Abstract
This article focuses on our current understanding of the role of activated coagulation factor VII (FVIIa) in coagulation, the current evidence regarding the efficacy and safety of recombinant FVIIa (rFVIIa), and thoughts regarding the use of rFVIIa in spine surgery. rFVIIa is approved in many countries (including the European Union and the USA) for patients with hemophilia and inhibitors (antibodies) to coagulation factors VIII or IX. High circulating concentrations of FVIIa, achieved by exogenous administration, initiate hemostasis by combining with tissue factor at the site of injury, producing thrombin, activating platelets and coagulation factors II, IX and X, thus providing for the full thrombin burst that is essential for hemostasis. This “bypass” therapy has led some clinicians to use rFVIIa “off-label” for disorders of hemostasis other than hemophilia. Based on clinical experience, case reports and limited information from clinical trials, rFVIIa may be efficacious in states of decreased concentration of coagulation factors, thrombocytopenia, and at least some states of altered platelet function. The former two can occur intra-operatively during spinal surgery as a consequence of substantial blood loss and normal consumption. Preliminary reports have indicated that rFVIIa does not increase the perioperative incidence of thromboembolic events. However, full reports from large clinical trials regarding the efficacy and safety of rFVIIa in settings other than hemophilia have yet to appear in peer-reviewed publications. Until adequate data demonstrating safety and efficacy are fully reported, it would seem appropriate to reserve the use of rFVIIa in spinal surgery to those instances where conventional therapy cannot provide adequate hemostasis, and “rescue” therapy is required.
Keywords: Blood loss, Coagulation, Coagulopathy, Hemostasis, Recombinant coagulation factor VIIa
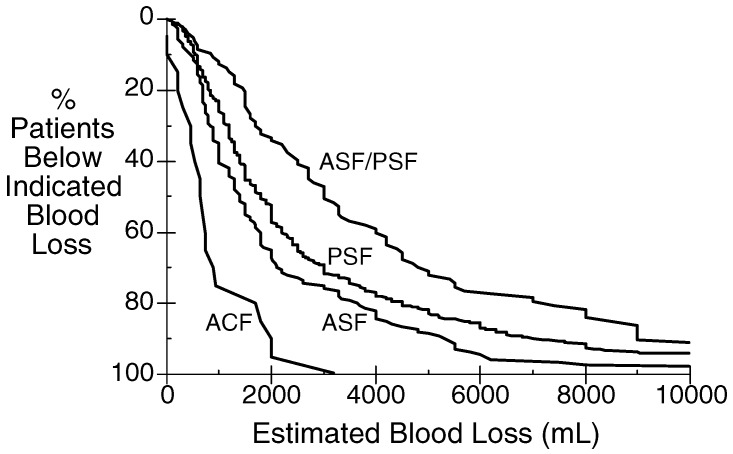
Surgery of the spine encompasses a very broad range of procedures. The extent and blood loss encountered during these procedures varies enormously (Fig. 1). A simple laminectomy or discectomy does not create much blood loss. Surgery for spinal deformity can entail blood loss of several liters. Even within this latter sub-set, the variability is great. Correction of scoliosis in an otherwise normal teenager can be associated with small-to-moderate blood loss, but is not usually associated with extensive blood loss, while correction in a child with a neuromuscular disorder, or an elderly adult frequently involves substantial blood loss. In a recent 3-year period at the University of California, San Francisco, among those patients for whom intra-operative cell salvage was used (that is, patients in whom substantial blood loss was anticipated), the range of estimated blood loss for patients undergoing spinal surgery was nil to more than 10 l (Fig. 1).
Fig. 1.
Estimated blood loss in spinal surgery of 1,003 patients (March 2000–May 2003) for whom intra-operative cell salvage was used. Data for each procedure (ACF anterior cervical fusion; ASF anterior spinal (thoracic and/or lumbar) fusion; PSF posterior spinal (thoracic and/ or lumbar) fusion; ASF/PSF anterior/posterior spinal fusion) show the cumulative number of patients who had that amount or less of blood loss. Data collected and analyzed with institutional review board approval (Committee on Human Research, University of California, San Francisco, CA, USA)
When blood loss approaches or exceeds one blood volume, and replacement does not include coagulation factors, sufficient dilution of circulating coagulation factors can produce a “dilutional” coagulopathy, adding to the potential for blood loss. Simultaneously, appropriate consumption of coagulation factors and platelets at the surgical site providing hemostasis can add some degree of a “consumptive” coagulopathy, further contributing to the potential for blood loss.
The usual appropriate treatment of dilution of coagulation factors or platelets, and their normal consumption during surgery, is to administer their replacement as needed: the former either as contained in whole blood or in fresh-frozen plasma, and the latter as either single-donor or pooled platelet concentrates. Some authors have concluded that excessive bleeding during spinal surgery can result from hyperfibrinolysis, that is, an abnormally activated fibrinolytic system with excessive fibrinolysis, and thus, they advocate the use of anti-fibrinolytic therapy during spinal surgery. It is my view that “hyperfibrinolysis” tends to be over-diagnosed and that in otherwise normal patients undergoing spinal surgery, observed “abnormal” fibrinolysis most likely is the result of a normal fibrinolytic system responding to an activated coagulation system. This, however, produces more than a normal degree of fibrinolysis, owing to a poorly formed clot and inadequate activation of coagulation factor XIII and thrombin activatable fibrinolytic inhibitor (TAFI), because of low concentrations of coagulation factors and platelets. Low concentrations of thrombin result in a poorly formed, less dense fibrin clot that is subject to rapid fibrinolysis. A denser fibrin clot formed with normal amounts of thrombin, is not vulnerable to this condition [8], which is produced by the dilution of coagulation factors that occurs with blood loss and asanguinous replacement.
This brief article focuses on our current understanding of the role of activated coagulation factor VII (FVIIa) in coagulation, the current evidence regarding the efficacy and safety of recombinant FVIIa (rFVIIa), and thoughts regarding the use of rFVIIa in spine surgery.
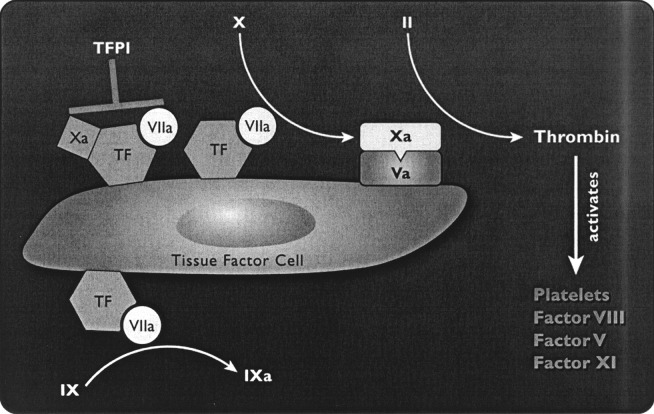
The vision of the process of coagulation, developed in the 1960s as a coagulation cascade, has a number of deficits, including the lack of involvement of cellular elements (for example, platelets and tissue factor bearing cells) and the inability to explain satisfactorily some clinical observations. More recent work has provided a better understanding of the events contributing to in vivo hemostasis. The initiating event is the formation of a complex of FVIIa and the tissue factor that is exposed on a tissue-factor-bearing cell (Fig. 2). The integrated concept of hemostasis has been recently reviewed by Roberts et al [34].
Fig. 2.
The two main functions of tissue factor (TF) are shown: to activate factor X and to activate factor IX. Factor Xa remains in the vicinity of the TF cell and activates factor V. The complex of factors Xa/Va can convert a small amount of prothrombin (factor II) to thrombin, with the results shown. Tissue-factor pathway inhibitor (TFPI) then inhibits the complex of TF/VIIa/Xa as a control mechanism. Reproduced with permission from Bernstein, Jeffers, Erhardtsen et al. [2]
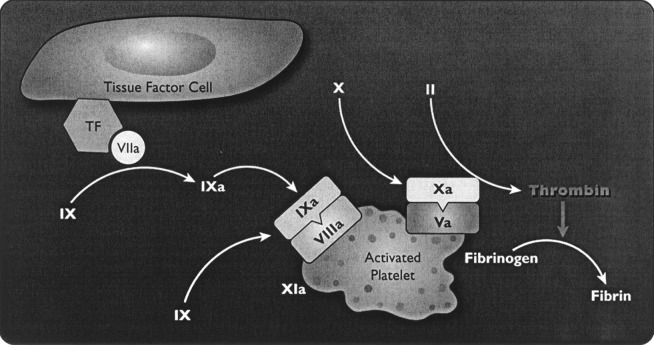
The development of an improved understanding of hemostasis has proceeded with, and been aided by, the development and understanding of the mechanism of action of activated coagulation factor VII. Circulating FVIIa accounts for approximately 1% of circulating coagulation factor VII (FVII) [41], and is enzymatically inactive until forming a complex with tissue factor (TF). When rFVIIa is administered exogenously, circulating concentrations of FVIIa greatly exceed the usual physiological concentrations. Activated coagulation factor VII initiates hemostasis by combining with tissue factor (a membrane-bound glycoprotein expressed by subendothelial cells) at the site of injury, forming a TF–FVIIa complex at the local site. The complex activates other factors, eventually producing limited thrombin generation and activated platelets (Fig. 2). Activated platelets and coagulation factors II, IX and X are critical for the development of a full thrombin burst. This thrombin burst is required to produce a stable, solid fibrin plug that is resistant to fibrinolysis [5, 8, 9, 40], and for the full activation of TAFI and of coagulation factor XIII, both of which are important for the stabilization of the fibrin plug and its resistance to fibrinolysis [27, 30]. Therapy with doses of rFVIIa that achieve supraphysiological concentrations saturate TF binding sites, activate platelets and produce clinically significant thrombin production, despite an absence of coagulation factors VIII or IX, or in the presence of antibodies to these factors (Fig. 3) [28]. Thrombin formation is impaired in thrombocytopenia and some types of platelet dysfunction [1]. rFVIIa increases thrombin generation in thrombocytopenia [23].
Fig. 3.
The activated platelet derived from un-activated, circulating platelets resulting from the thrombin generation on the TF cell. Activated co-factors Va and VIIIa occupy sites on the activated platelet before binding of the respective enzymes, factors IXa and Xa. Factor Xa on the activated platelets is recruited from circulating factor X and is different from the factor X on the TF cell. The burst of thrombin generation takes place on the platelet surface. Thrombin generation can be boosted by further activation of factor IX by factor XIa. The burst of thrombin is sufficient to convert fibrinogen to fibrin. Reproduced with permission from Bernstein, Jeffers, Erhardtsen et al. [2]
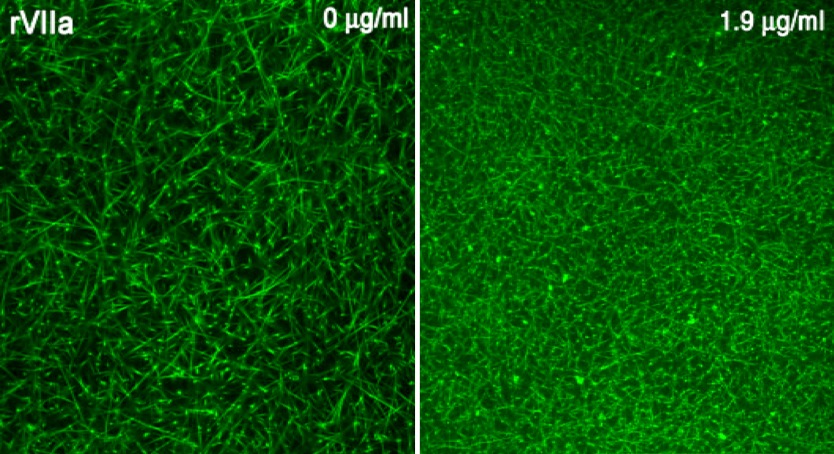
Hedner was the first to realize that FVIIa could be used as a “bypass” therapy for the treatment of patients with hemophilia and inhibitors (antibodies) to coagulation factors VIII or IX, which have developed either as a result of treatment of inherited hemophilia with these factors, or in patients with previously normal coagulation (acquired hemophilia), thus bypassing the need for these clotting factors [15, 16, 17, 18]. In an in vitro system, addition of rFVIIa to FVIII-deficient plasma, to achieve a concentration similar to that after administration of an in vivo dose of 90–100 µg/kg, restores to normal the abnormal clot permeability and the fibrin network (Fig. 4) [14]. rFVIIa has been demonstrated to be efficacious and safe for these indications, and until February 2004, use in patients with inhibitors to coagulation factors VIII or IX was the only use for which rFVIIa had regulatory approval. Hedner also suggested that rFVIIa might be efficacious for other disorders of hemostasis. Her suggestion resulted in an initial dramatic off-label use of rFVIIa, wherein rFVIIa provided hemostasis after clinicians had ceased all other therapies, including surgery, for a victim of major trauma with uncontrollable hemorrhage [22]. Following this apparent success, a great number of case reports have appeared, purporting to indicate that rFVIIa is efficacious for a broad group of disorders of hemostasis, including:
Thrombocytopenia [24]
Thrombocytopenia refractory to platelet transfusion owing to antibodies to platelet antigens [39]
Von Willebrand’s disease [7]
Cerebral injury-induced coagulopathy [29]
Hepatic dysfunction (with normalization of prothrombin time) [2]
Trauma [26]
Necrotizing pancreatitis [36]
Pulmonary hemorrhage following stem-cell transplantation [32] or cardiac surgery [25]
Other bleeding following cardiopulmonary bypass [35]
Gastrointestinal bleeding [20]
Postpartum hemorrhage [6]
Traumatic brain injury [42]
Dilutional coagulopathy during spinal surgery [38]
Fig. 4 a.
Plasma deficient in coagulation factor VIII produces a loose, porous clot that is readily subject to fibrinolysis; b addition of rFVIIa, 1.9 µg/ml, a concentration equivalent to that achieved with exogenous administration of approximately 90–100 µg/kg rFVIIa, to plasma deficient in coagulation factor VIII restores the fibrin clot to a tight, normal architecture, resistant to fibrinolysis. Reproduced with permission from Hedner and Kisiel [16]
These reports, and the substantial off-label use of rFVIIa has led to the unproven proposition that rFVIIa may be efficacious for providing hemostasis in abnormal states other than hemophilia. This has resulted in a substantial program to develop use of rFVIIa in non-hemophiliac populations, and the US National Institutes of Health has called for applications for research grants in this field. Recently, rFVIIa has been approved in the European Union (but not in the USA) for FVII-replacement therapy and for Glanzmann’s thrombasthenia.
There have been a limited number of clinical trials, reported in the peer-reviewed literature, of use of rFVIIa for conditions other than hemophilia. rFVIIa normalizes the international normalized register (INR) of volunteers given acenocoumarol to produce an INR greater than 2.0 [12]; it corrects the prothrombin time in patients with cirrhosis [2, 21], and it provided for hemostasis in within 10 min for 74% of 66 patients with Childs-Turcotte B or C hepatic cirrhosis undergoing laparoscopic liver biopsy [21]. In an open-label, pilot study of six patients undergoing hepatic transplantation, patients given rFVIIa had less blood loss and required less transfused fresh-frozen plasma (FFP) and red cells than did matched controls [19]. In a double-blind, randomized, dose-escalation study of 36 patients undergoing prostatectomy, rFVIIa decreased blood loss and red cell transfusion compared to placebo-treated patients[13]. Recently (December 2003), Novo Nordisk announced the results of a phase II double-blinded, placebo-controlled, multi-center trial of 280 patients with severe trauma. Patients treated with rFVIIa reportedly needed fewer transfusions, had fewer complications, and spent less time in intensive care units than did the patients given placebo. In February 2004 Novo Nordisk announced the preliminary results of their phase II, randomized double-blind trial of rFVIIa for hepatic transplantation. They stated that those patients treated with rFVIIa had a significantly lesser incidence of requiring red cell transfusion than did those not treated with rFVIIa, and that there was a similar incidence of thromboembolic events in those treated or not treated with rFVIIa. The data from these two studies had not appeared in the peer-reviewed literature at the time of the writing of this article (February-March 2004).
Taken together, these case reports, and the limited data from clinical trials appear to indicate promising hemostatic applications for rFVIIa in non-hemophilia-related states of altered hemostasis: thrombocytopenia, decreased coagulation-factor concentrations causing elevated prothrombin time and partial thromboplastin time, and altered platelet function. The former two are of direct relevance to spinal surgery, in which extensive blood loss can produce these altered states by both dilution and, to a lesser extent, normal consumption. That is not to say that administration of rFVIIa should, at this time, replace the standard therapies of administration of coagulation factors (via whole blood or FFP) and platelets. There are insufficient data to confirm that rFVIIa is superior to, or even as good as, these conventional therapies during spinal surgery. However, there are times when it is difficult to correct the deficiency of decreased coagulation factors by conventional therapy, especially during ongoing, massive blood loss. When conventional therapy does not, or cannot, succeed, it is reasonable to administer rFVIIa as an attempted rescue therapy.
The appropriate dose under these circumstances is not clear. The studies and case reports cited above report the use of a wide range of doses: from 10 µg/kg to 100 µg/kg or more. Doses at the low end of this range are not likely to be efficacious. Most clinicians using rFVIIa as a rescue drug for hemorrhage during surgery administer doses of approximately 90–100 µg/kg. However, it should be noted that the trauma trial discussed above used an initial dose of 200 µg/kg, followed by two additional doses of 100 µg/kg each, 2 h and 4 h after the initial dose. The hepatic-transplantation trial used a dose of 60 µg/kg or 120 µg/kg repeated every 2 h, while the prostatectomy study used doses of 20 µg/kg and 40 g/kg.
In addition to the uncertainty regarding the appropriate dose, the duration of action, and thus timing, of repetitive doses is not fully elucidated. The clearance of rFVIIa is approximately 30–35 ml/kg/hr in adults and greater in children [11]. This suggests that administration of rFVIIa should be repeated relatively frequently, if inadequate hemostasis persists; perhaps at 2 h intervals. However, it is not clear that the most important aspect of the mechanism of action relates to the plasma concentration of rFVIIa at any given time, rather than the peak concentration achieved shortly after administration. The latter may have greater importance for rFVIIa than for many drugs because of the need to produce the thrombin burst, which is essential to the mechanism of action of rFVIIa and hemostasis.
The issue of safety is also of importance in consideration of the use of rFVIIa. The safety profile of this biologic, following its approval in 1996 in the European Union and 1999 in the USA, has been excellent for the many doses and patients to which it has been given for treatment of hemophilia. Some of the reports cited above have described rare complications. However, in the absence of published full reports of large, randomized clinical trials, it is unknown if these complications are associated with rFVIIa administration more than they occur in patients not so treated, or in similar patients in whom altered hemostasis has been corrected by other means, or in patients with unaltered coagulation. The most important complications of theoretical concern are thromboembolic events. One of the six patients who underwent hepatic transplantation in the series reported by Hendriks et al. developed a hepatic artery thrombosis [19]. None of the 24 patients treated with rFVIIa in the double-blinded randomized trial of prostatectomy developed any adverse events during the study period, although one treated patient had a myocardial infarction on post-operative day 14, after the end of the 10-day study period [13]. Two clinical trials of substantial size have been completed: the trauma trial and the hepatic transplant trial discussed above. As these trials have been completed only recently, the results have yet to appear in the peer-reviewed literature. The sponsor, Novo Nordisk, reported that there were no differences in incidences of serious adverse events, including thromboembolic events, between rFVIIa and placebo-treated patients in both the trauma trial and the hepatic transplant trial.
In summary, rFVIIa appears likely to be efficacious in states of decreased concentration of coagulation factors, thrombocytopenia, and in at least some states of altered platelet function. While efficacy has been found in one small study, it has not been demonstrated in large, randomized, double-blinded, placebo-controlled trials published in the peer-reviewed literature. Many clinicians now use rFVIIa to treat intra-operative hemorrhages not easily controlled by conventional therapy. A trial of substantial size in spinal surgery may be undertaken in the near future. Until adequate data demonstrating safety and efficacy are fully reported, it would seem appropriate to reserve the use of rFVIIa in spinal surgery to those cases where conventional therapy cannot provide adequate hemostasis.
Acknowledgements
Supported, in part, by National Heart, Lung and Blood Institute Grant No. 1 P50 HL54476
Footnotes
The author serves as a consultant for Novo Nordisk A/S, but has not and is not participating in any sponsored investigations
References
- 1.BéguinHaemostasis 1999295010494034 [Google Scholar]
- 2.Bernstein Gastroenterology. 1997;113:1930. doi: 10.1016/s0016-5085(97)70013-1. [DOI] [PubMed] [Google Scholar]
- 3.Berntorp E, Stigendal L, Lethagen S, Olofsson L, Hedner U (2000) NovoSeven in warfarin-treated patients. Blood Coagul Fibrinolysis [Suppl 1] S113–S115 [DOI] [PubMed]
- 4.Billon Blood Coagul Fibrinolysis. 2001;12:551. doi: 10.1097/00001721-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Blomback Thromb Res. 1994;75:521. doi: 10.1016/0049-3848(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 6.Bouwmeester Obstet Gynecol. 2003;101:1174. doi: 10.1016/S0029-7844(03)00350-8. [DOI] [PubMed] [Google Scholar]
- 7.Ciavarella N, Schiavoni M, Valenzano E, Mangini F, Inchingolo F (1996) Use of recombinant factor VIIa (NovoSeven) in the treatment of two patients with type III von Willebrand’s disease and an inhibitor against von Willebrand factor. Haemostasis 26 [Suppl 1]:150–154 [DOI] [PubMed]
- 8.Collet Arterioscler Thromb Vasc Biol. 2000;20:1354. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 9.Collet J Biol Chem. 2003;278:21331. doi: 10.1074/jbc.M212734200. [DOI] [PubMed] [Google Scholar]
- 10.Deveras Ann Intern Med. 2002;137:884. doi: 10.7326/0003-4819-137-11-200212030-00009. [DOI] [PubMed] [Google Scholar]
- 11.Erhardtsen Semin Thromb Hemost. 2000;26:385. doi: 10.1055/s-2000-8457. [DOI] [PubMed] [Google Scholar]
- 12.Erhardtsen Blood Coagul Fibrinolysis. 1998;9:741. doi: 10.1097/00001721-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Friederich Lancet. 2003;361:201. doi: 10.1016/S0140-6736(03)12268-4. [DOI] [PubMed] [Google Scholar]
- 14.He J Thromb Haemost. 2003;1:1215. doi: 10.1046/j.1538-7836.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 15.Hedner Semin Thromb Hemost. 2000;26:363. doi: 10.1055/s-2000-8453. [DOI] [PubMed] [Google Scholar]
- 16.Hedner J Clin Invest. 1983;71:1836. doi: 10.1172/JCI110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HednerLancet 1988211932903400 [Google Scholar]
- 18.Hedner Haemostasis. 1989;19:335. doi: 10.1159/000216080. [DOI] [PubMed] [Google Scholar]
- 19.HendriksTransplantation 20017140211233901 [Google Scholar]
- 20.Hoffman J Thromb Haemost. 2003;1:606. doi: 10.1046/j.1538-7836.2003.t01-5-00177.x. [DOI] [PubMed] [Google Scholar]
- 21.Jeffers Gastroenterology. 2002;123:118. doi: 10.1053/gast.2002.34164. [DOI] [PubMed] [Google Scholar]
- 22.Kenet Lancet. 1999;354:1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- 23.Kjalke Br J Haematol. 2001;114:114. doi: 10.1046/j.1365-2141.2001.02870.x. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen J, Killander A, Hippe E, Hellegerg C, Ellegard J, Holm M, Kutt, J, Mellqvist U, Johansson J, Glazer S, Hedner U (1996) Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis 26 [Suppl 1]:159–164 [DOI] [PubMed]
- 25.Leibovitch Pediatr Crit Care Med. 2003;4:444. doi: 10.1097/01.PCC.0000074276.20537.0A. [DOI] [PubMed] [Google Scholar]
- 26.Martinowitz J Trauma. 2001;51:431. doi: 10.1097/00005373-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 27.McDonagh Semin Thromb Hemost. 1996;22:369. doi: 10.1055/s-2007-999034. [DOI] [PubMed] [Google Scholar]
- 28.Monroe Br J Haematol. 1997;99:542. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 29.Morenski J Neurosurg. 2003;98:611. doi: 10.3171/jns.2003.98.3.0611. [DOI] [PubMed] [Google Scholar]
- 30.Nesheim Thromb Haemost. 1997;78:386. [PubMed] [Google Scholar]
- 31.O’Connell Semin Hematol. 2004;41:76. doi: 10.1053/j.seminhematol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Pastores Chest. 2003;124:2400. doi: 10.1378/chest.124.6.2400. [DOI] [PubMed] [Google Scholar]
- 33.Peters Thromb Haemost. 1998;80:352. [PubMed] [Google Scholar]
- 34.Roberts Anesthesiology. 2004;100:722. doi: 10.1097/00000542-200403000-00036. [DOI] [PubMed] [Google Scholar]
- 35.Stratmann Ann Thorac Surg. 2003;76:2094. doi: 10.1016/S0003-4975(03)01052-X. [DOI] [PubMed] [Google Scholar]
- 36.Svartholm Anesthesiology. 2002;96:1528. doi: 10.1097/00000542-200206000-00041. [DOI] [PubMed] [Google Scholar]
- 37.Tengborn Thromb Haemost. 1996;75:981. [PubMed] [Google Scholar]
- 38.Tobias Anesthesiology. 2002;96:1522. doi: 10.1097/00000542-200206000-00039. [DOI] [PubMed] [Google Scholar]
- 39.Vidarsson Thromb Haemost. 2000;83:634. [PubMed] [Google Scholar]
- 40.Weisel J, Veklich Y, Collet J, Francis C (1999) Structural studies of fibrinolysis by electron and light microscopy. Thromb Haemost. 82:277–282 [PubMed]
- 41.WildgooseBlood 199280251611090 [Google Scholar]
- 42.Zaaroor M, Bar-Lavie M (2004) The use of recombinant factor VIIa in head injury: a report of five cases. Semin Hematol 41 [Suppl 1] :175–176