain epithelial cancers, EGFR expression plays a very important part in determining disease outcome. For example, some breast cancers overexpress ErbB2 by 100-fold, causing vigorous proliferation of the cancer cells. Clinical application of monoclonal anti-ErbB2 has been shown to reduce cellular expansion in breast cancer (5).
NRGs are a large family of alternatively spliced growth factors. Of the 4 human NRG genes discovered, NRG1 is the best understood. Most NRGs are initially synthesized as transmembrane precursors, called proneuregulin, and are later proteolytically cleaved, releasing a soluble protein through a process called ectodomain shedding (6). NRGs typically include an EGF-like domain, a glycosylation domain, and, in certain isoforms, an immunoglobulin (Ig)-like domain that is responsible for binding to heparin sulfate proteoglycans (HSPGs). NRGs are implicated in embryonic endocardium development (trabecula formation), Schwann and oligodendrocyte survival and differentiation (myelination), localized acetylcholine receptor expression at the neuromuscular junction (NMJ), and epithelial morphogenesis in breast cancer (3). The diverse developmental effects further complicate the issue of how NRGs are able to target certain tissues and not others. In most cases, NRGs are able to potentiate their effects through ligand and receptor expression in specific tissues. Other control mechanisms exist for the distribution of NRG1, involving HSPG interactions. Evidence for this notion was derived from the chicken embryo development model, where agrin (an HSPG) and NRG1 form localized complexes that synergistically concentrate to the basal lamina of the NMJ, potentiating a signal that upregulates expression of acetylcholine receptor genes (7, 8). Later studies have also suggested that NRGs target specific HSPGs, preferentially choosing N-sulfate over 2-O- and 6-O-sulfate groups (9).
Poxviruses have been instrumental in revealing numerous basic molecular processes associated with immune evasion and viral proliferation in vivo. Poxviral immune evasion strategies target a variety of extracellular and intracellular pathways, giving the virus a distinct advantage for establishing an infection. These viral gene products often mimic host cytokines or their cellular receptors, termed virokines and viroceptors, respectively. Among the poxviruses, the best characterized is vaccinia virus (VACV), which exploits a range of immunomodulatory strategies, including gamma interferon (IFN-γ) modulation, complement control, tumor necrosis factor (TNF) inactivation, and EGF-like molecules (reviewed in references 10, 11, and 12). Numerous other poxvirus EGF-like growth factors have been described. Viral gene knockout experiments have been important in identifying their effects on virus virulence and cellular proliferation and have revealed that most of these growth factors are nonessential for virus replication in vitro (13–15). These relatively well described poxvirus EGF-like growth factors include growth factors from VACV (vaccinia growth factor [VGF]), variola virus (smallpox growth factor [SPGF]), Shope fibroma virus (Shope growth factor [SGF]), myxoma virus (myxoma growth factor [MGF]), and cowpox virus (cowpox growth factor [CGF]) (16–24). Further characterization of these poxvirus EGF-like growth factors on A-431 cells transfected with ErbB receptor combinations revealed that the different viral growth factors displayed varied specificity for the different receptor combinations. For example, VGF preferentially activated ErbB1/1, -1/2, and -1/3, SFGF displayed broad-range specificity to ErbB1/1, -1/2, -1/3, -1/4, and -2/3, and MGF bound only ErbB2/3 (2). SPGF, on the other hand, has yet to be fully characterized, but some data suggest that ErbB1 is necessary for signal transduction (25). Here, we describe a fourth member of the EGF-like growth factor family from tanapoxvirus (TPV), encoded by the TPV-15L gene, that is capable of activating the neuregulin receptor heterodimer ErbB2/3 as shown in an established neuregulin bioassay (26). Our initial characterization of this protein has identified heparin binding ability, similar to the case for certain isoforms of mammalian neuregulin. TPV-15L knockout studies described here demonstrate a diminished replicative ability of the virus in human umbilical vein endothelial cells (HUVEC) containing neuregulin receptors, providing preliminary evidence that TPV-15L protein does play a stimulatory role in virus replication.
MATERIALS AND METHODS
Cells, viruses, and neuregulin.
Sf21 cells used for baculovirus protein expression were obtained from Gibco and cultured in SFII 900 SFM (Invitrogen). Owl monkey kidney (OMK) cells and TPV-Kenya (wtTPV) were cultured using previously established protocols in our lab (27). L6 rat myoblasts were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. Human umbilical vein endothelial cells (HUVEC) (ATCC CRL-1730) were cultured in F12K medium (Cellgro) containing 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mg/ml sodium heparin (Sigma H-0777), and 50 μg/ml endothelial cell growth supplement (BD Biosciences 354006). NRG1 was purchased from R&D Systems, MN.
Expression and purification of TPV-15L protein from baculovirus.
Recombinant baculovirus containing TPV-15L (AcTPV-15Lmyc/His) was generated by PCR amplifying the TPV-15L gene using a forward BamHI primer (GCGGGATCCATGAAAAACAAATTTATG) and reverse XhoI primer (CGCTCGAGATTTACTATTTTATTTTCAC). This amplicon was cloned into pcDNA3.1/myc/His ver C (Invitrogen), generating a fusion-tagged gene construct. This fusion gene was subsequently cloned into the pFastBac-Dual-eGFP cassette, containing the enhanced green fluorescent protein (GFP) gene. Recombinant viruses were then made using the Bac-to-Bac system (Invitrogen) following the manufacturer-supplied protocol. The resultant recombinant baculovirus was used to infect Sf21 insect cells cultured in serum-free medium (Invitrogen). The infected cell supernatant was harvested after complete infection of the culture vessel (3 to 5 days postinfection). The AcTPV-15Lmyc/His infected cell supernatant was collected, centrifuged (1,000 × g) to remove cell debris, and then subjected to a hexa-His Co2+ chelate resin affinity column (BD Biosciences). The column was equilibrated with 10 bed volumes of equilibrating buffer (50 mM sodium phosphate, 300 mM NaCl, pH 7.0), followed by 3 passes of the clarified supernatant, and washed with another 10 bed volumes of equilibrating buffer. The bound proteins were eluted in 4 to 8 fractions, using one bed volume of elution buffer (50 mM sodium phosphate, 300 mM NaCl, 150 mM imidazole, pH 7.0) each. The elution fractions were then subjected to Western blot analysis to confirm the presence of the expressed AcTPV-15Lmyc/His.
Western blot analysis.
AcTPV-15Lmyc/His-containing samples were mixed with 5× SDS gel loading buffer (25% glycerol, 5% SDS, 0.002% bromophenol blue, 15% β-mercaptoethanol) and boiled for 3 min. Samples were separated on a 12% Tris-glycine gel at a constant 100 V for 1 h 45 min. The proteins were transferred from the gel to an Immobilon-P polyvinylidene difluoride (PVDF) membrane using a semidry transfer system (Bio-Rad Trans-Blot SD) for 1 h 15 min at 14 V. The membrane was blocked in TBST (20 mM Tris, 137 mM NaCl [pH 7.6] plus 0.05% Tween 20) containing 5% nonfat dry milk for 1 h at room temperature. A 1:5,000 dilution of mouse anti-myc antibody (Delta Biolabs) in TBST plus 5% nonfat dry milk was applied to the membrane in a sealed bag overnight at 4°C. The membrane was washed three times for 10 min each with TBST. A 1:10,000 dilution of anti-mouse IgG–horseradish peroxidase (HRP) (Sigma) in TBST plus 5% nonfat dry milk was added to the membrane in a sealed bag for 1 h at room temperature with gentle agitation. The membrane was washed three times for 10 min each with TBST, and signal was detected using chemiluminescence reagents (Thermo Scientific) and exposure to X-ray film (Eastman Kodak).
Heparin binding analysis.
Using a slot blot apparatus, 50 μl of bovine intestinal sodium heparin (10 mg/ml Sigma), AcTPV-15L-infected supernatant, and 1% bovine serum albumin (BSA) (Sigma) in phosphate-buffered saline (PBS) and uninfected Sf21 cell supernatant was each vacuumed through slots on three triplicate PVDF membranes. The membranes were blocked using 5% milk in TBS and incubated in either TPV-15L supernatant, milk negative control, or uninfected cell supernatant for 1 h at room temperature. The membranes were washed, and detection was following the Western blot detection protocol described above. Additionally, a heparin-agarose affinity column (Sigma) was equilibrated using 10 bed volumes of 0.01 M Tris-HCl (pH 7.5). Subsequently, centrifugation-clarified AcTPV-15Lmyc/His-infected supernatant was passed through the column 3 times. Column-bound proteins were eluted in 10 fractions, containing an increasing gradient of NaCl (0.1 M to 1.0 M) in equilibration buffer. The fractions were then analyzed using the Western blot protocol described above. Elution fractions containing AcTPV-15Lmyc/His were dialyzed using a dialyzer cassette (3,000-molecular-weight [MW] cutoff; Pierce) with PBS overnight at 4°C to remove excess salt for subsequent analysis.
Neuregulin bioassay.
L6 cells were cultured and seeded at a density of 50,000 cells/well in 48-well dishes. After 8 days in culture, the L6 cells fused into myotubes and were incubated for 45 min at 37°C in various concentrations of NRG1 (10 to 1,000 pM) and 10-fold serial dilutions of dialyzed, heparin-column purified AcTPV-15Lmyc/His along with the appropriate negative controls. The plate was immediately put on ice, the assay medium was removed, and cells were mixed with 2× SDS sample buffer containing 0.1 M dithiothreitol (DTT). Samples were then run on a 6.5% Tris-glycine polyacrylamide gel at 200 V for 1 h and transferred onto a PVDF membrane using a 3-(cyclohexylamino)propane-1-sulfonic acid (CAPS) buffer tank transfer system (110 V for 1 h at 4°C). The membrane was blocked in 5% milk in PBS-Tween (PBST) and probed with 1:2,000 4G10 antiphosphotyrosine antibody (Millipore) overnight at 4°C. Membranes were washed 5 times with PBST and probed with 1:10,000 goat anti-mouse-HRP in 5% milk in PBST. The membrane was washed 5 times in PBST, incubated with chemiluminescence substrate (Perkin-Elmer), and exposed to X-ray film (Eastman Kodak). After film exposure, the membrane was incubated in stripping solution (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM β-mercaptoethanol) for 30 min and rinsed 10 times with deionized water and PBS. The stripped membrane was then blocked and reprobed with anti-ErbB2 and anti-ErbB3 antibodies (Santa Cruz) and anti-rabbit–HRP using the detection protocol. Densitometry quantitation was performed using the Metamorph imaging system (Universal Imaging Corp.) by calculating a ratio of p185 to ErbB2 and ErbB3 on nonsaturated images.
Generation of TPV-15L knockout virus.
To generate a TPV-15L knockout virus (TPVΔ15L), a plasmid derived from pBSII-KS+ was used created to include stretches of genomic TPV DNA flanking the left and the right sides of the TPV 15L open reading frame (ORF). The left flank (659 bp) was generated by PCR using XhoI forward (TAGGTACTCGAGAAAAACACCAATA) and ClaI reverse (GTTTAAATCGATGGACCTG) primers. The right flank (615 bp) was amplified using NotI forward (CATATTTTGCGGCCGCGGTAAACAATT) and SacI reverse (GTTAAAAATGGAAAAGAGCTCTAATTTTAACAACAG) primers. In between the flanking sequences, a synthetic early/late poxvirus promoter was used to drive the expression of mCherry (Clontech). This plasmid was transfected following the manufacturer's protocol (Superfect transfection reagent; Qiagen) into 60-mm tissue culture dishes containing OMK cells infected with TPV for 3 h at a multiplicity of infection (MOI) of approximately 1. The infection/transfection was incubated at 37°C with 5% CO2 for 8 days in maintenance medium (Eagle's minimal essential medium [EMEM] plus 2% newborn calf serum [NBCS]). The virus was harvested and serially diluted in a plaque assay to isolate recombinant knockout viruses. Recombinant knockout viruses were plaque purified three times and confirmed through PCR amplification of a stretch of DNA unique to TPV-15L (TPV-15L internal primers, CACACCTTTTTCCGTTAAATTGCC and GTTTTTTACTTTATCATGTGTCATTTTAGC). As a control, the internal primers to the TPV-2L gene (TPV-2L internal primers, CCATTGCATCCTTCAGAACAAG and GCATAACTTTAAAATATAATTATACTGTTACG) were also used to amplify a fragment to ensure a suitable viral DNA sample for amplification.
Replication of TPVΔ15L in HUVEC.
The isolated knockout virus TPVΔ15L was amplified in OMK cells and concentrated to 100× using ultracentrifugation (type 45Ti rotor at 40,000 rpm or 186,000 × g for 90 min), and the titer of the resultant virus was determined on OMK cells. HUVEC were plated at a density of 5 × 104 cells/well in a 24-well dish. After adherence, the cells were infected with TPVΔ15L and wtTPV by aspirating medium, adding 200 μl of inoculum suitable for an infection at MOIs of 0.1 and 5, and rocking for 1 h at room temperature. After infection, the virus inoculum was aspirated and the monolayer was rinsed three times using 200 μl of growth medium. Subsequently, the cells received 1 ml of HUVEC growth medium and were incubated at 37°C with 5% CO2 for 48 and 96 h. After incubation, infected cells were harvested and wells rinsed three times with 200 μl of sterile PBS. Cells were pelleted by centrifugation at 800 × g for 10 min, after which the cells were lysed with 400 μl of deionized water and subjected to three freeze-thaw cycles. The infected cell lysate was pooled with infected supernatant and serially diluted for virus titration in OMK cell monolayers.
RESULTS
Bioinformatic analyses of TPV-15L protein.
Our initial characterization of total TPV proteins using [35S]methionine-labeled TPV-infected cell supernatants found two major proteinaceous products (28). One protein had an apparent molecular mass of 38 kDa (29); we later described this protein to be the product of TPV-2L, which binds and inhibits TNF (30). The other protein was detected at approximately 12 kDa. When the TPV genomic sequence became available, all predicted secretory proteins were investigated for the potential gene for this 12-kDa protein, and the TPV 15L ORF appeared to be the best candidate gene that is most likely responsible for this protein.
TPV-15L is a 77-amino-acid secretory protein that is predicted to be an epidermal growth factor (EGF)-like growth factor with a predicted molecular mass of 8.8 kDa and an isoelectric point of 8.6 (31). Most poxviruses sequenced to date possess genes that encode EGF-like growth factors (2). Through genetic inactivation experiments, it was found that poxviruses typically utilize these secretory proteins for the enhancement of virulence and increase cell growth at the primary site of infection (15).
Sequence similarity comparison performed by BLASTP revealed orthologous open reading frames in Yaba-like disease virus (YLDV-15L), goatpox virus (GPTV_gp013), lumpy skin disease virus (LSDV016), and sheeppox virus (SPPV_13) (32–34). Similarity among orthologs ranges from 99% in YLDV-15L to 47% in SPPV_13. Detailed amino acid analysis using programs such as NetNGlyc 1.0 and SMART predicted that TPV-15L should be N-linked glycosylated at amino acid residue 54, with an EGF-like domain that consisted of 6 conserved cysteine residues (Fig. 1). Attempts to identify additional O-linked glycosylation (NetOGlyc 3.1) failed to identify additional glycosylation sites. Further analysis also revealed a putative transmembrane domain from amino acid 7 to 29 (TMHMM) and a signal peptide sequence with a predicted cleavage site between positions 16 and 17 (SignalP 4.0, WoLF PSORT). Aside from poxvirus-derived polypeptides, the protein sharing the highest similarity with TPV-15L as found in the BLAST result was mammalian neuregulin 1 (NRG1), sharing 37% identity. TPV-15L has a total of 77 amino acids, while hNRG1 has 422 amino acids. Figure 2 demonstrates a partial alignment of three proteins (TPV-15L, hNRG1, and EGF) indicating the regions of the highest homology. Heparin binding variants of NRG1 are known to exist, where the domain responsible for heparin binding resides on an Ig-like domain near the N terminus of the EGF-like domain (7, 9, 35, 36). This type of heparin binding motif is clearly absent from the amino acid sequence of TPV-15L. The BXB site in Fig. 2 reflects a predicted heparin binding site in TPV-15L, as stretches of basic amino acid residues (e.g., XBBBXXBX, XBBXBX, XBX, and BXB) in other proteins typically indicate the potential for heparin binding activity (37).
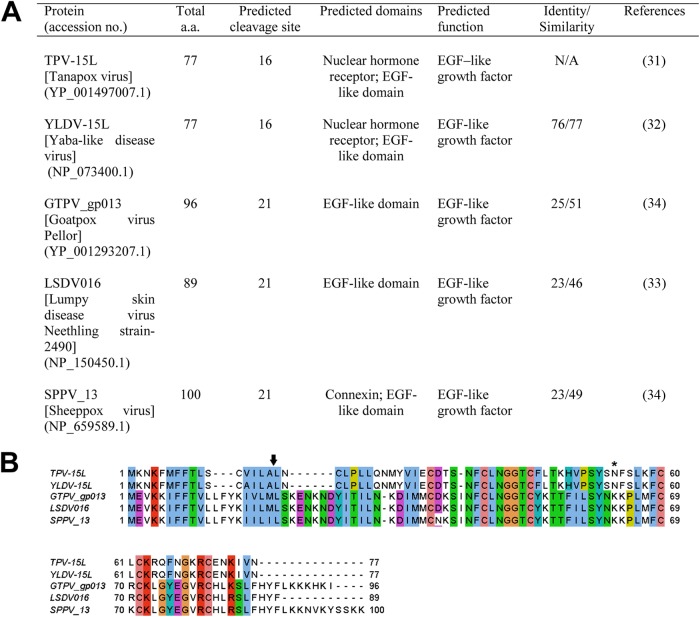
Fig 1.
Comparison of different poxvirus orthologs of TPV-15L. (A) Table summarizing different orthologs of TPV-15L from poxviruses. Cleavage sites of N-terminal signal peptides were predicted using SignalP 4.0, and domains were predicted using PFAM. Identity/similarity was determined by BLASTP. (B) Amino acid sequence alignment of poxvirus-derived orthologs of TPV-15L using ClustalW. The arrow indicates the predicted N terminus signal peptide cleavage site of TPV-15L, while the N-linked glycosylation site is denoted with an asterisk. Colored boxes represent amino acids: pink, cysteine residues; green, polar/hydrophilic; blue, hydrophobic; violet, acidic; red, basic; and magenta, large aromatic polar.
Fig 2.
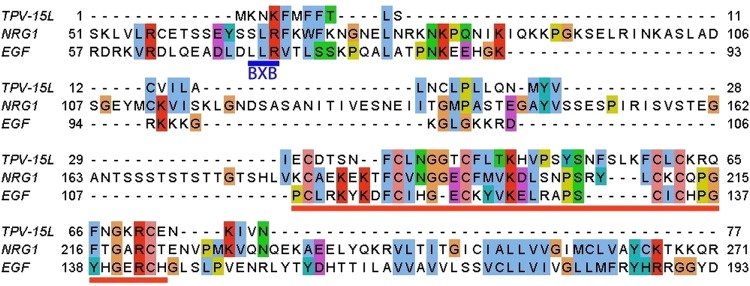
Partial amino acid sequence alignment between TPV-15L, human neuregulin 1, and human epidermal growth factor using ClustalW. Underlined in blue is a predicted heparin binding site (BXB) in TPV-15L, while the red underline denotes the EGF-like domain. Colored boxes represent amino acids: pink, cysteine residues; green, polar/hydrophilic; blue, hydrophobic; violet, acidic; red, basic; and magenta, large aromatic polar.
Baculovirus-expressed TPV-15L is a secreted protein.
Both secreted and transmembrane-bound forms of EGF-like growth factors have been identified (38). Furthermore, the proteolytic release called ectodomain shedding has shown to play a critical role in EGF-like ligand activity (39). Therefore, we needed to first identify experimentally whether the TPV-15L EGF-like growth factor was secreted to assess its function. From the sequence analysis of the TPV-15L ORF, it was predicted that the protein was not only secreted but also glycosylated. Experiments on nonglycosylated VGF demonstrated reduced mitogenic activity in certain cells expressing EGF receptors (40).Therefore, the baculovirus expression system was selected to express TPV-15L under the consideration of potential posttranslational modifications seen in eukaryotic systems. The added ability to culture insect cells in serum-free medium also enables rapid purification. In the TPV-15L-expressing baculovirus construct, we included a fluorescent reporter gene for rapid monitoring of infection, as well as a myc/His tag on the C terminus of TPV-15L. The tagged gene product enables us to use commercially available columns and antibodies for downstream analysis. To identify whether TPV-15L is a secretory protein, we expressed TPV-15L using AcTPV-15Lmyc/His in insect cells cultured in serum-free medium. The expressed protein was harvested prior to the lysis of cells to ensure uniform expression of posttranslational modified protein and assayed by SDS-PAGE and Western blotting using anti-myc antibody. As shown in Fig. 3, lane a, a major protein was detected in the supernatant of infected insect cells. To serve as a control, an unrelated recombinant baculovirus (AcTPV126Rmyc/His) was also included. Although the amino acid sequence suggests that the protein should appear in the 8.8-kDa range, the myc/His epitope tag and the predicted glycosylation of the expressed protein altered the detectable size to approximately 12 kDa. Taken together with previous experiments demonstrating the synthesis of a secreted, 12-kDa protein in TPV-infected cells in the presence of AraC (28), these results suggest that TPV-15L is a secreted early protein.
Fig 3.
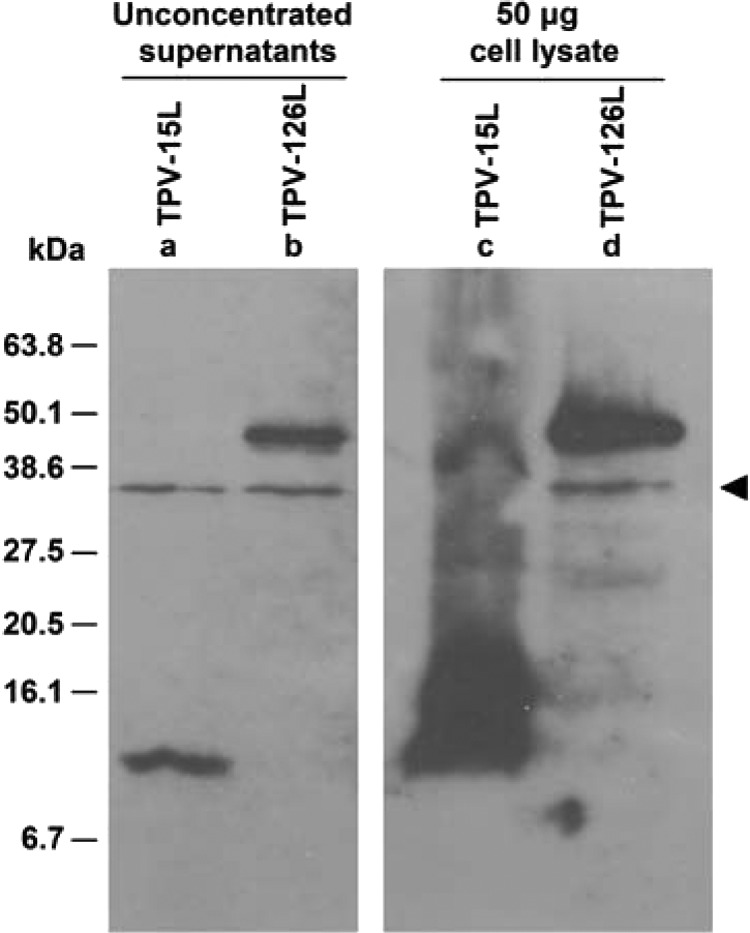
Expression of TPV-15L in baculovirus. A Western blot (anti-myc) comparing Sf21 cells infected with AcTPV-15Lmyc/His and AcTPV-126Rmyc/His at 5 days postinfection is shown. A nonspecific band is detected and denoted with an arrowhead. Lane a, TPV-15L myc/His with an apparent molecular mass of 12 kDa is present in unconcentrated supernatant. Lane b, AcTPV-126Rmyc/His is a control baculovirus, secreting a glycoprotein with an apparent molecular mass of 45 kDa. Lanes c and d, cell lysates of the respective baculovirus-infected cells.
TPV-15L possesses neuregulin activity.
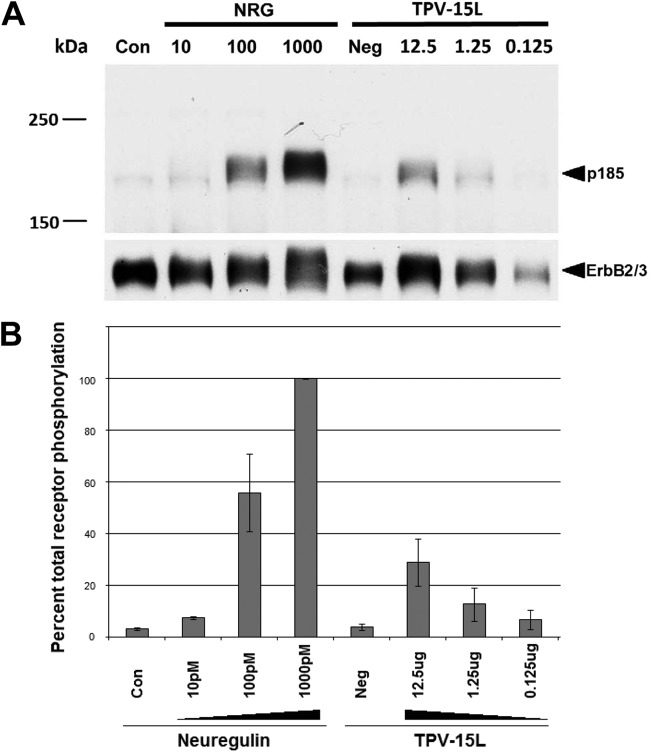
In light of the sequence similarities shared between TPV-15L and NRG1, we asked whether TPV could encode a protein that mimics NRG1. Neuregulins are natural ligands for the ErbB3 and ErbB4 receptors on a variety of different cells (3). The EGF-like domains present in neuregulins are capable of inducing receptor tyrosine phosphorylation, leading to upregulation of a specific subset of genes (reviewed in reference 41). Neuregulins are routinely assayed using the L6 cell bioassay, where rat myoblasts are serum starved for a week to induce myoblast differentiation into myotubes (42). These myotubes overexpress the neuregulin receptors. In the presence of neuregulin, these receptors become phosphorylated and can be detected using antiphosphotyrosine Western blot analysis (42). In addition, the amount of phosphorylated receptor could be quantitated to show a dose-dependent interaction and compare relative potencies of the ligand. To evaluate the ability of TPV-15L in phosphorylating NRG receptors, 12.5 μg of His column-purified TPV-15L was 10-fold serially diluted and incubated on L6 myotubes for 45 min. Various concentrations of neuregulin (10 pM to 1,000 pM) were used as positive controls, and growth medium served as a negative control. As shown in Fig. 4A, TPV15L clearly shows phosphorylation of neuregulin receptors, indicating that the EGF-like domain is functionally active. Quantitative results from 4 independent experiments are shown in Fig. 4B as a percentage of total phosphorylated receptors normalized to total receptors. This result clearly indicates that TPV-15L is functionally active, in a dose-dependent manner. Also, it must be noted that the dose of NRG is classically measured by pM concentration. The unavailability of the precise TPV-15L protein glycosylation pattern and other necessary information prevented us from calculating the molarity of TPV-15L protein. Hence, the dose of this protein is indicated in μg (Fig. 4). However, it is clear that TPV-15L protein was able to phosphorylate NRG receptors, as expected.
Fig 4.
Neuregulin L6 cell assay. (A) Western blot of tyrosine receptor (p185) phosphorylation, comparing neuregulin and TPV-15L. Lane Con, neuregulin negative control, consisting of medium only. Neuregulin concentrations are indicated in pM, and His column-purified TPV-15L concentrations are indicated in μg. Lane Neg, negative control taken from an elution fraction devoid of TPV-15L. The p185 was detected by antiphosphotyrosine antibody, and ErbB2/3 was detected by anti-ErbB2 and anti-ErbB3 antibodies. (B) Antiphosphotyrosine band intensities were quantified and normalized as a percentage of total ErbB2/3 receptors. Four separate experiments were performed, and error bars represent standard deviations.
TPV-15L protein binds to heparin.
As shown from the previous result, expressed TPV-15L protein can functionally mimic neuregulin. However, certain soluble forms of NRG1 have been previously shown to bind heparin and are partially responsible for the localization of NRG1 to different cells and tissues in the nervous system (9). A careful analysis of the amino acid sequence of TPV-15L indicates that the Ig-like domain responsible for heparin binding is absent, unlike in NRG. In light of this, we sought to identify whether TPV-15L could bind heparin through other motifs by performing a simple slot blot test (Fig. 5A). To perform this experiment, various solutions of heparin, TPV-15L supernatant (positive control), BSA (negative control), and uninfected cell supernatant (negative control) were vacuumed through slots on three triplicate PVDF membranes. The membranes were blocked and incubated in either TPV-15Lmyc/His supernatant, TPV-2Lmyc/His supernatant, or uninfected cell supernatant. The membranes were washed and detected following a standard anti-myc Western blot detection protocol. As shown in Fig. 5A, slot 1a, an apparent band is present where there is a detectable interaction with the membrane-immobilized heparin, incubated in TPV-15L supernatant. In contrast, no bands are found in the baculovirus control (TPV-2L, slot 3a) or the cell supernatant control (slot 5a). This indicates that baculovirus expressed TPV-15L is capable of binding to heparin and that this activity is not attributed to baculovirus proteins or other cellular proteins.
Fig 5.
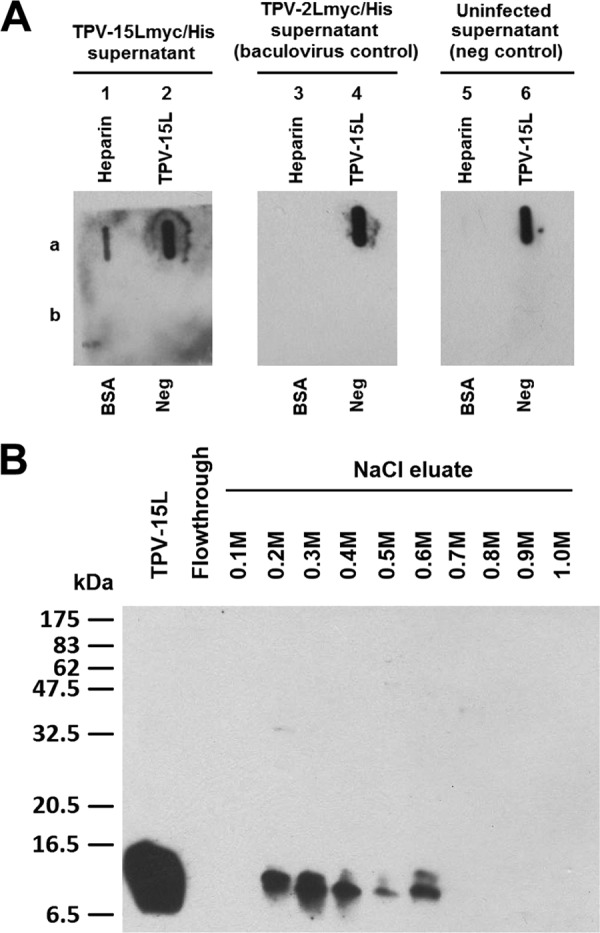
TPV-15L binds to heparin. (A) Unfractionated baculovirus-expressed TPV-15Lmyc/His slot blots. Three identical slot blots were performed, each aspirating heparin (1a, 3a, and 5a), TPV-15Lmyc/His (2a, 4a, and 6a), BSA (1b, 3b, and 5b), and uninfected Sf21 cell supernatant (Neg, 2b, 4b, and 6b). Blots were blocked and then incubated in either TPV-15Lmyc/His supernatant, TPV-2Lmyc/His supernatant or uninfected Sf21 cell supernatant as shown. Membranes were washed and detected using anti-myc antibodies. (B) TPV-15L isolated from His column was dialyzed with PBS, passed through a heparin column, and eluted with an increasing gradient of NaCl to assess relative affinity. In comparison, hNRG1elutes at 0.45 to 0.65 M NaCl on an identical column, suggesting a slightly weaker but comparable interaction with heparin.
Armed with the data indicating TPV-15L binding to heparin, we raised the question of how strong is this interaction is. By comparing TPV-15L to the heparin binding neuregulins, we could evaluate potential differences between the two proteins. Baculovirus-expressed TPV-15L was centrifuge clarified, passed through a heparin affinity column, and eluted in 10 fractions using a range of NaCl solutions (0.1 M to 1.0 M in 0.1 M increments). The 10 elution fractions were then evaluated using Western blot analysis. Figure 5B clearly shows that TPV-15L binds to heparin and starts eluting with 0.2 M NaCl.
TPVΔ15L knockout virus demonstrates reduced replication kinetics.
Previous studies on poxvirus-derived EGF-like growth factors revealed that these growth factors have a wide array of activity on multiple systems. In the case of VACV, VGF induces host cell proliferation and, if knocked out, can adversely affect VACV replication in mice (13, 14). Thus, we sought to identify whether TPV-15L played any role in viral infectivity. To accomplish this, we generated a TPV-15L knockout virus by transfecting TPV-infected cells with a knockout p15LCherry plasmid containing a fluorescent reporter gene (mCherry) under control of a synthetic early/late poxvirus promoter. The plaque-purified (3 times) recombinant knockout virus, named TPVΔ15L, was confirmed by PCR (Fig. 6A) demonstrating the deletion of the TPV-15L-encoding nucleotide sequence (absence of a 197-bp DNA fragment in Fig. 6A, lane c) and displayed no visible differences in cytopathic effect or plaque size (Fig. 6B and C) from wtTPV (Fig. 6D and E). HUVEC have been well characterized to overexpresses ErbB2, ErbB3, and ErbB4 receptors (43). Therefore, a growth curve was generated, using MOIs of 0.1 and 5, to compare wtTPV and TPVΔ15L on HUVEC. According to previous studies in our lab, a high-MOI TPV infection achieves maximum titer at approximately 96 h postinfection (27).Therefore, the progress of viral replication was monitored by harvesting the cells at two time points (48 and 96 h). As shown in Fig. 7, TPVΔ15L displayed a 10-fold difference in viral replication at both MOIs, suggesting (i) that TPV-15L is nonessential for virus replication in cell culture and (ii) that TPVΔ15L does not replicate as efficiently as wtTPV in HUVEC. In contrast, wtTPV displayed no such difference in viral replication at both MOIs, suggesting that the mutant virus replicates similarly to the wild-type virus in OMK cells (Fig. 8).
Fig 6.
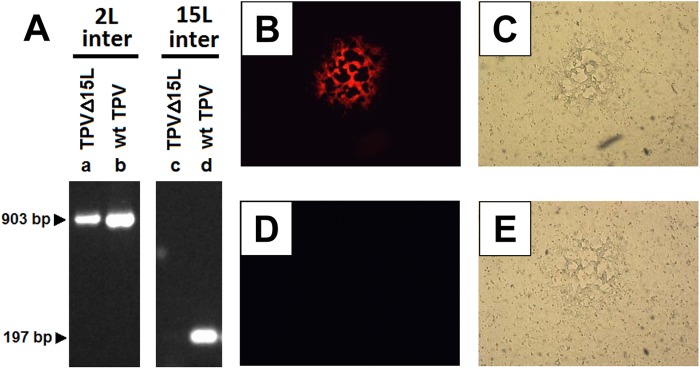
TPV-15L knockout virus. (A) Confirmatory PCR after three rounds of plaque purification using primers internal to TPV-2L (2L inter) and TPV-15L (15L inter) ORFs. As indicated by arrows, the TPV-2L internal fragment is expected to be 903 bp, and the TPV-15L internal fragment is expected at 197 bp. (B and C) An isolated plaque of TPVΔ15LCherry in OMK cells at 5 days postinfection (dpi), expressing mCherry as visualized by fluorescence (B) and phase contrast thereof (C). (D and E) An isolated plaque of wtTPV in OMK cells visualized by fluorescence (D) and phase contrast (E). Isolated plaques were selected from a 6-well titration plate.
Fig 7.
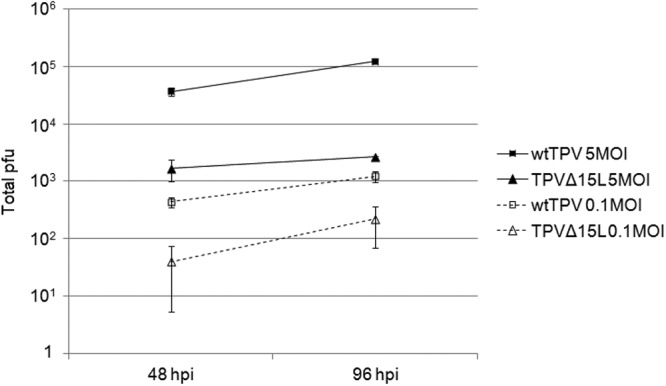
TPVΔ15L replication compared to that of wild-type TPV in HUVEC possessing neuregulin receptors. Cells were infected with either wtTPV or TPVΔ15LCherry at MOIs of 0.1 and 5 as indicated. The virus was harvested at 48 and 96 h postinfection and titrated on OMK cells. Shown are the averages from 3 independent experiments.
Fig 8.
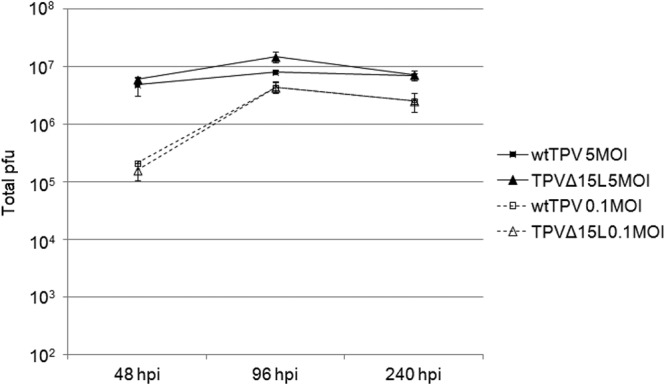
TPVΔ15L replication compared to that of wild-type TPV in OMK cells. Cells were infected with either wtTPV or TPVΔ15LCherry at MOIs of 0.1 and 5 as indicated. The virus was harvested at 48, 96, and 240 h postinfection and titrated on OMK cell monolayers. Shown are the averages from 3 independent experiments.
DISCUSSION
In this study, we analyzed TPV-15L using a combination of bioinformatics and assays to identify the role that it plays during TPV infection. The primary amino acid sequence of TPV-15L revealed an EGF-like domain, suggesting that it is a potential mimetic of EGF-like growth factors, such as neuregulin. Taking the results together, it is clear that TPV-15L is a so-called nonessential secreted glycoprotein that demonstrates a functional activity similar to that of neuregulin, despite sharing only 37% similarity with NRG1. Based on our data, we conclude that TPV-15L is capable of binding and phosphorylating the most potent NRG heterodimer receptor ErbB2/3. Limitations to this receptor heterodimer in our neuregulin bioassay prevented us from identifying other potential ErbB receptor interactions. The other NRG receptor heterodimer combinations involving ErbB4 were not evaluated, as this receptor is expressed primarily in the brain. In addition, it is still unclear whether TPV-15L is capable of phosphorylating the classical EGFR, ErbB1. Previous studies on other poxvirus-derived EGF-like growth factors have demonstrated ligand-specific receptor specificity (2). This suggests that although poxviral EGF-like molecules share a resemblance through the EGF-like domain, they differ in their ErbB receptor preference and functional capacity. Of all poxviral EGF-like molecules, only MGF has been previously shown to interact specifically with the ErbB2/3 heterodimer, while demonstrating no interaction with the respective individual homodimers (2). It is worthwhile to note that SFGF displays a broad range of receptor specificity, binding all varieties of ErbB1 heterodimers as well as the ErbB2/3 heterodimer. It has been previously proposed that specific virus growth factors may confer specialized advantages to target a specific subset of host cells that the virus invades (2). Yaba monkey tumor virus (YMTV), which targets histiocytes for proliferation, does not contain an EGF-like molecule in its genome (44). In contrast, TPV, which does contain an EGF-like molecule, preferentially replicates in epithelial cells, muscle cells, and fibroblasts (31, 45). The fact that ErbB1 can be found in most fibroblasts and epithelial cells while Erb3 and ErbB4 are expressed primarily in epithelial cells and brain represents only a possible connection with virus tissue tropism. The ErbB2/3 heterodimer is arguably one of the most potent of all ErbB receptor combinations, as indicated in numerous epithelial tumors overexpressing ErbB2. Whether or not TPV employs TPV-15L for this purpose is not yet resolved.
TPV-15L interaction with ErbB2/3 heterodimer may alter the microenvironment in which TPV proliferates. Overexpression of ErbB2 can result in resistance to TNF, as first shown in transfected NIH 3T3 cells (46). In addition, the presence of TNF can also reduce ErbB2 mRNA expression in human pancreatic cancers (47). In light of these observations, it is conceivable that TPV-15L, along with another TPV secretory protein called TPV-2L, which binds and neutralizes TNF with high affinity, could be working synergistically to prevent TNF activation of macrophages during virus replication.
Sequence analysis for the Ig-like heparin binding domain found on certain neuregulins shows that it is absent from TPV-15L. In fact, fusion proteins containing the Ig-like heparin binding domain from neuregulin retains heparin binding capacity, although at different affinities depending on the requirement of a spacer domain and where it fits into the context of the rest of the protein (26). Despite this, TPV-15L retains the functional capacity to bind heparin, which may be an indication of potential targeting and function of the molecule. Our results also indicate that there is a demonstrable but weak interaction between TPV-15L and heparin (0.2 to 0.6 M NaCl). Comparatively, human neuregulin typically begins to elute at 0.45 to 0.65 M NaCl (35, 48). However, it should be noted that even the shortest forms of neuregulin are still several times larger than TPV-15L, and this may be a potential reason for the apparent higher affinity for heparin. To date, other poxvirus-derived EGF-like growth factors have not been assessed for heparin binding, and such information may lead to additional insights on the functional activity of those molecules. Nevertheless, it is still quite interesting that despite the viral protein TPV-15L lacking the neuregulin-derived heparin binding domain (Ig-like domain), it still shares the dual ability to both activate ErbB receptors and bind heparin, in spite of a lower-affinity interaction with heparin.
One of the mysteries associated with certain viral infections, such as with TPV, is how the infection causes muscle pain and aches (49). One can hypothesize that perhaps a virally encoded secretory protein migrates from the primary site of infection and targets the neuromuscular junction, causing the muscle pain. In this case, one could speculate that TPV-15L, as a mimetic to neuregulin, could feasibly induce a localized increase in acetylcholine receptors at the neuromuscular junction, thus increasing sensitivity to muscle pain.
The precise role that TPV-15L plays in virus replication and host-virus interaction remains to be determined. At this point, our data on TPVΔ15L replication supports what others have previously shown from other poxvirus EGF-like growth factors (13, 14). Knocking out the TPV-15L protein reduced the replicative ability of the virus in at least certain cultured cells. To this end, we conclude the presence of a virally based neuregulin-like molecule may assist in TPV replication. If TPV-15L is a viral mimetic of human neuregulin and assists in TPV proliferation, perhaps neuregulin may play some additional role in the immunity that has yet to be described. The absence of a suitable experimental animal model for TPV infection poses major challenges in evaluating the precise function of this protein in vivo, but further studies of the ligand/receptor specificities of this viral protein may provide clues as to its biological role(s).
ACKNOWLEDGMENTS
This work was partially funded by NIH grants to K.E. (1R15CA156262-01) and J.A.L. (RO1NS059947). D.J. was a recipient of a Research Assistant award from WMU and a Graduate Research Assistant Scholarship from Pfizer Animal Health, administered by BIC at WMU.
We thank Steven Conrad and Bruce Bejcek for critically reviewing the manuscript.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Schneider MR, Wolf E. 2008. The epidermal growth factor receptor and its ligands in female reproduction: insights from rodent models. Cytokine Growth Factor Rev. 19:173–181 [DOI] [PubMed] [Google Scholar]
- 2. Tzahar E, Moyer JD, Waterman H, Barbacci EG, Bao J, Levkowitz G, Shelly M, Strano S, Pinkas-Kramarski R, Pierce JH, Andrews GC, Yarden Y. 1998. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 17:5948–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burden S, Yarden Y. 1997. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18:847–855 [DOI] [PubMed] [Google Scholar]
- 4. Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251–5257 [DOI] [PubMed] [Google Scholar]
- 5. Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. 1996. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14:737–744 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Mizushima H, Adachi S, Ohishi M, Iwamoto R, Mekada E. 2006. Cytoplasmic domain phosphorylation of heparin-binding EGF-like growth factor. Cell Struct. Funct. 31:15–27 [DOI] [PubMed] [Google Scholar]
- 7. Li Q, Esper RM, Loeb JA. 2004. Synergistic effects of neuregulin and agrin on muscle acetylcholine receptor expression. Mol. Cell Neurosci. 26:558–569 [DOI] [PubMed] [Google Scholar]
- 8. Loeb JA, Khurana TS, Robbins JT, Yee AG, Fischbach GD. 1999. Expression patterns of transmembrane and released forms of neuregulin during spinal cord and neuromuscular synapse development. Development 126:781–791 [DOI] [PubMed] [Google Scholar]
- 9. Pankonin MS, Gallagher JT, Loeb JA. 2005. Specific structural features of heparan sulfate proteoglycans potentiate neuregulin-1 signaling. J. Biol. Chem. 280:383–388 [DOI] [PubMed] [Google Scholar]
- 10. Jeng D, Rahman MM, McFadden G, Essani K. 2011. Tumor necrosis factor inhibitors from poxviruses with an emphasis on tanapoxvirus-2L protein. Recent Pat. DNA Gene Seq. 5:97–103 [DOI] [PubMed] [Google Scholar]
- 11. Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377–423 [DOI] [PubMed] [Google Scholar]
- 12. Smith GL. 1993. Vaccinia virus glycoproteins and immune evasion. J. Gen. Virol. 74:1725–1740 [DOI] [PubMed] [Google Scholar]
- 13. Buller RM, Chakrabarti S, Cooper JA, Twardzik DR, Moss B. 1988. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J. Virol. 62:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buller RM, Chakrabarti S, Moss B, Fredrickson T. 1988. Cell proliferative response to vaccinia virus is mediated by VGF. Virology 164:182–192 [DOI] [PubMed] [Google Scholar]
- 15. McFadden G, Graham K, Barry M. 1996. New strategies of immune modulation by DNA viruses. Transplant. Proc. 28:2085–2088 [PubMed] [Google Scholar]
- 16. Blomquist MC, Hunt LT, Barker WC. 1984. Vaccinia virus 19-kilodalton protein: relationship to several mammalian proteins, including two growth factors. Proc. Natl. Acad. Sci. U. S. A. 81:7363–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown JP, Twardzik DR, Marquardt H, Todaro GJ. 1985. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature 313:491–492 [DOI] [PubMed] [Google Scholar]
- 18. Chang W, Macaulay C, Hu SL, Tam JP, McFadden G. 1990. Tumorigenic poxviruses: characterization of the expression of an epidermal growth factor related gene in Shope fibroma virus. Virology 179:926–930 [DOI] [PubMed] [Google Scholar]
- 19. Chen HR, Barker WC. 1985. Similarity of vaccinia 28K, v-erb-B and EGF receptors. Nature 316:219–220 [DOI] [PubMed] [Google Scholar]
- 20. da Fonseca FG, Silva RL, Marques JT, Ferreira PC, Kroon EG. 1999. The genome of cowpox virus contains a gene related to those encoding the epidermal growth factor, transforming growth factor alpha and vaccinia growth factor. Virus Genes 18:151–160 [DOI] [PubMed] [Google Scholar]
- 21. Lin YZ, Ke XH, Tam JP. 1991. Synthesis and structure-activity study of myxoma virus growth factor. Biochemistry 30:3310–3314 [DOI] [PubMed] [Google Scholar]
- 22. Massung RF, Liu LI, Qi J, Knight JC, Yuran TE, Kerlavage AR, Parsons JM, Venter JC, Esposito JJ. 1994. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology 201:215–240 [DOI] [PubMed] [Google Scholar]
- 23. Reisner AH. 1985. Similarity between the vaccinia virus 19K early protein and epidermal growth factor. Nature 313:801–803 [DOI] [PubMed] [Google Scholar]
- 24. Twardzik DR, Brown JP, Ranchalis JE, Todaro GJ, Moss B. 1985. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc. Natl. Acad. Sci. U. S. A. 82:5300–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim M, Yang H, Kim SK, Reche PA, Tirabassi RS, Hussey RE, Chishti Y, Rheinwald JG, Morehead TJ, Zech T, Damon IK, Welsh RM, Reinherz EL. 2004. Biochemical and functional analysis of smallpox growth factor (SPGF) and anti-SPGF monoclonal antibodies J. Biol. Chem. 279:25838–25848 [DOI] [PubMed] [Google Scholar]
- 26. Ma Z, Li Q, An H, Pankonin MS, Wang J, Loeb JA. 2009. Targeting human epidermal growth factor receptor signaling with the neuregulin's heparin-binding domain J. Biol. Chem. 284:32108–32115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mediratta S, Essani K. 1999. The replication cycle of tanapox virus in owl monkey kidney cells. Can. J. Microbiol. 45:92–96 [DOI] [PubMed] [Google Scholar]
- 28. Essani K, Chalasani S, Eversole R, Beuving L, Birmingham L. 1994. Multiple anti-cytokine activities secreted from tanapox virus-infected cells. Microb. Pathog. 17:347–353 [DOI] [PubMed] [Google Scholar]
- 29. Paulose M, Bennett BL, Manning AM, Essani K. 1998. Selective inhibition of TNF-alpha induced cell adhesion molecule gene expression by tanapox virus. Microb. Pathog. 25:33–41 [DOI] [PubMed] [Google Scholar]
- 30. Brunetti CR, Paulose-Murphy M, Singh R, Qin J, Barrett JW, Tardivel A, Schneider P, Essani K, McFadden G. 2003. A secreted high-affinity inhibitor of human TNF from Tanapox virus. Proc. Natl. Acad. Sci. U. S. A. 100:4831–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nazarian SH, Barrett JW, Frace AM, Olsen-Rasmussen M, Khristova M, Shaban M, Neering S, Li Y, Damon IK, Esposito JJ, Essani K, McFadden G. 2007. Comparative genetic analysis of genomic DNA sequences of two human isolates of tanapox virus. Virus Res. 129:11–25 [DOI] [PubMed] [Google Scholar]
- 32. Lee HJ, Essani K, Smith GL. 2001. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281:170–192 [DOI] [PubMed] [Google Scholar]
- 33. Tulman ER, Afonso CL, Lu Z, Zsak L, Kutish GF, Rock DL. 2001. Genome of lumpy skin disease virus. J. Virol. 75:7122–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tulman ER, Afonso CL, Lu Z, Zsak L, Sur JH, Sandybaev NT, Kerembekova UZ, Zaitsev VL, Kutish GF, Rock DL. 2002. The genomes of sheeppox and goatpox viruses. J. Virol. 76:6054–6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loeb JA, Fischbach GD. 1995. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J. Cell Biol. 130:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meier T, Masciulli F, Moore C, Schoumacher F, Eppenberger U, Denzer AJ, Jones G, Brenner HR. 1998. Agrin can mediate acetylcholine receptor gene expression in muscle by aggregation of muscle-derived neuregulins. J. Cell Biol. 141:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fromm JR, Hileman RE, Caldwell EE, Weiler JM, Linhardt RJ. 1997. Pattern and spacing of basic amino acids in heparin binding sites. Arch. Biochem. Biophys. 343:92–100 [DOI] [PubMed] [Google Scholar]
- 38. Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. 1995. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity Mol. Biol. Cell 6:967–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong J, Wiley HS. 2000. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains J. Biol. Chem. 275:557–564 [DOI] [PubMed] [Google Scholar]
- 40. Lin YZ, Ke XH, Tam JP. 1990. Growth inhibition by vaccinia virus growth factor. J. Biol. Chem. 265:18884–18890 [PubMed] [Google Scholar]
- 41. Esper RM, Pankonin MS, Loeb JA. 2006. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res. Rev. 51:161–175 [DOI] [PubMed] [Google Scholar]
- 42. Li Q, Loeb JA. 2001. Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. J. Biol. Chem. 276:38068–38075 [DOI] [PubMed] [Google Scholar]
- 43. Russell KS, Stern DF, Polverini PJ, Bender JR. 1999. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis Am. J. Physiol. 277:H2205–H2211 [DOI] [PubMed] [Google Scholar]
- 44. Brunetti CR, Amano H, Ueda Y, Qin J, Miyamura T, Suzuki T, Li X, Barrett JW, McFadden G. 2003. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus Yaba monkey tumor virus J. Virol. 77:13335–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Espana C. 1971. A pox disease of monkeys transmissible to man, p 694–708 In Goldsmith EI, Moor-Jankowski J. (ed), Medical primatology. Karger, Basel, Switzerland [Google Scholar]
- 46. Hudziak RM, Lewis GD, Shalaby MR, Eessalu TE, Aggarwal BB, Ullrich A, Shepard HM. 1988. Amplified expression of the HER2/ERBB2 oncogene induces resistance to tumor necrosis factor alpha in NIH 3T3 cells Proc. Natl. Acad. Sci. U. S. A. 85:5102–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalthoff H, Roeder C, Gieseking J, Humburg I, Schmiegel W. 1993. Inverse regulation of human ERBB2 and epidermal growth factor receptors by tumor necrosis factor alpha Proc. Natl. Acad. Sci. U. S. A. 90:8972–8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. 1993. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell 72:801–815 [DOI] [PubMed] [Google Scholar]
- 49. Downie AW, Taylor-Robinson CH, Caunt AE, Nelson GS, Manson-Bahr PE, Matthews TC. 1971. Tanapox: a new disease caused by a pox virus. Br. Med. J. 1:363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]