Abstract
Positioning on the surgical table is one of the most important steps in any spinal surgical procedure. The “prone position” has traditionally been and remains the most common position used to access the dorsolumbar-sacral spine. Over the years, several authors have focused their attention on the anatomy and pathophysiology of both the vascular system and ventilation in order to reduce the amount of venous bleeding, as well as to prevent other complications and facilitate safe posterior approaches. The present paper reviews the pertinent literature with the aim of highlighting the advantages and disadvantages of various frames and positions currently used in posterior spinal surgery.
Keywords: Spinal frame, Prone position, Batson plexus, Blood loss, Posterior spinal approach
Introduction
The ideal position for spinal surgery should facilitate exposure, minimize both bleeding and the likelihood of damage to vital structures, and allow proper ventilation of the anesthetized patient. Additionally, it is imperative to avoid any postoperative morbidity secondary to the position during surgery.
These goals are more important, or potentially more difficult to achieve, in spinal surgery because of the deep exposures, and occasionally related difficulties, the accuracy required to identify the correct level, and the inherent risks of the various positions [4].
Neither supine nor lateral positioning directly affects intraoperative bleeding, because neither alters the physiology of the cardio-pulmonary system. On the contrary, a common complication of the prone position is increased bleeding, mostly due to damage to engorged vertebral veins or to excessive stretching of muscles. This decubitus is frequently required for patients undergoing posterior spinal operations for lumbar disc herniation, fusion surgery, and surgical correction of scoliosis. The prone position is comfortable for surgeons, providing an adequate vision of both bone and neural structures. Surgical frames, kneeling attachments and special operating tables have been designed over the years to promote good prone positioning, lower intra-abdominal pressure, and reduce epidural bleeding.
Factors affecting blood loss
To avoid complications in spinal surgery it is necessary to consider the anatomy and physiology of vertebral veins and the effects of their engorgement or damage during spinal operations. As a matter of fact, there are several plexuses of thin-walled, valve-less veins in relationship to the vertebrae. They normally contain blood at low pressure, and the direction of flow is reversible. The vertebral veins are connected with those in the chest through the vertebral canal, and with the ones in the abdomen and pelvis through the intercostals, lumbar and other connecting veins.
Oscar Batson’s pioneering experiments on monkeys, confirmed a few years later by Norgore, showed that, in cases of vena cava obstruction, the venous return from the lower parts of the body could be diverted into the vertebral venous system. This demonstrated definitively that this vertebral venous system acts as a supplementary channel of blood discharge [2, 22].
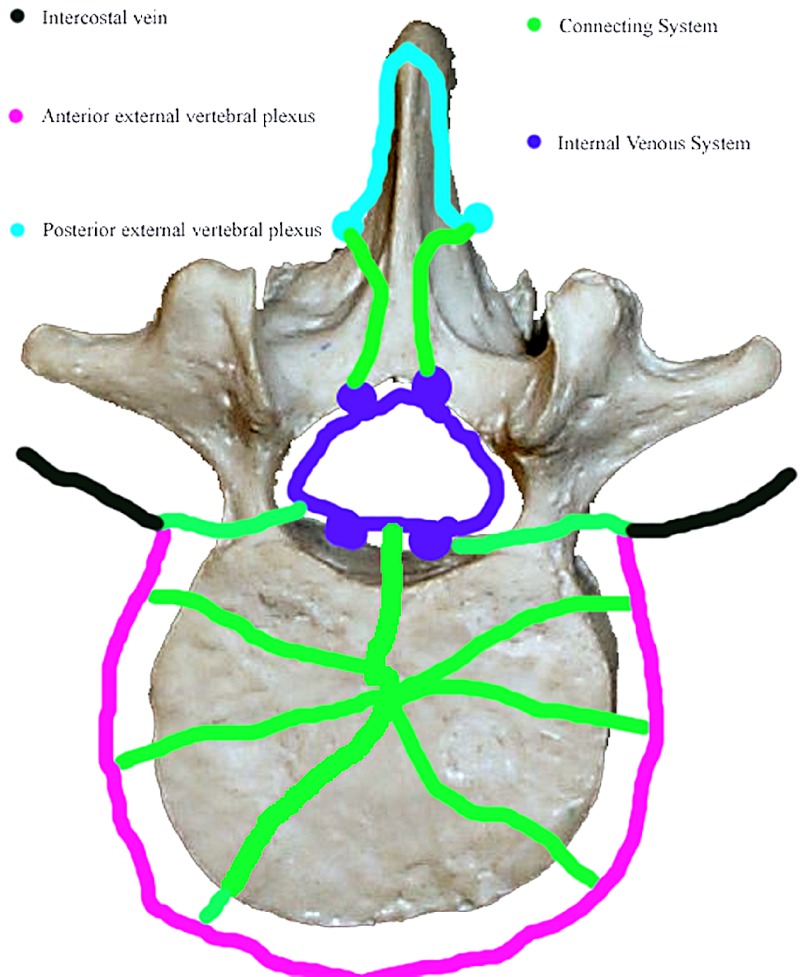
There are three components to Batson’s plexus: the internal venous system, the external venous system and the rich net of connecting or anastomotic veins (Fig. 1).
Fig. 1.
Batson’s plexus
The internal venous system
Within the spinal canal there can be found:
The anterior internal veins (AIVV) on the posterior surface of the vertebral bodies (the basivertebral vein drains into this part of the system)
The posterior internal vertebral veins (PIVV) on the anterior surface of the lamina (in the posterior part of the canal)
The anastomotic veins connecting the two systems within the spinal canal
The internal venous system represents a continuous venous pathway from the sacrococcygeal region to the base of the skull [18].
The external venous system
Longitudinally traveling veins lie anterior to the vertebral bodies, on the outer aspect of the lamina (posterior external vertebral plexus) and on the outer aspect of the transverse process.
Connecting or anastomotic veins
There is a rich anastomotic system of veins connecting the internal to the external vertebral system and connecting both parts of the vertebral venous system to the systemic vena cava circulation. It consists of the following: a basivertebral branch that passes laterally and anteriorly to penetrate vertebral bodies, radicular branches to veins lying along the spinal roots (intervertebral veins), posterior anastomotic channels that penetrate the ligamentum flavum, and anastomotic links between the AIVV, between the PIVV, and the AIVV and the PIVV within the spinal canal [18].
Bearing in mind these anatomic considerations, many factors may be responsible for causing partial or complete obstruction of the inferior vena cava during operations, thus causing a significant rise in the caval pressure and diversion of blood into the vertebral veins. Pressure on the anterior abdominal wall is transmitted to the inferior vena cava and only moderate external pressure is needed to cause a big rise in caval pressure [25]. A rise of intra-abdominal pressure may be caused by extrinsic factors such as sandbags, bolsters or the mattress of the operating table or by excessive abdominal muscle tension.
Moreover, if abdominal compression occurs while the patient is in the prone position, particularly in obese patients, the respiratory dynamic can be altered because of a decreased respiratory compliance. In the setting of reduced compliance, very high airway pressures may be required to ensure an adequate ventilation for the patient. High airway pressures may, in turn, impair venous return to the heart, decrease cardiac output and increase systemic venous pressure [23].
In addition, high venous pressure may result in decreased spinal cord perfusion pressure (mean arterial pressure—spinal venous pressure), putting the patient at increased risk of neurological complications [23]. Thus, less bleeding may be expected if the patient is supported with the abdomen pendulous and free from external pressure.
Methods to reduce blood loss (positions without frames)
Spinal posterior surgery has essentially two requirements: an adequate position of the column and an unrestricted abdomen with reduction of bleeding due to engorged vertebral veins. Yet, the prone position in spinal surgery may be complicated by the need to use a C-arm.
Good exposure of the contents of the segmental spinal canal is a sine qua non condition in surgery for lumbar disc herniation. In these cases, a position that decreases the lordosis of the lumbar spine and opens the posterior intervertebral spaces, facilitates the access to the inner spinal canal.
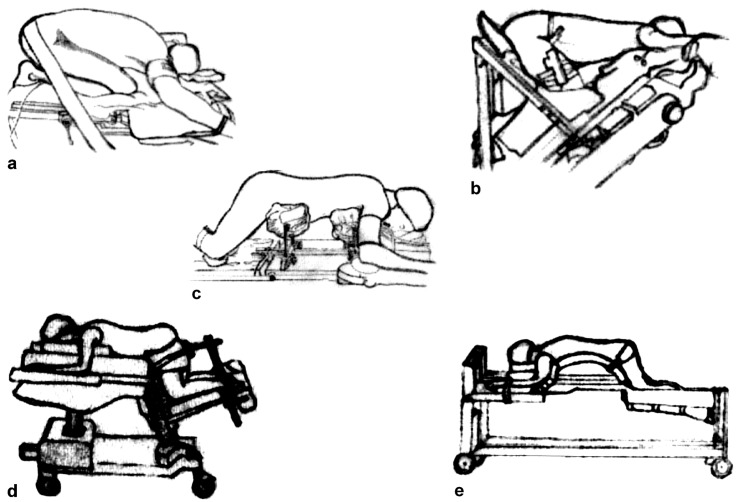
Use of chest rolls remains an effective and inexpensive technique to obtain an unrestricted abdomen during prone spinal surgery. This can also be achieved by the kneeling position, first described by Ecker in 1949 [9]. Lipton in 1950 described a variant of the Ecker position, the so-called Mohammedan praying position [17]. The knee-chest position, another evolution of the first position reported, was than described by Tarlov in 1967 [31]. The tuck position, an extreme fixed position, was than described in the same year by Wayne [32] (Fig. 2a). However, considerable flexion of the spine, hips, and knees occurs in this extreme tucked position, and this may produce vascular and nerve compression in the popliteal compartment. Moreover, after prolonged spinal surgical procedures, massive release of myoglobin can cause acute renal failure [11, 16]. In addition, this extreme flexed position may tighten the posterior paraspinal muscles so that lateral retraction, particularly important in cases of lateral stenosis, may be quite difficult [11]. Finally, prolonged maximal joint flexion is potentially dangerous in patients with hip or knee disorders, joint degeneration, or implanted prostheses [26].
Fig. 2.
Positioning for spinal surgery. a Tuck position; b Canadian frame; c Relton Hall type frame; d Andrews frame; e Wilson frame
Methods to reduce blood loss (the jungle of frames)
Preservation of the normal sagittal spinal alignment is critical in spinal reconstructive surgery. In these cases, positioning devices have to balance the goals of both abdominal decompression and lordosis preservation [12, 28]. Relton and Hall in 1969 described a frame that may be still considered a standard for comparison (Imperial Surgical, Halpern Dorval, Quebec, Canada). Their device consists of four padded supports arranged in two V-shaped pairs. The rostral pair supports the lateral aspects of the upper thoracic cage—below the clavicles and as far down as the xiphisternum. The caudal pair supports the anterolateral aspects of the pelvic girdle between the iliac crests and the greater trochanters of the femora, so that they do not encroach on the lower parts of the anterior abdominal wall. The supports are set at a 45° inward tilt and are individually adjustable for length and width. With suitable adjustment they give adequate support and prevent external pressures from being applied to the anterior abdominal wall during the procedure. The tendency towards hyperextension of the vertebral column is partially counteracted by lowering the legs [27], (Fig. 2c).
Furthermore, many devices were designed to obtain an abdomen free of restriction and to decrease lordosis during posterior spinal operation for lumbar disc herniation. Hastings described in 1969 the so-called Canadian frame (Fig. 2b) [13]. This complex device allowed the patient to be placed in a knee-chest position, without overstretching the joints. (The Cloward surgical saddle and Heffington frame were proposed by neurosurgeons with the same objective [29]).
However, two popular devices actually warrant a safe prone surgical position for lumbar disc herniation: the “Andrews” and “Wilson” frames. On the Andrews table, patients are positioned in a modified knee-chest position with a chest pad and an adjustable tibial support lowered to obtain 90° hip flexion. The tibial support may be adjusted to produce 60° hip flexion for spinal fusion operations. It allows C-Arm integration for both A/P and lateral intraoperative views (OSI, Union City, CA, USA) (Fig. 2d).
The Wilson supporting frame provides a convenient and stable method of maintaining patients in a flexed position for spinal surgery. It has two curved full-length pads, which provide continuous support for chest and pelvis and adjust laterally to improve ventilation and relieve pressure from the abdomen. A recent evolution of this frame (Wilson Plus) offers 360° of unobstructed radiolucency, for easily obtainable images by either C-arm or X-ray (OSI, Union City, CA, USA) (Fig. 2e).
The Jackson surgical table has a 360° axis rotational capability and thus facilitates safe and efficient rotation of particularly traumatized patients during combined approaches. It also offers 360° of unobstructed radiolucency for easily obtainable images with either C-arm or X-ray (OSI, Union City, CA, USA).
Human studies
In 1967 Wayne et al. first measured the pressure in the vena cava (intra-caval venous pressure ICVP) in six male patients, by introducing a long venous catheter through the femoral vein. Two patients representing each of the three body types (tall muscular, medium, and obese) were tested in each of the following positions: supine, prone, prone with rolls supporting the shoulders and iliac crests, prone on a foam rubber horseshoe pad, and in the tuck position. Only in the tuck position were consistently favorable readings obtained even in the obese subjects, whose abdominal contents produced a venous pressure of 220 mm of water in the supine position. In the tuck position it was possible to reduce pressure significantly, whereas, pressure increased markedly in the other prone positions [32].
In 1969 DiStefano et al. measured intraoperatively the ICVP in ten patients affected by spinal instability or disc herniation, using the same technique as Wayne. Venous pressure determinations were recorded in six positions: prone with bolsters; on a Wilson frame; kneeling; lateral decubitus; in the tuck position; and on a Canadian Frame. Hastings’s Canadian frame was found to result in significantly lower ICVP, with negative pressure recordings in three occasions. However, the comparison of the effects of different support systems was not made in the same patient, and no obese subjects were included in the study [7].
In 1992, McNulty et al. measured IVCP in 18 patients undergoing elective lumbar laminectomy. Those patients were assigned randomly to one of the three prone support systems (Andrews frame, Cloward surgical saddle, and longitudinal bolsters). The IVCP in the group with their abdomens extremely pendulous (on an Andrews frame) was significantly lower than that in the other two groups. However, again in this series, the comparison of the effects of different support systems was not made in the same patient [19].
In 1990 Botsman et al. reported a retrospective analysis of 436 standard operations for herniated lumbar discs during a period of 8 years. The aim of this study was to evaluate the relation between blood loss and operating time with various positions. Prone position on bolsters was used in 216 cases and a frame-supported position (with a modified Hastings frame) was used in 192. The choice between these two positions was based solely on the personal preference of the operating surgeon. First and second operations, irrespective of the vertebral spaces explored were included, but cases requiring complete laminectomy were excluded, to obtain an homogeneous study group. The blood loss was assessed by weighting the gauze packs used and by measuring the amount drained by suction from the operating field. The mean calculated blood loss in prone position was 376 ml (interquartile range 150−450 ml) in the first operations and 504 ml (interquartile range 200–110 ml) in reoperations. In the kneeling, supported position the calculated mean blood loss was 150 ml (50–300 ml) and 218 ml (100–400 ml), respectively. The mean operating time with prone position was 74 min (standard deviation (SD) 32) in the first operations and 97 min (SD, 36) in reoperations. With kneeling position it was 52 minutes (SD, 23) and 82 min (SD, 29), respectively [3].
In 2000 Park et al. conducted a detailed studied of the effects of the width of Wilson-frame-pad supports for posterior lumbar spinal fusion operations. His study was a prospective analysis of 40 patients undergoing surgery. Patients were randomly assigned to group 1, narrow (36.6±1.2 cm), or group 2, wide (43.8±1.2 cm) support. There were no significant differences between groups for sex, weight, height and preoperative MAP (mean arterial pressure). IAP (intra abdominal pressure) by rectal balloon was measured as estimation of ICVP for the following positions: supine, prone on a gurney, prone on the Wilson frame before and after incision, and supine after tracheal extubation. Moreover, intraoperative blood loss was calculated by weighing blood-soaked gauzes as they were passed off the surgical field. Blood contents of the suction bottle were also measured, excluding the irrigation solution. IAP in the prone position on the gurney was not different from that in the supine position in each group. IAP in the prone position on the Wilson frame before incision (8.8 cmH2O) was significantly more than that in the supine position after the induction (6.9 cmH2O) in group 1 (p<0.05). However, in group 2, IAP in the prone position on the Wilson frame before incision (3.6 cmH2O) was significantly less than in the supine position after induction (7.0 cmH2O) (p<0.05). IAP in the prone position on the Wilson frame after incision was 10.6 cmH2O in group 1 and 4.7 cmH2O in group 2 and was higher than that for pre-incision in each group (p<0.05). IAP in the supine position after tracheal extubation was the highest in each group. Comparing the different groups, IAPs in the prone position on the Wilson frame before and after incision in group 2 were significantly less than those in group 1 (p<0.05). Intraoperative blood loss in group 2 (436±159 ml) was significantly less than in group 1 (878±521 ml) (p<0.05). In conclusion, blood loss and IAP were less in the group positioned on a wider Wilson pad support. [24].
Tao-Chen Lee et al. in 1998 reported a prospective study including 20 patients undergoing lumbar spinal surgery in a prone position under controlled isoflurane-induced hypotension. For each patient, IVCP was measured: with the patient positioned supine, prone on a conventional pad, and, subsequently, prone on a Relton-Hall frame. The mean IVCP was 15.3 mmHg (range, 8.2–23.4 mmHg) when patients were positioned prone on a conventional pad, and this dropped to 8.2 mmHg (range, 4.6–13.6 mmHg) when they were subsequently positioned on a Relton-Hall frame. It is important to note that in every case, the measured IVCP in patients on a conventional pad was 1.5× higher (range, 1.5–2.4×; mean, 1.9×) than that measured in those on a Relton-Hall frame. They concluded that a device allowing the patient’s abdominal viscera to hang freely in a prone position significantly reduces IVCP, and isoflurane-induced hypotension, with reduction of the patient’s mean arterial pressure by 20 mmHg, does not influence IVCP [30].
Complications of positioning
Injury to the lateral femoral cutaneous nerve (LFCN) was found to be a common complication during spine surgery and occurs in 20% of the patients [20]. Neuropathy of this nerve is usually associated only with hypoesthesia, but in some patients it may cause pain and dysesthesia in the anterolateral aspect of the thigh [10]. As the LFCN is sensitive, the signs of injury may be missed, mainly in the first postoperative days when the patient has pain at the surgical site and is not completely alert under the influence of analgesics and narcotics.
Compression neurapraxia is most probably the cause of injury in patients undergoing operation on frames. In those positions, in fact, the posts supporting the pelvis can compress the nerve at the exit below the anterior superior iliac spine [20]. This complication, also known as meralgia paresthetica, usually has a benign course, and 89% of patients reported by Mirowski recovered completely 3 months after surgery [20]. Because of the relatively high probability of meralgia paresthetica after spine surgery, patients should be informed about the occurrence of this complication.
Direct pressure on the eye, especially as a result of a patient malposition, has been cited as a factor contributing to visual loss, often irreversible, in several published reports. The incidence of significant visual complications after spine surgery, according to a recent review, could be on the order of one case per 100 spine surgeons per year [21]. Long operative times, substantial intraoperative blood loss [14, 15] and intraoperative hypotension could be associated risk factors for this complication [6, 8]. Vigilance regarding eye protection during positioning by both the surgeon and the anesthesiologist is compulsory to avoid this dramatic event.
Shoulder dislocation [1], massive release of myoglobin with acute renal failure [11, 16] and ischemic medullary syndromes [5] are other sporadically reported complications.
References
- 1.Ali J Clin Anesth. 2003;15:471. doi: 10.1016/S0952-8180(03)00103-X. [DOI] [PubMed] [Google Scholar]
- 2.Batson Ann Surg. 1940;112:139. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BotsmanSpine 1990153602363066 [Google Scholar]
- 4.CallahanClin Orthop 1981154227471559 [Google Scholar]
- 5.Chu Anesth Analg. 2002;95:1451. doi: 10.1097/00000539-200211000-00065. [DOI] [PubMed] [Google Scholar]
- 6.Connolly Am J Ophthalmol. 1994;117:235. doi: 10.1016/s0002-9394(14)73082-x. [DOI] [PubMed] [Google Scholar]
- 7.DiStefanoClin Orthop 197499514825719 [Google Scholar]
- 8.Drance N Engl J Med. 1973;288:392. doi: 10.1056/NEJM197302222880804. [DOI] [PubMed] [Google Scholar]
- 9.Ecker Surgery. 1949;25:112. [PubMed] [Google Scholar]
- 10.Edelson J Bone Joint Surg Am. 1994;76:993. doi: 10.2106/00004623-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ford Clin Orthop. 1977;123:104. doi: 10.1097/00003086-197703000-00042. [DOI] [PubMed] [Google Scholar]
- 12.Guanciale Spine. 1996;21:964. doi: 10.1097/00007632-199604150-00012. [DOI] [PubMed] [Google Scholar]
- 13.HastingsCan J Surg 1969122515776927 [Google Scholar]
- 14.Hayreh Ophthalmology. 1980;87:75. doi: 10.1016/s0161-6420(80)35283-4. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh Ophthalmology. 1987;94:1488. doi: 10.1016/s0161-6420(87)33273-7. [DOI] [PubMed] [Google Scholar]
- 16.Keim J Bone Joint Surg Am. 1970;52A:1248. [PubMed] [Google Scholar]
- 17.LiptonAnaesthesia 1950520814783279 [Google Scholar]
- 18.McCulloch JA, Young PH (1998) Microsurgery for lumbar disc herniation. In: McCulloch JA, Young PH (eds) Essentials of spinal microsurgery. Lippincott, Philadelphia pp 329–382
- 19.McNulty J Clin Anesth. 1992;4:220. doi: 10.1016/0952-8180(92)90070-H. [DOI] [PubMed] [Google Scholar]
- 20.Mirovsky Spine. 2000;25:1266. doi: 10.1097/00007632-200005150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Myers Spine. 1997;22:1325. doi: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Norgore Surgery. 1945;17:606. [Google Scholar]
- 23.Palmon Anesth Analg. 1998;87:1175. doi: 10.1097/00000539-199811000-00037. [DOI] [PubMed] [Google Scholar]
- 24.Park Anesth Analg. 2000;91:552. doi: 10.1097/00000539-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Pearce Proc R Soc. 1957;Med:109. [Google Scholar]
- 26.RayNeurosurgery 1987202663561734 [Google Scholar]
- 27.Relton J Bone Joint Surg Br. 1967;49:327. [PubMed] [Google Scholar]
- 28.Stephens Spine. 1996;21:1802. doi: 10.1097/00007632-199608010-00016. [DOI] [PubMed] [Google Scholar]
- 29.Sutterlin Orthop Rev. 1988;17:597. [PubMed] [Google Scholar]
- 30.Tao-Chen Spine. 1998;23:941. doi: 10.1097/00007632-199804150-00019. [DOI] [PubMed] [Google Scholar]
- 31.Tarlov J Bone Joint Surg Am. 1967;49:1193. [PubMed] [Google Scholar]
- 32.Wayne J Bone Joint Surg Am. 1967;49:1195. [PubMed] [Google Scholar]