Abstract
In preparing for the threat of a pandemic of avian H5N1 influenza virus, we need to consider the significant delay (4 to 6 months) necessary to produce a strain-matched vaccine. As some degree of cross-reactivity between seasonal influenza vaccines and H5N1 virus has been reported, this was further explored in the ferret model to determine the targets of protective immunity. Ferrets were vaccinated with two intramuscular inoculations of trivalent inactivated split influenza vaccine or subcomponent vaccines, with and without adjuvant, and later challenged with a lethal dose of A/Vietnam/1203/2004 (H5N1) influenza virus. We confirmed that vaccination with seasonal influenza vaccine afforded partial protection against lethal H5N1 challenge and showed that use of either AlPO4 or Iscomatrix adjuvant with the vaccine resulted in complete protection against disease and death. The protection was due exclusively to the H1N1 vaccine component, and although the hemagglutinin contributed to protection, the dominant protective response was targeted toward the neuraminidase (NA) and correlated with sialic acid cleavage-inhibiting antibody titers. Purified heterologous NA formulated with Iscomatrix adjuvant was also protective. These results suggest that adjuvanted seasonal trivalent vaccine could be used as an interim measure to decrease morbidity and mortality from H5N1 prior to the availability of a specific vaccine. The data also highlight that an inducer of cross-protective immunity is the NA, a protein whose levels are not normally monitored in vaccines and whose capacity to induce immunity in recipients is not normally assessed.
INTRODUCTION
The recent announcement of the engineering of avian H5N1 influenza virus to become readily transmissible by air in ferrets (1, 2) has caused considerable concern and refocusing on issues regarding how vulnerable we are to infection with such a virus and whether we have the tools available to lessen the impact should this virus become pandemic. An influenza pandemic due to highly pathogenic H5N1 could be significantly more severe than that recently experienced with pandemic H1N1/09 virus of swine origin, in part due to its capacity to infect beyond the respiratory tract in animal models (3, 4) and humans (5, 6). Unlike the pandemic H1N1/09 virus, the emergence of which was not predicted, we are alert to the potential threat from avian H5N1 and need to develop appropriate control measures to reduce morbidity and mortality in the event that this virus will gain the ability for efficient human-to-human spread.
Although vaccination is considered the best approach to prevent disease and limit transmission, the effectiveness of vaccination in the face of a pandemic is dictated by the length of time taken to produce a pandemic strain-matched vaccine in sufficient quantities for mass vaccination (7). The lengthy gap between the outbreak of pandemic H1N1/09 and the supply of vaccine has prompted studies aimed at investigating the possible benefits of using the existing seasonal influenza vaccine, either alone or in combination with an adjuvant, as a stopgap measure to reduce severe illness and fatalities while the pandemic vaccine is being manufactured.
Cross-reactive immunity, particularly against influenza virus of a heterologous hemagglutinin (HA) subtype, as would be expected to emerge as a severe pandemic, is efficiently induced by prior infection through the action of cytotoxic T cells to the conserved internal components of the virus, but such immunity is much less demonstrable after vaccination with current inactivated vaccines (8). Nevertheless, a number of groups have investigated the ability of a seasonal influenza vaccine (or components thereof) to induce protection against H5N1 infection. Thus far, partial protection has been demonstrated in the mouse model (9, 10) and in pigs (11) following parenteral delivery of a seasonal trivalent vaccine in the absence of an adjuvant. Cross-reactive responses to seasonal influenza vaccines that could potentially confer this partial benefit have been attributed to antibodies against HA (12) or neuraminidase (NA) (13–15).
In the present study, we use highly pathogenic H5N1 influenza virus infection of the naïve ferret to examine whether seasonal influenza vaccines, with and without adjuvant, could be employed as an interim control measure for prevention of severe disease. To fully appreciate the targets of cross-reactive immunity, the individual components of seasonal influenza vaccines were used to examine heterologous protection in this setting. These studies have provided further insight into the immune mediators of heterologous protection and suggest that an adjuvanted seasonal influenza vaccine could be deployed immediately after an H5N1 pandemic is declared to afford protection before the pandemic strain-matched vaccine becomes available.
MATERIALS AND METHODS
Ferrets.
Healthy juvenile ferrets less than 12 months old and weighing 800 to 1,700 g were obtained from the Institute of Medical and Veterinary Science, Adelaide, South Australia. Ferrets were allocated to groups in a manner to minimize the potential impact of age and weight on the response to vaccination and challenge and were determined to be seronegative for the currently circulating seasonal strains (H1N1, H3N2, B viruses) using standard hemagglutination inhibition (HI) tests prior to the commencement of the study. All experiments were performed under biosafety level 3+ (BSL3+) containment at the Australian Animal Health Laboratories (AAHL) with the approval of the CSIRO AAHL Animal Ethics Committee.
Viruses.
The wild-type H5N1 human influenza isolate A/Vietnam/1203/04 was used as the challenge virus, as previously documented (16). The seasonal viruses A/New Caledonia/20/1999 (H1N1), A/Brisbane/59/2007 (H1N1), A/Hiroshima/52/2005 (H3N2), and B/Malaysia/2506/2004 were obtained from the Department of Influenza Development (CSL Limited, Melbourne, Australia). The H3N1 virus was obtained by reassortment of A/New Caledonia/20/99 (H1N1) and A/Wisconsin/67/2005 (H3N2) in MDCK cells by using a plaque assay that included both a sheep polyclonal and mouse monoclonal antibody in the overlay to select against parental A/New Caledonia/20/99 virus. Real-time PCR assays specific for H3 and N1 were used to confirm the identity of the gene segments contained in the resulting reassortant virus.
Vaccine formulations.
The seasonal influenza vaccine used was the trivalent inactivated detergent-disrupted (“split”) vaccine Fluvax, a product of CSL Limited, prepared as described previously (17) from influenza virus strains bearing the surface antigens of A/New Caledonia/20/99 (H1N1), A/Hiroshima/52/2005 (H3N2), and B/Malaysia/2506/2004. This vaccine was recommended for the Northern Hemisphere 2006-to-2007 influenza seasons and the Southern Hemisphere 2007 season. Additional split virus vaccines were monovalent preparations of the individual H1N1 (A/New Caledonia/20/99) and H3N2 (A/Wisconsin/67/2005, which is antigenically equivalent to A/Hiroshima/52/2005) components of this seasonal influenza vaccine and also from vaccines based on A/Brisbane/59/2007 (H1N1), the reassortant H3N1 virus, and A/Vietnam/1194/2004 (H5N1). The concentration of viral antigen, expressed in terms of HA protein content, was determined by a standard single radial immunodiffusion assay and compared to that of a known standard of the relevant strain.
Recombinant NA (rNA) (A/Brevig mission/1/1918) and NP (rNP) (A/Indonesia/5/2005) were purchased from R&D systems (Minneapolis, MN) and Sapphire Biosciences (Australia), respectively. Recombinant HA derived from influenza virus strains A/Vietnam/1203/04 (H5), A/Indonesia/59/07 (H5), A/Brisbane/59/07 (H1), and A/Wisconsin/67/05 (H3) was purchased from Protein Sciences (Meriden, CT). H1 (A/Brisbane/59/07) and H3 (A/Wisconsin/67/05) viral HAs were released from inactivated purified virus preparations by bromelain cleavage and isolated by purification on a sucrose gradient. HA content was confirmed by Lowry protein estimation and purity by SDS-PAGE analysis.
Vaccines were tested at the antigen dose indicated, either alone or in formulation with either Iscomatrix adjuvant (60 μg/dose) (18, 19) or AlPO4.
Vaccination and viral challenge of ferrets.
Two equivalent 0.5-ml doses of vaccine were given 21 days apart and administered intramuscularly into the quadriceps using a 1-ml syringe with a 27-gauge needle. Three to 4 weeks after the last inoculation, the ferrets were challenged intranasally with 106 50% egg infectious doses of the A/Vietnam/1203/04 (H5N1) challenge virus in 0.5 ml. Vaccination and challenge were performed under ketamine-medetomidine anesthesia (50:50, 0.1 ml/kg of body weight, reversed with atipemazole).
Monitoring.
General clinical observations were made prior to and following challenge, and a detailed clinical signs sheet and an evaluation of activity based on a five-level score (16) were recorded at each inspection. Animals were weighed while under sedation at the time of vaccination and challenge and on days 3, 5, 7, and 14 postchallenge. Rectal temperature was also determined at sedation. Ferrets were euthanized 14 days after challenge or upon reaching a predetermined humane endpoint defined as 10% body weight loss or exhibition of signs consistent with involvement of other organ systems (e.g., tremor or abdominal discomfort).
Sampling.
Blood samples were collected immediately prior to each vaccination and prior to viral challenge. A further blood sample was taken 14 days postchallenge or at the time of euthanasia for serology. Blood collections were performed on anesthetized animals (ketamine-medetomidine, 50:50, 0.1 ml/kg, reversed with atipemazole) via the axillary veins. Nasal washes were collected into 1 ml of phosphate-buffered saline (PBS), on days 3, 5, and 7 postchallenge for virus isolation.
Immunological and virological evaluation.
Serum samples were heat inactivated at 56°C for 1 h prior to testing. The samples were assessed by a hemagglutination inhibition (HI) test using chicken red blood cells and virus neutralization (VN) by standard methods. NA activity was determined using an enzyme-linked microplate assay where Arachis hypogaea (peanut) lectin labeled with horseradish peroxidase was used to detect β-d-galactose-N-acetylglucosamine sequences exposed after the removal of sialic acid from fetuin, as described by Lambre et al. (20). Enzyme-linked immunosorbent assays (ELISAs) were performed on wells coated with detergent-solubilized purified virus preparations (21).
Western blotting analyses were performed after protein separation on a nonreducing SDS-PAGE 4-to-20% gradient gel (Invitrogen) and transferred onto nitrocellulose using an iBlotgel transfer device (Invitrogen, Carlsbad, CA). Membranes were blocked with 1% casein in PBS (pH 7.0) and washed with 0.05% Tween 20 in PBS (pH 7.2) prior to incubation with monoclonal antibody for 1 h at room temperature. Immunoreactive proteins were visualized with a peroxidase-labeled goat anti-mouse antibody (KPL) and Opti-4CN peroxidase substrate kit (Bio-Rad Life Sciences) following the manufacturer's instructions.
The antibody forensics (AF) method was used for measuring the levels of strain-specific IgG to HA in ferret sera (22). This is a multiplexed bead array technique based on the BioPlex/Luminex platform, in which serum is incubated with arrays of fluorescent beads coated with recombinant hemagglutinins derived from different influenza strains. Fluorescent -COOH beads no. 25, 27, 42, and 45 (Bio-Rad Life Science Group) were coated individually with recombinant HA of either A/Vietnam/1203/04 (H5N1), A/Brisbane/59/07 (H1N1), or A/Wisconsin/67/05 (H3N2), all from Protein Sciences, Meriden, CT. The coating was performed using carbodiimide-based linking of the -COOH groups on the bead surface with primary amino groups of the antigen according to the Bio-Rad protocol modified to slow down the reaction in order to reduce undesired cross-linking of the HA. Reading of the control and tested samples was performed using a BioPlex-100 bead array reader (Bio-Rad). The individual sensitivity of the beads coated with various HAs was determined by comparing the results to those of standard antibody solutions using the bead array in the BioPlex reader and using ELISAs with the same HA antigens that were used for coating the beads. The standard antibody for such calibration was ViroStat 1307 anti-influenza A polyclonal biotinylated antibody. Fluorescence in BioPlex readings was measured as the mean fluorescent index (MFI), which is proportional to the level of the antibody binding to the influenza virus strain-specific bead. All MFI values were corrected using the previously determined coefficients of bead sensitivity (MFIcorrected), and the final results are presented as the normalized mean fluorescence index, nMFI, where nMFI = (MFIcorrected) × (sample dilution factor). All serum samples were diluted 1:3,000 and read in duplicate.
RESULTS
Seasonal influenza vaccine formulated with adjuvant provides complete protection against H5N1 virus disease.
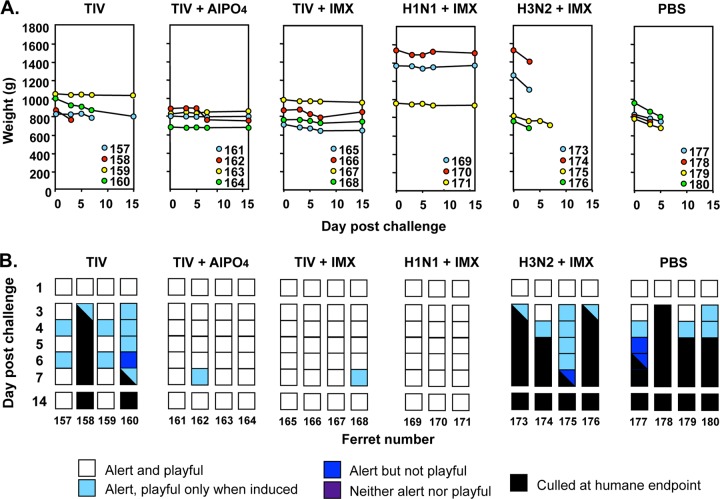
The ferret model was used to investigate the ability of seasonal influenza vaccines to protect against a lethal H5N1 challenge. Ferrets were immunized twice with either PBS or the trivalent influenza vaccine (TIV), containing 30 μg HA of each strain, either alone or formulated with the AlPO4 or Iscomatrix adjuvant. Three weeks later, the ferrets were challenged with a lethal dose of A/Vietnam/1203/2004 (H5N1). All four PBS control ferrets rapidly lost weight (Fig. 1A) and reached the humane endpoint on days 3 to 6 postchallenge, as in our previous studies (16). Ferrets that were inoculated with two doses of seasonal influenza vaccine showed partial protection against severe disease, with 2 of 4 showing minimal disease and the remaining two displaying fever (data not shown), weight loss greater than 10% (Fig. 1A), and reduced activity scores, meeting the humane endpoint on days 3 and 7 postchallenge, respectively (Fig. 1B).
Fig 1.
Response of ferrets vaccinated with seasonal trivalent or monovalent split influenza vaccines after challenge with a lethal dose of H5N1 virus. Ferrets were immunized with Fluvax seasonal trivalent split inactivated influenza vaccine (TIV), TIV formulated with AlPO4 or Iscomatrix adjuvant (IMX), or the individual monovalent H1N1 or H3N2 components of the TIV with Iscomatrix adjuvant or PBS alone, as indicated. The vaccines contained 30 μg HA of each component (i.e., 30 μg of each strain in the case of TIV) and were delivered as two inoculations 3 weeks apart. The ferrets were challenged with 106 50% egg infectious doses of wild-type A/Vietnam/1203/04 (H5N1) virus 4 weeks after the last immunization. (A) Weights of individual ferrets on days 0, 3, 4, 5, 6, 7, and 14 after challenge. (B) Activity scores for the indicated days after challenge out to day 14. Scores are depicted for each ferret by a strip of colored squares corresponding to the activity of the animal on the different days as indicated in the legend. Squares that are half-black indicate the activity score on the day of culling at the humane endpoint. Data are for individual ferrets, each assigned a number.
Improved survival was observed with the incorporation of adjuvant with the seasonal influenza vaccine. Complete protection from severe disease and death was observed in the two groups of ferrets that were vaccinated with seasonal influenza vaccine combined with either AlPO4 or Iscomatrix adjuvant (Fig. 1B); all animals showed minimal to no weight loss (Fig. 1A) and were fully alert and playful throughout the study, with the exception of one animal from each group (no. 162 and 168) that displayed a slight decrease in activity on day 7 postchallenge (Fig. 1B).
Despite this marked improvement in overall well-being, there was no significant impact of the vaccines on viral shedding, as measured by virus titers in the nasal washings on days 3, 5, and 7 postchallenge (repeated-measures analysis of variance [ANOVA]; data not shown). This is in contrast to our previous findings with homologous vaccine in the same model, which demonstrated that virus was cleared more rapidly in animals inoculated with vaccines formulated with Iscomatrix adjuvant than in those inoculated with unadjuvanted vaccines (16).
The H1N1 not the H3N2 component of seasonal influenza vaccine provides cross-protection against H5N1 virus challenge despite the apparent lack of cross-reactive antibody in classical tests.
To investigate the nature of the antigenic component(s) in the seasonal influenza vaccine that conferred cross-protection, monovalent vaccines containing a 30-μg HA dose of the individual H1N1 (A/New Caledonia/20/1999) and H3N2 (A/Wisconsin/67/2005) seasonal influenza vaccine components were formulated with Iscomatrix adjuvant and tested in the challenge model. A Mantel-Cox analysis of all the survival curves indicated a significant difference (P = 0.0019) between the vaccine groups, with the H1N1 monovalent vaccine, like the seasonal influenza vaccine group, being significantly different from the PBS control group. In contrast, there was no significant difference between the H3N2 monovalent vaccine and control groups. The improved survival of ferrets vaccinated with two doses of H1N1 plus Iscomatrix adjuvant was accompanied by less weight loss following challenge (Fig. 1A), and there was no disruption of activity (Fig. 1B).
Preboost, prechallenge, and endpoint sera from all vaccinated animals were screened by the classical serological methods, HI and VN, for the presence of antibodies cross-reactive with the H5N1 challenge virus (Table 1). No serological responses to H5N1 were detected by these tests in any of the ferrets vaccinated with seasonal trivalent influenza vaccines or monovalent vaccines. In addition, no antibodies to H5N1 were detected by either ELISA or Western blot analysis (data not shown).
Table 1.
Serum HI and VN responses to H5N1 virus of ferrets immunized with seasonal influenza vaccine or its individual type A strain components
| No. of ferrets | Vaccine preparation or controla | Geometric mean titerb |
|||||
|---|---|---|---|---|---|---|---|
| Primary |
Secondary |
Terminal bleedc |
|||||
| HI | VN | HI | VN | HI | VN | ||
| 4 | Fluvax | <4 | <4 | <4 | <4 | 13.5 | 11.3 |
| 4 | Fluvax + AlPO4 | <4 | <4 | <4 | <4 | 107.6 | 32 |
| 4 | Fluvax + IMX | <4 | <4 | <4 | <4 | 107.6 | 32 |
| 3d | H1N1 + IMX | <4 | <4 | <4 | <4 | 64 | 50.9 |
| 4 | H3N2 + IMX | <4 | <4 | <4 | <4 | 4.7 | <4 |
| 4 | Control (PBS) | <4 | <4 | <4 | <4 | <4 | <4 |
Vaccines were administered on days 0 and 21. IMX, Iscomatrix adjuvant.
Hemagglutination inhibition (HI) and virus neutralization (VN) tests were performed with A/Vietnam/1203/04 virus on sera taken 3 weeks after the primary immunization and 4 weeks after the secondary immunization, immediately prior to viral challenge. Data are expressed as the geometric mean titer.
Sera were tested again on day 14 postchallenge or earlier at the time of culling.
One immunized ferret needed to be euthanized prior to challenge for unrelated reasons, and data were removed from the group.
If NA is a target of cross-protection in the H1N1 component of seasonal influenza vaccine, dose and context may be important.
The analysis of monovalent seasonal influenza vaccine preparations indicated that cross-protection was induced by some element specific to the H1N1 virus and not shared with the H3N2 virus. As both of these vaccine components are derived from high-growth reassortants containing the internal genes of the egg-adapted A/Puerto Rico/8/34 (PR8) virus, this would implicate the H1 HA and/or the N1 NA as the target(s) of cross-protection. We hypothesized that because the H1N1 virus has the same subtype of NA as H5N1, this protein is most likely to be the target of cross-protective responses. In order to test this, we generated an H3N1 virus by reassortment of the H1N1 vaccine strain with the H3N2 vaccine strain and predicted that vaccination with an adjuvanted split preparation of this virus would induce a protective response. Groups of ferrets were vaccinated with two inoculations (15 μg HA) of monovalent preparations of either H1N1 (A/Brisbane/59/2007), H3N2 (A/Wisconsin/67/2005), or the reassortant H3N1 virus, all of which were formulated with Iscomatrix adjuvant. Additional ferret groups received two inoculations with either PBS or 1.5 μg of baculovirus-expressed recombinant N1 NA (rNA) from A/Brevig Mission/1/18 (H1N1) or recombinant NP (rNP) from A/Indonesia/5/2005 (H5N1) formulated with Iscomatrix adjuvant.
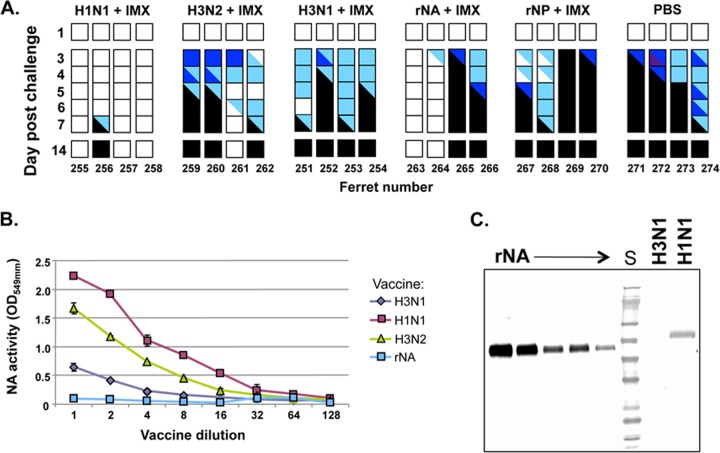
Consistent with the previous experiment, all of the PBS control animals and 3 of 4 H3N2-vaccinated animals succumbed to the H5N1 challenge within 7 days (Fig. 2A). In addition, 3 of 4 ferrets inoculated with A/Brisbane/57/2009 (H1N1) plus Iscomatrix adjuvant were free of disease, reflected by the maintenance of weight following challenge (average, 0.3% gain, range, 0.2 to 0.3%) and normal activity scores (Fig. 2A). It should be noted that in this experiment, the H1N1 vaccine used was derived from a different strain (A/Brisbane/59/2007) from that used in the first experiment (A/New Caledonia/20/99), indicating that the cross-protection observed was not strain specific. The dose of antigen was half of that used in the first experiment, which might explain the death of one ferret in the group (Fig. 2A).
Fig 2.
Response of ferrets to H5N1 challenge after vaccination with monovalent split or recombinant protein vaccines and the NA composition of these vaccines. (A) Activity scores of ferrets vaccinated with two doses, 3 weeks apart, of monovalent H1N1, H3N2, or H3N1 vaccines containing 15 μg HA or 1.5 μg rNA or rNP, all of which were formulated with Iscomatrix (IMX) adjuvant. Ferrets were challenged 4 weeks later with wild-type A/Vietnam/1203/04 virus and assigned activity scores as described in the legend to Fig. 1. (B) Relative neuraminidase activity in vaccine preparations was assessed in the fetuin cleavage assay. All split virus vaccine preparations were standardized to equivalent protein concentrations of 45.5 μg/well and the rNA to 6.25 μg/well in the first well, and then serial 2-fold dilutions were performed. (C) The level of neuraminidase protein in the vaccine preparations was determined by Western blotting. Lanes were loaded with 20 μg per split virus preparation. To estimate the level of NA content, rNA was used as a standard starting at 2 μg per lane and with further 2-fold serial dilutions of this preparation. A monoclonal antibody to a conserved region of N1 NA present in all viruses examined was used to probe the blots (S. Rockman, unpublished data).
Despite our predictions, all ferrets vaccinated with the H3N1 reassortant virus experienced a rise in temperature from day 3 that did not return to baseline (data not shown) and a similar drop in weight to the controls (average, 12%; range, 10 to 13%) and loss of activity, with all four animals reaching the humane endpoint by day 7 (Fig. 2A). Half of the animals inoculated with rNA plus Iscomatrix survived the challenge but continued to lose weight throughout the observation period (9% at day 12). The activity of these two animals was normal (no. 263) or recovered to normal at day 3 (no. 264). The remaining animals in this group (no. 265 and 266), reached the humane endpoint, with significant weight loss (11 and 12%), combined with reduced activity and fever, and were euthanized on days 3 and 5, respectively (Fig. 2A). Ferrets that received two doses of the rNP-Iscomatrix vaccine could not be distinguished from control animals with respect to weight loss, activity, temperature, and survival (Fig. 2A).
To investigate the differences in the levels of protection against death afforded by the preparations H1N1 (6 of 7), H3N1 (0 of 4), and rNA (2 of 4) with Iscomatrix adjuvant in these studies, the activity of NA in the different vaccines was determined using a fetuin-based enzyme assay (23). For this analysis, the vaccines were normalized with respect to HA content and tested in 2-fold serial dilutions for the ability to cleave sialic acid from the fetuin substrate (Fig. 2B). The H1N1 vaccine had the highest NA activity, whereas no NA activity was detected in the rNA vaccine, suggesting that the functional integrity of the active site was compromised in the recombinant protein. The H3N2 vaccine showed approximately half the NA activity of the H1N1 vaccine, whereas the NA activity in the H3N1 vaccine was estimated as 12-fold less than that of the H1N1 vaccine (Fig. 2B).
The differences in NA activity raised the question of whether the NA protein contents of the vaccines also differed. To address this, each of the viral vaccines was evaluated for NA content by Western blotting and densitometry, using a monoclonal anti-N1 antibody that was raised to a 14-amino-acid conserved carboxy-terminal peptide that is 100% identical between the NAs of the H1N1, H3N1, and rN1 preparations (Steven Rockman, unpublished data). The rN1 protein was used to generate a standard curve (0.24 μg to 62.5 μg) against which the level of NA protein in the H1N1 and H3N1 vaccines was determined (Fig. 2C). Based on this analysis, the cross-protective preparation containing H1N1 (A/New Caledonia/20/1999) and Iscomatrix adjuvant was estimated to contain 0.6 μg NA per 30 μg of HA. This is 2.5-fold lower than the dose of NA in the rN1 vaccine (1.5 μg NA per inoculation). The NA content of the H3N1 vaccine could not be visualized, indicating it had an NA content of <0.24 μg. Subsequent analysis of a more concentrated preparation of the H3N1 vaccine by the same method (data not shown) revealed that it contained approximately 0.55 μg NA/330 μg HA (corresponding to 0.025 μg NA per 15 μg HA used in each inoculation in Fig. 2A). This represents 12-fold less A/New Caledonia/20/99 NA in the H3N1 reassortant vaccine than in the equivalent amount of A/New Caledonia/20/99 monovalent H1N1 vaccine compared on an equal HA basis. This is in accordance with the relative NA activities of the two vaccines (Fig. 2B) and may explain the inability of the H3N1 vaccine to induce a protective response.
Neuraminidase-inhibiting antibody level corresponds with cross-protection.
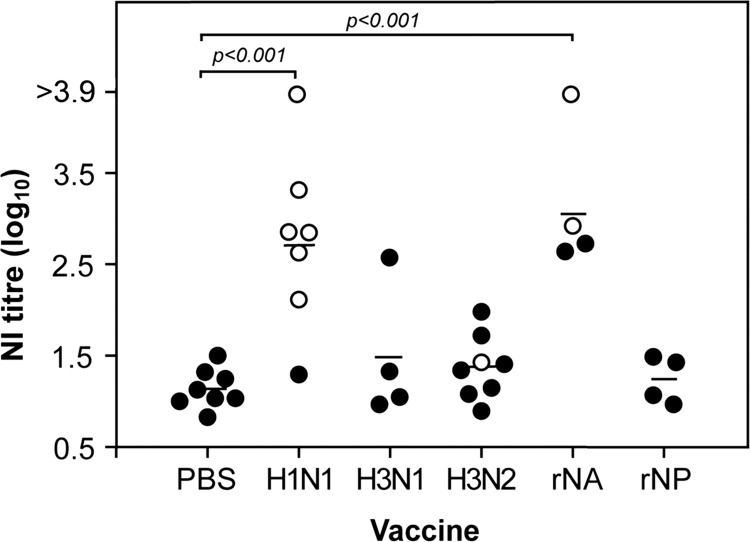
Lower protein content or loss of functional integrity may result in poor immunogenicity of the NA. We therefore tested the prechallenge serum from the groups of ferrets receiving monovalent virus vaccines or recombinant protein vaccines in the previous two experiments, for cross-reactive neuraminidase-inhibiting (NI) antibodies that prevented the cleavage of the substrate fetuin by infectious A/Vietnam/1203/04 (H5N1) virus (Fig. 3). Only serum from ferrets vaccinated with the H1N1 monovalent vaccines or rNA showed significant (P < 0.001) cross-reactive NI antibody levels compared to those in the PBS-vaccinated animals. These data indicate that despite being enzymatically inactive, the recombinant NA protein was still immunogenic and induced antibodies that blocked the H5N1 NA enzyme activity. It was also apparent that the low levels of NA in the H3N1 vaccine represented a subimmunogenic dose, with a possible exception in one animal. We then examined these data in the context of which animals had been protected by the vaccine (Fig. 3, open circles). The NI titers of the ferrets that survived were significantly greater than that of the ferrets euthanized as a consequence of reaching the humane endpoint (P < 0.0001, Mann-Whitney test), and ferrets with prechallenge NI antibody titers greater than 101.5 were significantly more likely to survive challenge (P = 0.004, Fisher's exact test), implying that these antibodies are either directly responsible for the cross-protection or are highly correlative with protective immunity.
Fig 3.
The presence of serum antibodies inhibiting the neuraminidase activity of H5N1 virus in prechallenge ferret sera. Sera from ferrets given monovalent vaccines, recombinant proteins, or PBS in the experiments shown in Fig. 1 and 2 were titrated for neuraminidase inhibition activity. The titers of individual ferrets that did not survive the challenge are shown as filled circles, while the survivors are shown in open circles. The geometric mean of each group is indicated by a line.
Increasing the overall dose of H3N1 vaccine only slightly improved cross-protection.
In order to provide more NA in the vaccine, the simplest method would be to increase the overall vaccine dose. To examine the effects of this, an H3N1-Iscomatrix vaccine was formulated that contained 0.55 μg NA (330 μg HA) per dose, which is a 22-fold higher HA level than the standard human adult vaccine dose of 15 μg HA and similar to the amount of A/New Caledonia/20/99 NA in the fully protective vaccine. This vaccine was compared to the monovalent H3N2-Iscomatrix vaccine at the same HA dose (330 μg). Additional groups tested were the monovalent H1N1 (A/Brisbane/59/2007) vaccine containing either 0.34 μg NA (15 μg HA) or 0.68 μg NA (30 μg HA), each with Iscomatrix adjuvant. Data for survival, percentage of weight change, and NI activity of prechallenge serum are shown in Table 2. All animals given the H1N1 vaccine, at either dose level, survived challenge, with only a transient loss of activity during the first 24 h postchallenge (data not shown) and either no significant loss of weight or mild weight gain. In contrast, 7 of 8 animals vaccinated with two doses of the H3N2 vaccine plus Iscomatrix adjuvant and all PBS controls reached a humane endpoint by day 6 and were euthanized. In the H3N1-Iscomatrix adjuvant group, 3 of 8 animals (41%) survived challenge with no loss of activity, which is an improvement above the lower-dose H3N1 vaccine that showed no protection (Fig. 2). The remaining ferrets in this H3N1 group were not different in any measurement of disease from those in the PBS control group. As in the previous experiment, the NI titers of the serum of individual ferrets that survived were significantly greater than that of the ferrets that needed to be euthanized at the humane endpoint (P = 0.0002, Mann-Whitney test) and ferrets with prechallenge NI antibody titers greater than 101.5 were significantly more likely to survive challenge (P = 0.049, Fisher's exact test).
Table 2.
Response of ferrets to vaccination with monovalent vaccines of a known N1 NA amount
| Vaccine or controla | Dose (μg) |
No. of ferrets surviving H5N1 challenge/total (%) | % wt changeb | NI activity (log10) against H5N1c | |
|---|---|---|---|---|---|
| HA | N1 | ||||
| H1N1 | 15 | 0.34 | 4/4 (100) | −3.48 ± 2.34 | 2.55 ± 0.74 |
| H1N1 | 30 | 0.68 | 4/4 (100) | −0.16 ± 1.57 | 2.02 ± 0.49 |
| H3N1 | 330 | 0.55 | 3/8 (41) | −9.36 ± 7.58 | 1.57 ± 0.67 |
| H3N2 | 330 | 1/8 (12) | −12.93 ± 4.94 | 1.28 ± 0.38 | |
| Control (PBS) | 0/4 (0) | −12.57 ± 3.02 | <0.9 | ||
Ferrets were inoculated and boosted with the indicated split virus preparations of monovalent seasonal influenza vaccine H1N1 (A/Brisbane/59/2007) or H3N2 (A/Wisconsin/67/2005) components or the reassortant H3N1 vaccine (A/Wisconsin/67/2005 × A/New Caledonia/20/1999), each with Iscomatrix adjuvant, prior to challenge with A/Vietnam/1203/04 virus.
Weight change is shown at day 7 or on the day of euthanasia at the humane endpoint and represents the mean ± standard deviation (SD) of the individual percentage changes relative to prechallenge weights.
The NI activity of prechallenge sera was assessed against A/Vietnam/1203/04 virus using fetuin as the substrate and is expressed as the geometric mean titer ± SD.
H1 HA provides an additional contribution as a target for cross-protective responses.
Having ascertained that the N1 NA is a target of cross-protective responses, the contribution of the H1 HA was explored. The HA was purified from A/Brisbane/59/2007, and Western blot analysis was used to confirm that this preparation was free of any detectable NA (not shown). Purified H1 HA and the rNA (from A/Brevig mission/1/1918) formulated in Iscomatrix adjuvant were compared at an equivalent dose of 2 μg per inoculation, for their ability to induce protective immunity against H5N1 challenge. Also included were purified H3 HA (from A/Brisbane/10/2007) at an equivalent dose as a negative control and the monovalent H1N1 vaccine plus Iscomatrix adjuvant (15 μg HA per dose) as a positive control. An additional group received a mixture of 2 μg each of H1 HA and rNA. The results, shown in Table 3, indicate that all animals given monovalent H1N1 split virus vaccine or rNA and Iscomatrix adjuvant survived the H5N1 challenge. In addition, 3 of 4 animals vaccinated with purified H1 HA survived challenge, whereas only a single animal in the H3 HA group survived. The temperature at the peak of fever on day 3 postchallenge was significantly greater in this group (Kruskal-Wallis test with Dunn's multiple comparison posttest).
Table 3.
Response of ferrets to vaccination with purified HA and recombinant NA vaccines
| Vaccine preparationa | Dose (μg) |
No. of ferrets surviving H5N1 challenge/total (%) | % wt changeb | Day 3 temp (no. of ferrets with temp of >40°C/total)c | |
|---|---|---|---|---|---|
| HA | N1 | ||||
| pH1 HA | 2 | 3/4 (75) | 1.20 ± 4.25 | 39.2 ± 0.52 (0/4) | |
| rN1 NA | 2 | 4/4 (100) | 1.80 ± 2.46 | 39.7 ± 0.29 (0/4) | |
| pH1 HA + rN1 NA | 2 | 2 | 4/4 (100) | 1.15 ± 4.17 | 39.4 ± 0.50 (0/4) |
| pH3 HA | 2 | 1/4 (25) | −3.35 ± 5.66 | 40.2 ± 0.25 (3/4) | |
| Split H1N1 | 15 | 0.34 | 4/4 (100) | −1.28 ± 3.38 | 39.4 ± 0.32 (0/4) |
Ferrets were inoculated and boosted with the indicated purified H1 (pH1) HA (A/Brisbane/59/2007), purified H3 (pH3) HA (A/Wisconsin/67/2005), recombinant N1 NA (A/Brevig mission/1/1918), or a split virus preparation of monovalent seasonal H1N1 (A/Brisbane/59/2007) vaccine in Iscomatrix adjuvant, prior to challenge with A/Vietnam/1203/04 virus (H5N1).
Weight change is shown at day 7 or on the day of euthanasia at the humane endpoint and represents the mean ± standard deviation (SD) of the individual percentage changes relative to prechallenge weights.
Mean ± SD of individual rectal temperatures of ferrets taken 3 days after challenge at the height of fever. In parentheses are the numbers of ferrets with temperatures of >40°C at this time.
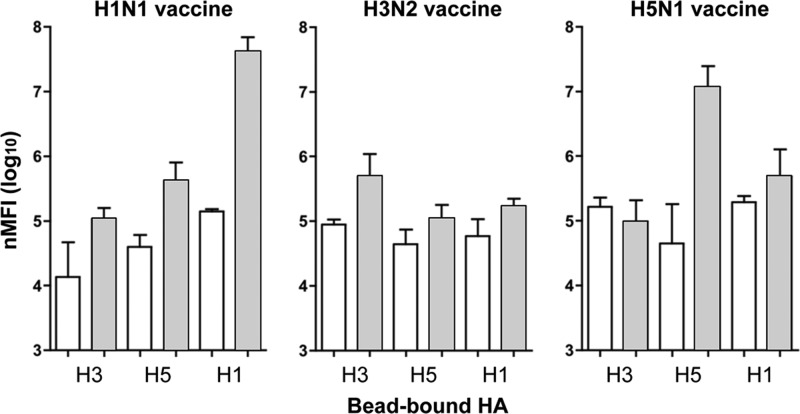
These results suggest that immune responses to H1 HA in addition to N1 NA contribute to protection in this model; however, none of the assay systems we had tested were useful to assess correlates of HA-dependent cross-protection. Although we could not detect the presence of cross-reactive antibodies by classical HI tests, this is a reasonably insensitive test and does not always correlate well with protection in this model, even with homologous vaccine (16). Therefore, a recently developed, highly sensitive method of AF (22) that uses Luminex beads coated with recombinant hemagglutinins for capturing influenza-specific antibody was employed. Beads coated with recombinant HA from either A/Vietnam/1203/04 (H5N1), A/Brisbane/59/07 (H1N1), or A/Wisconsin/67/05 (H3N2) were used to determine the presence of HA-specific antibodies in pre- and postvaccine sera from the ferrets immunized with PBS or two 30-μg HA inoculations of H1N1 or H3N2 (as described in Fig. 1) or H5N1 split virus preparations formulated with Iscomatrix adjuvant from our previous study (16). As shown in Fig. 4, reactivity of the postvaccination sera with the homologous HA was observed. However, the significance of the higher levels of binding of antiserum raised in ferrets vaccinated with the H1N1 vaccine to H5 HA compared to those of the prebleed sera from the same ferrets was not supported by statistical analysis, and thus the correlate of HA-induced cross-protection remained elusive.
Fig 4.
Reactivity of prechallenge ferret sera in the AF assay. Pre- and postvaccination sera were collected from the ferrets immunized with PBS or two of the H1N1 or H3N2 preparations containing 30 μg HA (described in the legend to Fig. 1) or from ferrets immunized with an equivalent dose of H5N1 split virus preparations formulated with Iscomatrix adjuvant for our previous study (16). The sera were tested by the AF method for reactivity on Luminex beads coated with recombinant HA from either A/Vietnam/1203/04 (H5N1), A/Brisbane/59/07 (H1N1), or A/Wisconsin/67/05 (H3N2). The normalized mean fluorescence intensity, nMFI, is plotted as the geometric mean of the group of 4 ferrets (or 3 in the case of H1N1), and the error bars represent the standard errors of the means. White bars, prevaccination serum; gray bars, postvaccination serum.
DISCUSSION
In our evaluation of the effectiveness of a seasonal trivalent split inactivated influenza vaccine (Fluvax) to protect ferrets against lethal H5N1 influenza challenge, we have confirmed the results of other studies demonstrating partial protection (9–15). However, in this study we have shown that the addition of an adjuvant (either Iscomatrix adjuvant or AlPO4) to the seasonal influenza vaccine affords complete protection in ferrets against severe disease and death due to the highly pathogenic H5N1 virus A/Vietnam/1203/04. Protection was induced by the H1N1 and not the H3N2 component of the trivalent vaccine and is therefore unlikely to be due to cross-reactive responses to viral antigens other than HA and NA, as these are derived from PR8 virus in both cases. This would tend to rule out certain targets, such as the M2 protein or conserved epitopes for T cells on internal proteins, as the dominant inducers of protection in this model and implicate the H1 HA and/or N1 NA.
Extensive analysis using a variety of classical assay approaches (HI, VN, ELISA, Western blotting) and a newly developed sensitive assay (AF) failed to reveal antibodies in prechallenge sera from ferrets that cross-reacted with the challenge virus. This is consistent with our previous studies that have shown protection from a lethal homologous H5N1 challenge could be induced in the absence of a classical HI and VN serological response (16). Furthermore, our findings with seasonal adjuvanted vaccines are consistent with those from other studies where cross-protection was demonstrated in the absence of detectable cross-reactive antibodies (10, 24). Nevertheless, we show here that antibodies to the H1N1 NA could inhibit the cleavage of fetuin by H5N1 virus, and the levels of these antibodies provided a strong correlate of protection against severe disease and death following H5N1 challenge. This implies that the N1 NAs of seasonal H1N1 and avian H5N1 viruses have one or more epitopes in common in the region affecting the enzyme-active site. The cross-reactivity with H5N1 NA was induced by a broad range of NAs, illustrated here by NA from the A/Brevig Mission/1/1918, A/New Caledonia/20/99, and A/Brisbane/59/2007 viruses. Alignment of the amino acid sequences of the NAs of these H1N1 strains with the sequence of the NA from the A/Vietnam/1203/2004 strain demonstrated overall identities in the protein sequence of 89.8% (Brevig Mission/1/18), 84.0% (A/Brisbane/59/2007), and 84.7% (A/New Caledonia/20/99) sequence and 77%, 53%, and 56%, respectively, within the putative antigenic sites (25). It would be important to extend this panel of viruses in future studies, which may aid in the definition of the common antigenic site by sequence and structural comparisons.
Responses to the H1 HA appeared to play an additional role in cross-protection, but the nature of these responses remains elusive. Compared on an equivalent weight basis, the recombinant N1 NA appeared to be slightly more cross-protective than the purified H1 HA, but more animals would need to be tested to be confident in this conclusion. Despite the finding that the recombinant NA was essentially devoid of sialidase activity, sufficient structural integrity of the critical epitope(s) around the cleavage site appears to have been maintained, in order to induce antibodies that inhibit the sialidase activity of H5N1 virus. Whether these NI antibodies provide the effector mechanism for protection is unknown, but this is certainly a possibility. NI antibodies, like NA inhibitor antiviral drugs, may function by reducing the efficiency of release of nascent virus from the infected host cell. This occurs by blocking the ability of NA to remove sialic acid from receptors on the cell that would otherwise trap the virus during exit. Furthermore, the carbohydrate side chains of newly formed NA and HA glycoproteins have terminal sialic acids that need to be removed in order to prevent clumping of the virus during budding. Additional but untested mechanisms by which NI antibodies may contribute to the protection against severe disease and death may include binding to NA expressed on the surface of infected cells (26), resulting in cytotoxicity via either complement-mediated or antibody-dependent cellular cytotoxicity (ADCC) mechanisms (27), thereby reducing the overall viral load on the host. In turn this may allow the developing H5N1-specific immune responses to achieve clearance more easily. Alternately, the antibodies could bind to virus particles and promote their opsonization, also leading to reduced viral loads.
Our findings are supported by those of other researchers using different modes of delivery of NA in experimental vaccines. Easterbrook et al. (28) showed that mice could be protected from a lethal H5N1 infection by intranasal vaccination with virus-like particles (VLPs) containing pandemic H1N1/09 NA (A/California/04/2009) and matrix proteins from a seasonal H1N1 virus (A/New York/312/2001); however, if the NA was replaced with a seasonal N1 (A/Bethesda/NIH50/2009), this protection was lost. Sandbulte et al. (13) used DNA vaccines encoding the NA from A/New Caledonia/20/99 (the same viral antigen used in this study) to protect 50% of mice from lethal H5N1 infection, even though detectable N1 antibody was only present in 12.5% of mice. Similar studies were reported with cross-reactive NA antibodies using live attenuated vaccines in ferrets (29). These studies demonstrate that the degree of homology of the NA used in the vaccine compared to that of the NA in the challenge virus may also be an important determinant in protection or survival.
In the initial challenge study, ferrets received two vaccine doses (21 days apart), each containing 30 μg of HA in order to maximize the likelihood of an outcome in naive animals. Having demonstrated protection using this level of antigen, all subsequent challenge experiments were undertaken using vaccines containing 15 μg of HA. Although we have not performed a formal dosing study, the level of NA-specific antibodies induced by the vaccine appears to be critical to achieving heterologous protection in this model. Without adjuvant, only half the ferrets vaccinated with seasonal trivalent vaccine survived challenge, and these showed reduced activity. The same vaccine formulated with either AlPO4 or Iscomatrix adjuvant, both of which are known to boost antibody responses (30), led to survival of all animals, with only a very slight reduction in activity. This infers that cross-reactive antibodies to NA may well be generated by routine seasonal vaccination of humans, but their levels cannot be assumed to be sufficient to ensure protection against a possible H5N1 challenge. The levels of cross-reactive NI antibody in humans and the ability to boost this by infection or seasonal vaccination warrant further investigation.
The context of the NA in the vaccine in relation to the other antigens present may also be important. In the monovalent H1N1 vaccine based on A/Brisbane/59/2007, protection against death and severe disease was seen with vaccines with NA content as low as 0.34 μg (and 15 μg HA), but the monovalent H3N1 vaccine given at a dose that resulted in a similar NA content of 0.55 μg (and 330 μg HA) gave only 41% survival. This may be due solely to the added benefit of the H1 HA. However, we postulate that an additional consideration is the low NA/HA ratio in the H3N1 virus, which can arise in these reassortants (J. Cobbin, S. Rockman, and L. E. Brown, unpublished data) to restore the correct balance of activities between HA and NA during replication (31). We showed that the sialidase activity of H3N1 virus was approximately 12-fold less than that of the H1N1 virus, and this was due to the presence of correspondingly less NA in the H3N1 virion. The dominance of the HA in terms of amount is thought to lead to its immunodominance in wild-type viruses (32), and this may become even more pronounced by the further reduction of the NA/HA ratio in the reassortant, resulting in even less antibodies to the NA being produced. Thus, the context of the NA in the vaccine, in terms of the subtype and/or amount of the HA with which it is paired, may well be important.
Unlike previous studies, this study has investigated the subcomponents of seasonal influenza vaccines and concluded that the cross-protection against H5N1 observed with trivalent seasonal or H1N1 preparations formulated in adjuvant can be attributed to both NA and HA, but the production of neuraminidase-inhibiting antibodies was a strong correlate for protection in this model. These findings suggest that consideration should be given to the development of adjuvanted NA-based or seasonal influenza vaccines to provide ongoing additional immunity to reduce the mortality and morbidity should a highly pathogenic H5N1 virus become the next pandemic. Given that ferrets in this study were protected from disease but not infection by the challenge virus, the benefit afforded by these vaccines may be a decrease in overall disease burden, which would potentially provide a window for the development of strain-specific immunity to the new virus, but they are unlikely to stop transmission. Our observations also strengthen the case for better monitoring of the NA content of vaccines and of the NA-specific responses that are induced by them. However, prior to doing so, it will be important to develop and validate appropriate assays that can be used globally so that results can be compared in a meaningful manner.
ACKNOWLEDGMENTS
We thank Tim Hancock for participation in the ferret experiments, Eleanor Cummins for formulation of vaccines, and Jesse Bodle for performance of ELISA and Western blotting.
This work was supported in part by the National Health and Medical Research Council of Australia's Urgent Research into a Potential Avian Influenza-Induced Pandemic grant 400583 and Program grant 567122. The WHO Collaborating Centre for Influenza Reference and Research is supported by the Australian Government Department of Health and Ageing.
Footnotes
Published ahead of print 2 January 2013
REFERENCES
- 1. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Zhao J, Tang S, Ye Z, Hewlett I. 2010. Viremia associated with fatal outcomes in ferrets infected with avian H5N1 influenza virus. PLoS One 5:e12099 doi:10.1371/journal.pone.0012099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao R, Dong L, Dong J, Wen L, Zhang Y, Yu H, Feng Z, Chen M, Tan Y, Mo Z, Liu H, Fan Y, Li K, Li CK, Li D, Yang W, Shu Y. 2010. A systematic molecular pathology study of a laboratory confirmed H5N1 human case. PLoS One 5:e13315 doi:10.1371/journal.pone.0013315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Z, Zhang J, Huang K, Li KS, Yuen KY, Guan Y, Chen H, Ng WF. 2009. Systemic infection of avian influenza A virus H5N1 subtype in humans. Hum. Pathol. 40:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hessel L. 2009. Pandemic influenza vaccines: meeting the supply, distribution and deployment challenges. Influenza Other Respir. Viruses 3:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown LE, Kelso A. 2009. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol. Cell Biol. 87:300–308 [DOI] [PubMed] [Google Scholar]
- 9. van Maurik A, Sabarth N, Dacho HS, Bruhl P, Schwendinger M, Crowe BA, Noel Barrett P, Kistner O, Keith Howard M. 2010. Seasonal influenza vaccine elicits heterosubtypic immunity against H5N1 that can be further boosted by H5N1 vaccination. Vaccine 28:1778–1785 [DOI] [PubMed] [Google Scholar]
- 10. Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, Itamura S, Odagiri T, Tashiro M, Takahashi H, Sawa H, Mitchell WM, Strayer DR, Carter WA, Chiba J, Kurata T, Sata T, Hasegawa H. 2007. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J. Infect. Dis. 196:1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Reeth K, Braeckmans D, Cox E, Van Borm S, van den Berg T, Goddeeris B, De Vleeschauwer A. 2009. Prior infection with an H1N1 swine influenza virus partially protects pigs against a low pathogenic H5N1 avian influenza virus. Vaccine 27:6330–6339 [DOI] [PubMed] [Google Scholar]
- 12. Garcia JM, Pepin S, Lagarde N, Ma ES, Vogel FR, Chan KH, Chiu SS, Peiris JS. 2009. Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin. PLoS One 4:e7918 doi:10.1371/journal.pone.0007918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. 2007. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4:e59 doi:10.1371/journal.pmed.0040059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gioia C, Castilletti C, Tempestilli M, Piacentini P, Bordi L, Chiappini R, Agrati C, Squarcione S, Ippolito G, Puro V, Capobianchi MR, Poccia F. 2008. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg. Infect. Dis. 14:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin CF, Qin ED. 2008. Seasonal influenza vaccination may mitigate the potential impact of an H5N1 pandemic. Chin. Med. J. (Engl.). 121:1481–1483 [PubMed] [Google Scholar]
- 16. Middleton D, Rockman S, Pearse M, Barr I, Lowther S, Klippel J, Ryan D, Brown L. 2009. Evaluation of vaccines for H5N1 influenza virus in ferrets reveals the potential for protective single-shot immunization. J. Virol. 83:7770–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coulter A, Wong TY, Drane D, Bates J, Macfarlan R, Cox J. 1998. Studies on experimental adjuvanted influenza vaccines: comparison of immune stimulating complexes (Iscoms) and oil-in-water vaccines. Vaccine 16:1243–1253 [DOI] [PubMed] [Google Scholar]
- 18. Boyle J, Eastman D, Millar C, Camuglia S, Cox J, Pearse M, Good J, Drane D. 2007. The utility of ISCOMATRIX adjuvant for dose reduction of antigen for vaccines requiring antibody responses. Vaccine 25:2541–2544 [DOI] [PubMed] [Google Scholar]
- 19. Pearse MJ, Drane D. 2005. ISCOMATRIX adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 57:465–474 [DOI] [PubMed] [Google Scholar]
- 20. Lambre CR, Chauvaux S, Pilatte Y. 1989. Fluorometric assay for the measurement of viral neuraminidase in influenza vaccines. Vaccine 7:104–105 [DOI] [PubMed] [Google Scholar]
- 21. Bodle J, Verity EE, Ong C, Vandenberg K, Shaw R, Barr IG, Rockman S. 15 May 2012. Development of an enzyme-linked immunoassay for the quantitation of influenza haemagglutinin: an alternative method to single radial immunodiffusion. Influenza Other Respir. Viruses [Epub ahead of print.] doi:10.1111/j.1750-2659.2012.00375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drake D, Singh I, Nguyen N, Kachurin A, Wittman V, Parkhill R, Kachurina O, Moser J, Burdin N, Moreau M, Misretta N, Byers A, Dhir V, Tapia T, Vernhes C, Gangur J, Kamala T, Swaminathan N, Warren W. 2012. In vitro biomimetic model of the human immune system for predictive vaccine assessments. Disruptive Sci. Technol. 1:28–40 [Google Scholar]
- 23. Aymard-Henry M, Coleman MT, Dowdle WR, Laver WG, Schild GC, Webster RG. 1973. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull. World Health Organ. 48:199–202 [PMC free article] [PubMed] [Google Scholar]
- 24. Xie H, Liu TM, Lu X, Wu Z, Belser JA, Katz JM, Tumpey TM, Ye Z. 2009. A live attenuated H1N1 M1 mutant provides broad cross-protection against influenza A viruses, including highly pathogenic A/Vietnam/1203/2004, in mice. J. Infect. Dis. 200:1874–1883 [DOI] [PubMed] [Google Scholar]
- 25. Soundararajan V, Tharakaraman K, Raman R, Raguram S, Shriver Z, Sasisekharan V, Sasisekharan R. 2009. Extrapolating from sequence—the 2009 H1N1 ‘swine’ influenza virus. Nat. Biotechnol. 27:510–513 [DOI] [PubMed] [Google Scholar]
- 26. Dowdle WR, Downie JC, Laver WG. 1974. Inhibition of virus release by antibodies to surface antigens of influenza viruses. J. Virol. 13:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashimoto G, Wright PF, Karzon DT. 1983. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J. Infect. Dis. 148:785–794 [DOI] [PubMed] [Google Scholar]
- 28. Easterbrook JD, Schwartzman LM, Gao J, Kash JC, Morens DM, Couzens L, Wan H, Eichelberger MC, Taubenberger JK. 2012. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Kim L, Subbarao K, Jin H. 2012. The 2009 pandemic H1N1 virus induces anti-neuraminidase (NA) antibodies that cross-react with the NA of H5N1 viruses in ferrets. Vaccine 30:2516–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baz Morelli A, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. 2012. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 61:935–943 [DOI] [PubMed] [Google Scholar]
- 31. Wagner R, Matrosovich M, Klenk HD. 2002. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12:159–166 [DOI] [PubMed] [Google Scholar]
- 32. Johansson BE, Moran TM, Kilbourne ED. 1987. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl. Acad. Sci. U. S. A. 84:6869–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]